Abstract
A simple and efficient one pot synthesis of β-acetamido ketones from reaction of aldehydes, enolizable ketones, alkyl/aryl nitriles, and BF3/Et2O catalyst under microwave irradiations is described. This method allows synthesis of β-acetamido ketones without using corrosive and hazardous acetyl chloride. It is applicable for diversified aldehydes and active methylene ketones supported by synthesizing varieties of β-acetamido ketones. As per literature, majority of synthetic methods of β-acetamido ketones are restricted to use of acetonitrile lonely, as a nitrile component. This method is found to be equally effective for range of nitriles, also. Key features of reported method are simple reaction protocol, better yields, shorter reaction time, and nonhazardous reaction conditions which support the “Green Chemistry approach.”
Introduction
β-Amido ketones are important building blocks in Medicinal Chemistry because of their wide biological and pharmaceutical properties Citation1,Citation2. These are valuable intermediates for preparation of β-amino ketones/β-amino alcohols such as antibiotic nikkomycins Citation3,Citation4 and neopolyoxines Citation5. The best known and commonly used route for synthesis of these compounds is the Dakin–West reaction, Citation6 which involves condensation of an α-amino acid with acetic anhydride in presence of base, providing an α-acetamido ketones via azalactone intermediate Citation7. Common method for synthesis of β-acetamido ketones involves coupling of aryl aldehyde, enolizable ketone, and acetonitrile using acetyl chloride and varieties of Lewis acid catalysts such as CoCl2 Citation8–10, SiO2/H2SO4 Citation11, BiCl3 Citation13 Citation14, ZrOCl2.8H2O Citation11, Sc(OTf)3 Citation15, FeCl3.6H2O Citation16, ZnO Citation17, H6P2W18O62 Citation18, H3PW12O40 Citation19–24, Co(OAc)2 Citation25, p-TSA Citation26, Cu(BF4)2 Citation27–29, SnCl2.2H2O Citation30–33. Although, plethora of methods are reported for such transformations, some of these are lacking ability to produce versatile β-amido ketones as most of them are restricted to acetonitrile and benzonitrile moieties (8–14, 19, 20, 22, 25). Most of literature reported methods Citation8–33 comprise acetyl chloride usage as a key reagent for this transformation in spite of its corrosive and lacrimetric nature. Sometimes, it creates emulsion formation issue in reaction work up which end up with lower yields. So, it is noteworthy to develop an improved method for preparation of β-acetamido carbonyl compounds using better substituent for acetyl chloride or avoiding its use completely. In continuation of our research work to develop new synthetic methods for preparation of biologically active heterocyclic compounds Citation34,Citation35, herein we pleased to report microwave assisted neat synthesis of β-acetamido ketones using BF3/Et2O catalyst. This is an unique synthetic method wherein acetyl chloride is not required for desired transformation. The reported method is advantageous over known methods in terms of short reaction time, ease of reaction protocol and work up protocol, solvent-free reaction conditions and avoiding usage of acetyl chloride.
Results and discussion
A range of catalysts were screened to synthesize representative β-acetamido ketone (N-(3-oxo-1,3-diphenylpropyl) acetamide (4a) from reaction of benzaldehyde, acetophenone, and acetonitrile in absence of acetyl chloride (). Out of screened catalysts (CuCl2, CuI, ZnCl2, ZrOCl2.8H2O, FeCl3.6H2O, SnCl2.2H2O); formation of 4a was observed using catalysts BF3/Et2O (Conversion: 45%) and ZrOCl2.8H2O (Conversion: 17%) catalysts only. To improve isolated yield of product using BF3/Et2O catalyst, various reactions were checked using different solvents and temperatures. But, isolated yields improved up to 64% only, in optimized reaction conditions (Reaction condition: benzaldehyde (1 equi.), acetophenone (1 equi.), BF3.Et2O (20 mol%.) in acetonitrile solvent under reflux for 8 hr). For further improvement of yield and reaction time, reaction was checked using microwave irradiation. Using microwave, reaction went to completion within 30 min at 25°C which on work up afford 76% desired product. Reaction conditions were standardized in terms of solvent and reaction temperature ().
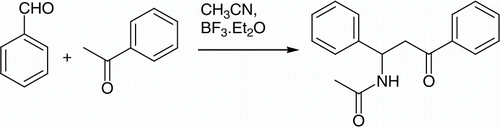
Table 1. Reaction conditions optimization for preparation of N-(3-oxo-1,3-diphenylpropyl) acetamide (4a) under microwave irradiations.
Among solvents checked for optimization of reaction conditions (acetonitrile, THF, ethanol, water, ethanol–water, and THF–water), water (, entry 6) was found to be superior at 25°C end up with 84% yield.. More fascinatingly, in solvent-free reaction conditions (, entry 7), product 4a was isolated with 92% yield as an off-yellow solid product. Further tuning of reaction conditions, end up with optimized reaction conditions, that is use of benzaldehyde (1 equi.), acetophenone (1 equi.), BF3.Et2O (20 mol%) catalyst at 70°C for 5–10 min reaction time (, entry 10).
Variety of β-acetamido ketones were synthesized using different substituted aldehydes, acetophenone, and acetonitrile using optimized reaction conditions. Results obtained are summarized in . Yields obtained were ranging from 91% to 98%. 5–10 min reaction time was found to be sufficient to drive reaction toward completion. Method found to be advantageous over reported methods Citation11–30 in terms of shorter reaction time and ease of reaction protocol. Literature reveals, majority reported methods required 4–15 hr reaction time depending on substrates (11, 15, 16), while present method require 5–10 min only for range of substrate. This is possible most probably due to usage of energetic microwave irradiations.
Table 2. Synthesis of β-acetamido ketones from aldehydesa, acetonitrile, and acetophenone and BF3.Et2O catalyst under microwave irradiations.
Especially for synthesis of compounds 4b, 4f, and 4i using ZrOCl2.8H2O catalyst, yields obtained were 85%, 80%, and 92%, respectively and reaction times required were 8, 12, and 7 hr, respectively Citation13,Citation14. While these products (4b, 4f, and 4i) were isolated in 95%, 93%, and 94% yield (respectively, entry 2, entry 6, and entry 9 in ) in 5 min reaction time (). Work up protocol of reaction includes, the triturating reaction mass with 2% aqueous Na2CO3 solution (5 vol) at 40–45°C to get precipitation of the product followed by filtration and water washings (2×2 vol) to afford desired product of sufficient HPLC purity (>90%). Crude products were further purified by recrystallization using suitable solvent like ethanol and MTBE.
Applicability of method was checked for synthesis various β-acetamido ketones using substituted acetophenones and different nitrile components. Present method was found to be versatile and equally effective for range of substituted aldehydes, acetophenones, and substituted nitrile components (). Yields obtained were in the range of 91%–96% and 5–10 min reaction time. Literature reveals, synthesis of 5l, 5m, and 5n with 86% yield (reaction time – 3.5 hr), 88% yield (reaction time – 3.0 hr), and 89% yield (reaction time – 3.5 hr), respectively using Cu(BF4)2 catalyst along with acetyl chloride Citation28,Citation29.. Whereas using present method, these compounds were isolated in 93%, 91%, and 94% yields, respectively in 5 min reaction time in absence of acetyl chloride reagent. It indicates the use of reported method for diversified substituents affording better yields in shorter reaction time.
Table 3. Synthesis of β-acetamido ketones from different enolizable ketones, aldehydes, nitriles, and BF3.Et2O catalyst under microwave irradiations.
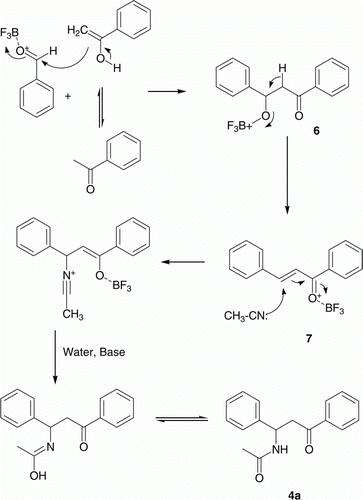
Sufficient data on mechanistic study for this reaction in presence of acid catalyst and acetyl chloride is published in literature. It proceeds via knoevenagel condensation of aldehyde and ketone to afford α-hydroxy ketone which is subsequently being protected with acetyl group (from acetyl chloride) followed by the nucleophilic displacement by alkyl/aryl nitrile to from the desired product (17, 19, 20, 24). Therefore, acetyl chloride plays a vital role to allow nucleophilic attack of nitrile component. To write a plausible mechanism of this reaction in absence of acetyl chloride, initially we considered the formation of α-hydroxy ketone via knoevenagel condensation of aldehyde and ketone in presence of BF3/Et2O. Later on, one aspect to get desired product is hydrogen bonding between hydroxyl (−OH) group and BF3/Et2O to convert free −OH group to a good leaving group. This will allow the nucleophilic attack of nitrile component to get formation of desired product. If this is a reaction path, the desired products should be obtained with various acid catalysts in absence of acetyl chloride, also. Since this is not a case, this aspect was discarded. To get idea about an intermediate formation (if any), we set up one reaction for preparation of 4a using benzaldehyde, acetophenone, and acetonitrile under microwave irradiations at 20°C. Reaction was taken for work up at intermittent stage only and reaction mass was analyzed on TLC and HPLC. It showed formation of two additional products apart from benzaldehyde and ketone. Out of these two, one was knoevenagel product from benzaldehyde & ketone and second was found to be a corresponding α,β-unsaturated ketone (Matching with standard on TLC and HPLC). It clearly indicates formation of knoevenagel product from aldehyde and ketone followed by α,β-unsaturated ketones. The subsequent step should be 1,4-Michael addition of nitriles in presence of BF3/Et2O to afford desired product. Chalcone formation (α,β-unsaturated ketones) is well reported in literature Citation36 using various acid catalysts including BF3/Et2O which support first two steps of reaction path Citation36. To support nucleophilic Michael addition of nitrile to chalcone, representative chalcone (7a) was treated with acetonitrile in presence of 10 mol% BF3/Et2O under microwave irradiations. The formation of desired 4a product was observed in 95% yield. This reaction was explored by treating representative chalcones (7a and 7b) with acetonitrile and benzonitrile () to support the plausible reaction pathway as depicted in reaction .
Table 4. Synthesis of β-acetamido ketones using BF3.Et2O catalyst using various chalcones and nitriles under microwave irradiations.
In conclusion, an efficient method for synthesis of β-acetamido ketones has been developed using BF3.Et2O catalyst under microwave irradiation. Advantageous of present method are higher yields, shorter reaction time, ease of reaction protocol, and solvent-free reaction conditions. Present method does not require use of acetyl chloride like reported methods and leading to avoid usage of corrosive and hazardous chemicals. So, this is an effective and successful approach for synthesizing β-amido ketones in absence of acetyl chloride reagent.
Experimental
All chemicals/reagents used for reactions were procured from either Sigma Aldrich or Across chemical suppliers. Melting points were obtained with an SRS Optimelt melting point apparatus and are uncorrected. Microwave reactions were carried out using Ethusi Milestone (MicroSynth) Labstation with temperature control. Infra-red (IR) spectra were recorded using JASCO FT-IR 4000 in KBr powder. NMR spectra were recorded on 400 MHz Bruker spectrometer using tetramethylsilane as an internal standard. The abbreviations used for NMR interpretation are: singlet – s, doublet – d, triplet – t, quartet – q, multiplate – m, and broad singlet – bs. Mass spectra were recorded on Micromass-QUATTRO-II of WATER mass spectrometer.
General procedure for the synthesis of β acetamido ketones derivatives using microwave
A stirred solution of aldehyde (10 mmol), nitrile (15 mmol), ketone (10 mmol), and BF3/ Et2O (2 mmol) was irradiated in microwave at 70°C till completion of reaction. Reaction monitored on TLC (mobile phase = 20% Ethyl Acetate in Hexane). After completion of reaction, product was triturated with 3% aqueous sodium carbonate solution and resulted reaction mass was stirred for 30 min. Reaction mass filtered on Buchner funnel and washed with water (4V) for twice and suck dried to get the crude product. The crude products were recrystallized using suitable solvent like ethanol/isopropanol.
Spectral data for representative β-acetamido ketones
N-(3-oxo-1,3-diphenylpropyl)acetamide (4a):
White Crystal, IR (KBr): 3290, 3080, 2950, 1705, 1646, 1570, 1480, 1350, 1225, 995, 750 cm−1; ES-MS m/z (%):268 (M + H); 1H NMR (400 MHz, CDCl3)1.95 (3H, s), 3.63 (1H, dd, J=6.0 and 16.8 Hz),3.85 (1H, dd, J= 5.2 and 16.8 Hz), 5.63 (1H, m), 6.85 (1H, d, J=7.6 Hz), 7.25 (5H, m),7.53 (2H, t, J=8.0 Hz), 7.65 (1H, t, J =0.6 Hz, Ph), 7.85(2H, d, J=8.0 Hz); 13C NMR (400 MHz, CDCl3) 24.8, 43.8, 51.5, 126.3, 127.4, 128.3, 128.8, 133.6, 135.8, 141.7, 168.3, 194.3.
N-[1-(4-Chlorophenyl)-3-oxo-3-phenyl-propyl]acetamide (4b)
White Crystal, IR (KBr): 3180, 2977, 1690, 1705, 1555, 1465, 1380, 1240, 1095, 780 cm−1; ES-MS m/z (%): 302 (M + H); 1 H NMR (400 MHz, CDCl3) 1.90(3H, s), 3.65 (1H, dd, J=6.0 and 16.8 Hz), 3.95(1H, dd, J=5.2 and 17.2 Hz), 5.70 (1H, m), 6.74 (1H, d, J=7.2 Hz), 7.35 (4H, d, J = 4.4 Hz), 7.50 (2H, t, J=8.0 Hz), 7.70(1H, t, J=7.6 Hz),7.95 (2H, d, J=8.4 Hz); 13C NMR (400 MHz, CDCl3) 23.4, 43.7, 49.9, 127.7, 128.2, 128.5, 133.1, 133.6, 140.5, 142.8, 148.5, 165.2, 194.7.
N-(3-oxo-1-phenyl-3-p-tolylpropyl)acetamide (5a)
Off white solid, IR (KBr): 3285, 2954, 1685, 1631, 1365, 1241, 1159, 990, 774 cm−1; ES-MS m/z (%):282 (M + H); 1H NMR (400 MHz, CDCl3) 1.92 (s, 3H), 2.26 (s, 3H), 2.96 (dd, 1H, J =6.4 and 16.8 Hz), 3.14 (2H, J=8.8 Hz), 7.16 (m, 7H), 7.38 (d, 2H, J =7.6 Hz);13C NMR (400 MHz, CDCl3) 23.1, 24.6, 49.1, 63.5, 54.5, 115.4, 126.8, 127.4, 133.9, 141.0, 143.3, 171.2, 201.3.
N-(3-(4-nitrophenyl)-3-oxo-1-phenylpropyl)acetamide (5b)
Off white solid, IR (KBr): 3265, 3126, 2952, 1665, 1616, 1492, 1359, 986, 665 cm−1; ES-MS m/z (%):311(M–H); 1H NMR (400 MHz, CDCl3) 2.03(s, 3H), 3.10 (dd, 1H, J =7.5 and 12.2 Hz), 3.23(2H, J=9.6 Hz), 7.21 (m, 5H), 7.56 (d, 2H, J=7.2 Hz), 8.01 (d, 2H, J=7.2 Hz); 13C NMR (400 MHz, CDCl3) 23.4, 45.2, 71.5, 122.0, 126.8, 127.1, 131.0, 142.3, 158.1, 176.4, 199.3.
Acknowledgements
Authors are thankful to the Head, Department of Chemical Technology, Dr Babasaheb Ambedkar Marathwada University, Aurangabad- 431004 (MS), India for providing the laboratory facility.
References
- Casimir , J.R. ; Turetta , C. ; Ettouati , L. ; Paris , J. Tetrahedron Lett . 1995 , 36 , 4797 .
- Godfrey , A.G. ; Brooks , D.A. ; Hay , L.A. ; Peters , M. ; McCarthy , J.R. ; Mitchell , D. J. Org. Chem . 2003 , 68 , 2623 .
- Barluenga , J. ; Viado , A.L. ; Aguilar , E. ; Fustero , S. ; Olano , B. J. Org. Chem . 1993 , 58 , 5972 .
- Henderson , D.P. ; Shelton , M.C. ; Cotterill , I.C. ; Toone , E.J. J. Org. Chem . 1997 , 62 , 7910 .
- Uramoto , M. ; Kobinata , K. ; Isono , K. ; Higashijima , T. ; Miyazawa , T. ; Jenkins , E.E. ; McCloskey , J. Tetrahedron Lett . 1980 , 21 , 3395 .
- Dakin , H.D. ; West , R. J. Biol. Chem . 1982 , 78 , 745 .
- Buchanan , G.L. Chem. Soc. Rev . 1988 , 17 , 91 .
- Mukhopadhyay , M. ; Bhatia , B. ; Iqbal , J. Tetrahedron Lett . 1997 , 38 , 1083 .
- Bhatia , B. ; Reddy , M.M. ; Iqbal , J. J. Chem. Soc. Chem. Commun . 1994 , 713 .
- Rao , I.N. ; Prabhakaran , E.N. ; Das , S.K. ; Iqbal , J. J. Org. Chem . 2003 , 68 , 4079 .
- Khodaei , M.M. ; Khosropour , A.R. ; Fattahpour , P. Tetrahedron Lett . 2005 , 46 , 2105 .
- Bahulayan , D. ; Das , S.K. ; Iqbal , J. J. Org. Chem . 2003 , 68 , 5735 .
- Ghosh , R. ; Maity , S. ; Chakraborty , A. Synlett 2005 , 1 , 115 .
- Ghosh , R. ; Maiti , S. ; Chakraborty , A. ; Chakraborty , S. ; Mukherjee , A.K. Tetrahedron 2006 , 62 , 4059 .
- Pandey , G. ; Singh , R.P. ; Garg , A. ; Singh , V.K. Tetrahedron Lett . 2005 , 46 , 2137 .
- Khan , A.T. ; Parvin , T. ; Choudhury , L.H. Tetrahedron 2007 , 63 , 5593 .
- Maghsoodlou , M.T. ; Hasssankhani , A. ; Shaterian , H.R. ; Habibi-Khorasani , S.M. ; Mosaddegh , E. Tetrahedron Lett . 2007 , 48 , 1729 .
- Heravi , M.M. ; Ranjbar , L. ; Derikvand , F. ; Bamoharram , F.F. Catal. Commun . 2007 , 8 , 289 .
- Rafiee , E. ; Shahbazi , F. ; Joshaghani , M. ; Tork , F. J. Mol. Catl. A . 2005 , 242 , 129 .
- Rafiee , E. ; Tork , F. ; Joshaghani , M. Bioorg. Med. Chem. Lett . 2006 , 16 , 1221 .
- Das , B. ; Reddy , K.R. Helv. Chim. Acta . 2006 , 89 , 3109 .
- Yakaiah , T. ; Reddy , G. ; Lingaiah , P.V. ; Marcia , B. ; Rao , P.S. Synth. Commun . 2005 , 35 , 1307 .
- Salama , T.A. ; Elmorsy , S.S. ; Khalil , G.M. ; Ismail , M.A. Tetrahedron Lett . 2007 , 48 , 6199 .
- Khan , A.T. ; Choudhury , L.H. ; Parvin , T. ; Ali , M.A. Tetrahedron Lett . 2006 , 47 , 8137 .
- Prabhakaran , E.N. ; Iqbal , J. J. Org. Chem . 1999 , 64 , 3339 .
- Das , B. ; Reddy , K.R. ; Srinivas , Y. ; Kumar , R.A. Can. J. Chem . 2007 , 85 , 479 .
- Yadav , J.S. ; Subba Reddy , B.V. ; Shankar , K.S. ; Premalatha , K. Org. Commun . 2008 , 14 , 76 .
- Shaterian , H.R. ; Yarahmadi , H. ; Ghashang , M. Arkivoc 2007 , Xvi , 298 .
- Nagarajan , P.S. ; et al. Arkivoc 2009 , X , 265 – 282 .
- Nagarapu , L. ; Bantu , R. ; Puttireddy , R. Appl. Catal. A: Gen . 2007 , 332 ( 2 ), 304 .
- Yakaiah , T.V. ; Lingaiah , B.P. ; Reddy , V.G. ; Narsaiah , B. ; Rao , P.S. Arkivoc . 2007 , 13 , 227 .
- Bhat , R.P. ; Raje , V.P. ; Alexander , V.M. ; Patil , S.B. ; Samant , S.D. Tetrahedron Lett . 2005 , 46 , 4801 .
- Mirjalilia , B.F. ; Bamonirib , A. ; Karimi Zarchia , M.A. ; Emtiazi , H. J. Iran Chem. Soc . 2010 , 7 , 95 .
- Kokare , N.D. ; Nagawade , R.R. ; Rane , V.P. ; Shinde , D.B. Synthesis . 2007 , 4 , 766 .
- Sangshetti , J.N. ; Shinde , D.B. Lett. Drug Design & Disc . 2010 , 7 , 171 .
- Narender , T. ; Reddy , P.K. Tetrahedr. Lett . 2007 , 48 , 3177 .