Abstract
There is a growing interest in applying green chemistry for nanocatalysis applications. On the basis of a Scifinder Scholar search, the field of applying green chemistry to catalysis with nanoparticles has undergone an explosive growth from year 2002 to present. It can be seen that green chemistry applied to nanocatalysis is a relatively hot area with much room for growth. I discuss several review articles written about the use of green nanocatalysts as well as green reactions. I discuss studies involving the synthesis of green nanocatalysts and application of metal nanocatalysts in green reactions. I have organized the discussion of green nanocatalysts by the type of nanoparticles that are synthesized and used as catalysts. I have organized discussions of green reactions by the type of green reaction that is being conducted. Overall, our review article discusses developments in new types of green nanocatalysts as well as developments in green catalytic reactions.
1. Introduction
There has been an explosive growth in the field of green chemistry both in preparing green nanocatalysts as well as green conditions during catalysis of industrially important reactions. Preparing green nanocatalysts refers to synthesizing the nanocatalysts using green solvents or processing the nanocatalysts so that they are finally dispersed in green solvents. Green nanocatalysis refers to doing the catalytic reaction in green solvents and preferably by the use of green nanocatalysts for these reactions. There have been many review articles in green nanocatalysts and industrially important green reactions which I will discuss here. The typical size of green nanocatalysts is 2–3 nm since this is the optimal size range for many catalytic reactions. Of course, there are also many exceptions to this general trend and other larger sizes of green nanocatalysts have also been synthesized for these purposes. Dendritic catalysts are discussed in the literature context, and organo-iron redox catalysts are used for nitrate and nitrile cathodic reduction in water Citation1. The combination of chiral and nanotechnologies is a promising strategy for green catalytic conversions Citation2. The different types of recyclable nanostructured catalytic systems that are used in environmentally friendly organic synthesis is discussed Citation3. Catalysis with nanoparticles using supercritical solvents with catalyst separation techniques is promising for the development of green chemical processes Citation4. Imidazolium-based ionic liquids have been shown to be a good media for the synthesis of metal nanoparticles. These nanocatalysts are efficient green catalysts for several reactions in multiphase conditions Citation5. The recent progress in the use of supported metal nanoparticles for sustainable green catalysis is described Citation6.
The preparation, characterization, and catalytic performance of bimetallic nanoparticles for solvent-free hydrogenation reactions have been reviewed Citation7. Heterogeneous gold, silver, and copper nanoparticles using hydrotalcite as the support material have been used for environmentally benign organic transformations such as aerobic oxidation of alcohols, lactonization of diols, and deoxygenation of epoxides and nitro aromatic compounds Citation8. One review article focuses on nanocatalysis for green chemistry development such as using microwave heating with nanocatalysis in benign aqueous reaction media Citation9. A variety of new nanocatalysts that have high relevance to green chemistry are discussed such as molecular sieve catalysts for regioselective oxyfunctionalization of alkanes, Fischer–Tropsch catalysts for the production of fuel, enantioselective catalysts, etc Citation10. There is a review which focuses on the green aspects that arises when catalytically active metal nanoparticles are present in ionic liquid media Citation11. Transition metal nanoparticles in environmentally friendly solvents such as ionic liquids have been reviewed Citation12.
In this article, I focus on green nanocatalysts as well as industrially important green reactions. There are two parts in this article. The first part involves green nanocatalysts and the second part involves green reactions.
2. Synthesis of green nanocatalysts
2.1. Silica nanoparticles
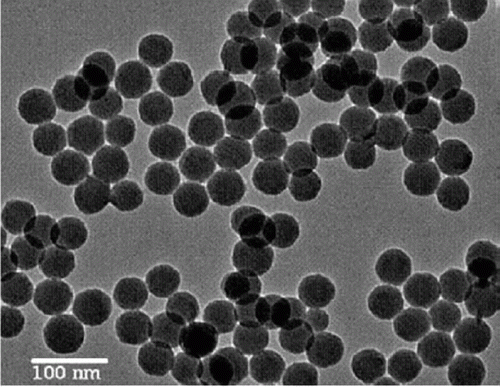
Silica nanoparticles that are mild and environmentally benign have been used as catalysts for synthesis of highly substituted pyridines Citation13. These catalysts retained most of their catalytic activity after being reused three times. shows a TEM image of the silica nanoparticles that are used as catalysts for the synthesis of highly substituted pyridines Citation13.
Figure 1. TEM image of silica nanoparticles used as catalysts for the one step, three-component synthesis of highly substituted pyridines (13). Reprinted with permission from Elsevier © 2009.
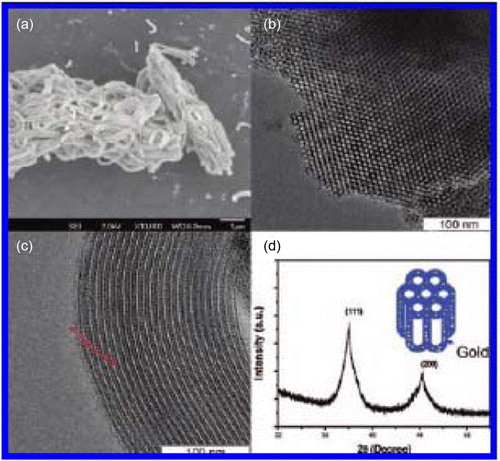
Silica colloids and nanoparticles can be easily synthesized using the Stoeber synthesis method and redispersed in water which is an environmentally friendly solvent. Environmentally friendly silica nanoparticles have been used as catalysts for the synthesis of thioethers, thioesters, vinylthioethers, and thio-Michael adducts Citation14. Very mild, neutral, and reusable silica nanoparticles have been used as catalysts for the synthesis of 4H-pyrans and polysubstituted aniline derivatives Citation15. Tubular silica nanostructures are synthesized by environmentally benign and mild conditions. These catalysts are found to be very active and selective in the microwave-assisted homocoupling of terminal alkynes. The three-component synthesis of highly substituted pyridines has been conducted using mild, effective, and environmentally benign silica nanoparticles Citation13.
2.2. Titania nanoparticles
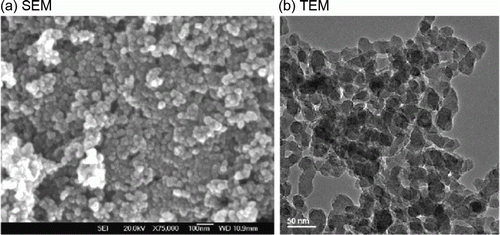
The titania nanoparticles are used as catalyst using a supercritical carbon dioxide anti-solvent process synthesis method Citation16. The size of the titania nanoparticles varies from 27 to 78 nm. shows the SEM and TEM images of the titania nanoparticles Citation16.
2.3. Palladium nanoparticles
Palladium nanoparticles can be synthesized using a variety of green methods such as the hydrogen reduction method in water as solvent, ethanol reduction method in an ethanol–water mixture in which the ethanol can be rotovaped, and several other green methods. Palladium nanoparticles capped with polyoxometalate have been used as green electrocatalysts Citation17. It was observed that the palladium nanoparticles are highly efficient catalysts for the hydrogen evolution reaction. Palladium nanoparticles that are entrapped in aluminum oxy-hydroxide in solvent-free condition have been used as catalysts for the hydrogenation of alkenes and aromatic nitro compounds to form the corresponding alkanes and amino compounds Citation18. A green synthesis method has been used to prepare palladium nanoparticles which are used as catalysts for the hydrodechlorination of trichloroethene Citation19. The palladium nanoparticles are synthesized by using sodium cellulose as the stabilizer and ascorbic acid as the reducing agent. The Pd nanoparticles have high catalytic activity for the hydrodechlorination reaction of trichloroethene.
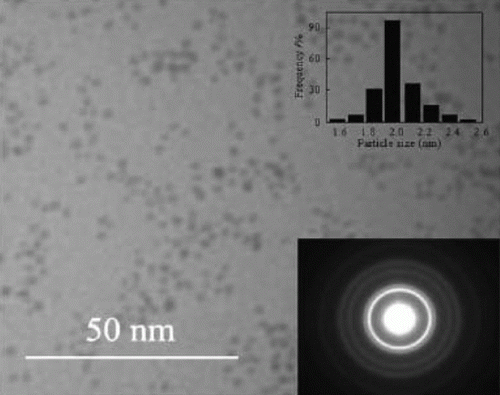
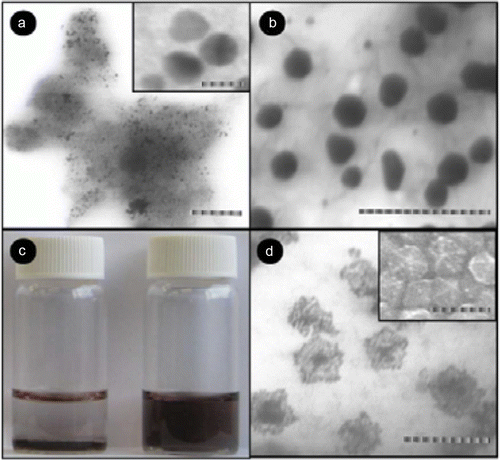
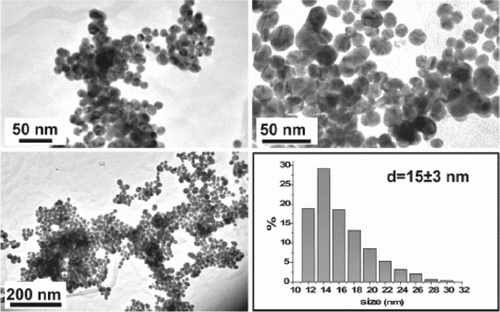
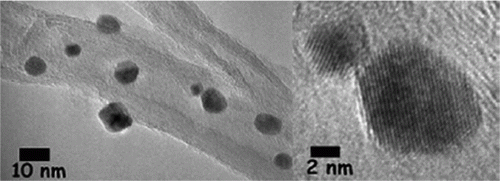
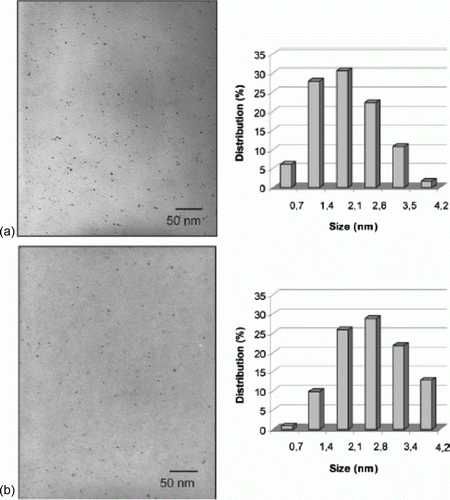
The palladium nanoparticles are stabilized using various types of ionic liquids which are green solvents Citation20. The palladium nanoparticles have been used as catalysts for the hydrogenation of acetophenone and were observed to be the most efficient catalysts for this reaction. Palladium nanoparticles of different shapes such as nanobelts, nanorods, nanoplates, and nanotrees were synthesized using water as the solvent and vitamin B1 as a reducing agent Citation21. These palladium nanoparticles showed excellent catalytic activity for Suzuki, Heck, and Sonogashira reactions. shows SEM images of the palladium nanoparticles synthesized using vitamin B1 as a reducing agent Citation21. A biomimetic method has been used for the synthesis of palladium nanoparticles as model green catalytic systems Citation22. These palladium nanoparticles were used as catalysts for the Stille coupling reaction in aqueous solvent. shows the TEM image and size distribution of the palladium nanoparticles that are synthesized using the biomimetic synthesis method Citation22.
Figure 3. SEM images of palladium nanoparticles of different shapes that are used as catalysts for the carbon–carbon coupling reactions (21). Reproduced by permission of the Royal Society of Chemistry.
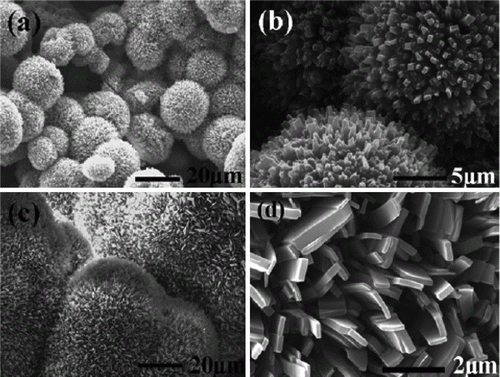
Figure 4. TEM image of the palladium nanoparticles prepared using the biomimetic synthesis method (22). Reprinted with permission from American Chemical Society © 2009.
Palladium nanoparticles prepared in the presence of ionic liquids have been used as catalysts for the Heck reaction of haloarenes and olefins Citation23. Palladium–sepiolite catalysts are synthesized in the presence of ionic liquids Citation24. They have been used as catalysts for hydrogenation of alkenes and the Heck cross-coupling reactions. Palladium nanoparticles were used as catalysts for the hydrodechlorination of chloroarenes in water Citation25. They have also been used for aerobic oxidation of different alcohols in water to form ketones, aldehydes, and carboxylic acids. Palladium nanoparticles have been synthesized using supercritical carbon dioxide as the solvent and this is a green process since supercritical carbon dioxide is an environmentally benign solvent Citation26. Palladium nanoparticles are prepared using a mixture of water and ionic liquid as the solvent and are used to catalyze the Heck cross-coupling reaction Citation27. The palladium nanoparticles were found to be very efficient catalysts for the Heck reaction. shows TEM images and electron diffraction data for the palladium nanoparticles prepared in a mixture of water and ionic liquid Citation27.
Figure 5. TEM images of the palladium nanoparticles after removing ionic liquid in solution, size distribution histogram and electron diffraction patterns (27). Reproduced by permission from the Royal Society of Chemistry.
Green palladium nanoparticles prepared using CM-cellulose as the stabilizer and ascorbic acid as the reducing agent were used as catalysts for the hydrodechlorination of trichloroethene and were found to have high catalytic activity Citation19. Ionic liquids and supercritical carbon dioxide have been used for solventless palladium nanoparticle catalyzed hydrogenations Citation20. The nanoparticles are stabilized by the ionic liquids and supercritical carbon dioxide extraction was used for the removal of the metal precursor ligands in the catalyst synthesis step. The catalysts are used for the palladium nanoparticle catalyzed hydrogenation of acetophenone.
2.4. Platinum nanoparticles
Platinum nanoparticles can be synthesized using a variety of green methods such as the hydrogen reduction method in water as solvent, ethanol reduction method in an ethanol–water mixture in which the ethanol can be rotovaped, and several other green methods. Monodisperse green platinum nanoparticles were synthesized by using glucose as the reducing agent and starch as the stabilizer Citation28. This synthesis method is simple, environmentally friendly, highly reproducible, and easy to scale up. These nanocatalysts were tested for reduction and oxidation reactions and were found to have high catalytic activity. Monodisperse platinum nanoparticles that are stabilized with ionic liquids have been used as catalysts for four-electron reduction of dioxygen to water Citation29.
shows a TEM image of the platinum nanoparticles that are stabilized using ionic liquids Citation29. The environmentally friendly synthesis of platinum nanoparticles decorated carbon nanotubes is reported Citation30. The nanohybrid catalyst shows higher electrocatalytic activity for the methanol oxidation reaction compared to traditional platinum-based catalysts. Platinum nanoparticles deposited onto expandable grapheme sheet were synthesized using a green electrochemical route Citation31. This catalyst is found to have high catalytic activity for the oxidation of methanol.
2.5. Gold nanoparticles
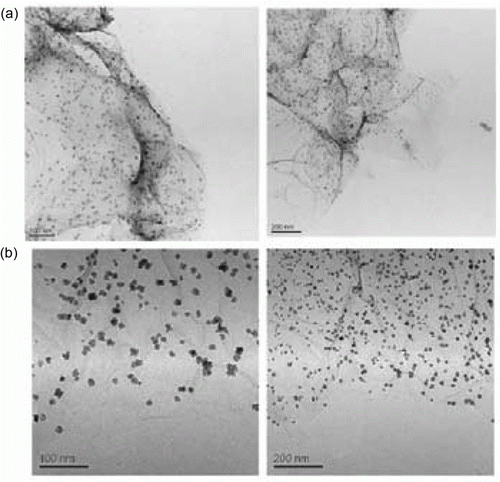
Gold nanoparticles have been synthesized using several green methods such as the seed-mediated growth method, doing the synthesis in the presence of ionic liquids, and other reduction methods such as hydrazine reduction method, and sodium borohydride reduction method. Gold nanoparticles supported on mesoporous silica have been used as catalysts for the alkane oxidation reaction Citation32. The gold nanoparticles are found to be robust and can be recycled. shows SEM and TEM images of the gold nanoparticles that are formed Citation32. Room temperature green imidazolium-based ionic liquids such as 1-butyl-3-methylimidazolium hexafluorophosphate (BMIMPF6) are used as liquid media for the synthesis of gold nanoparticles Citation33. It was found that the PVP-capped gold nanoparticles synthesized in the presence of DMIMPF6 are the most stable against aggregation. Gold nanoparticles that are 20 nm prepared by addition of HAuCl4 to green tea leaves extract at room temperature. The synthesis of the gold nanoparticles does not involve any toxic chemicals or organic solvents so it is a green synthetic process. The gold nanoparticles are used as catalysts for the reduction of methylene blue dye Citation34. Gold nanoparticles have been synthesized by a green photocatalytic method in which the synthesis is conducted in water Citation35. Calcium-alginate stabilized gold nanoparticles are prepared using a photochemical green synthetic method Citation36. These nanoparticles are used as catalysts for the 4-nitrophenol reduction reaction. Gold/TS-1 nanoparticles are prepared using two green routes which are sol-immobilization method and adsorption–reduction method Citation37. This gold nanoparticle catalyst was found to have excellent performance for the propylene oxidation reaction.
2.6. Silver nanoparticles
Silver nanoparticles have been synthesized using several green methods such as the seed-mediated growth method, doing the synthesis in the presence of ionic liquids, and other reduction methods such as hydrazine reduction method, and sodium borohydride reduction method. Silver nanoparticles have been synthesized by a green photocatalytic method in which the synthesis is conducted in water Citation35. shows silver nanoparticles that are synthesized using a photocatalytic process Citation35. Calcium-alginate stabilized silver nanoparticles are prepared using a photochemical green synthetic method Citation36. These nanoparticles are used as catalysts for the 4-nitrophenol reduction reaction.
2.7. Palladium–gold nanoparticles
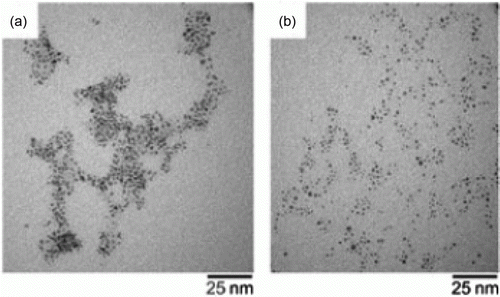
Room temperature green imidazolium-based ionic liquids such as BMIMPF6 have been used as liquid media for the synthesis of palladium–gold nanoparticles Citation33. The catalytic activity of the PdAu nanoparticles were tested for the hydrogenation of 1,3-cyclooctadiene and 3-buten-1-ol and it was observed that the PVP-capped PdAu nanoparticles are the most catalytically active. Gold–palladium nanoparticles that are catalytically active are prepared in the absence of organic ligands and adsorbed onto titanium dioxide supports and is found to be stable in oxidative catalysis conditions Citation38. It was found that 70% gold, 30% Pd composition of the bimetallic nanoparticles results in the highest catalytic activity for the oxidative catalysis.
shows TEM images of the gold–palladium nanoparticles which are synthesized without the use of organic liquids as stabilizers Citation38. Gold nanoparticles are prepared by using a green synthesis method by using 2,3-dihydroxyfumaric acid (DHFA) as the reducing agent and PVP as the stabilizer Citation39. These gold nanoparticles were found to have good catalytic activity for aerial oxidation of hydroxybenzyl alcohols. Ceria is prepared by using supercritical anti-solvent precipitation and serves as a support for gold–palladium nanoparticles for the oxidation of alcohols Citation40.
2.8. Nickel–platinum nanoparticles
Bimetallic nanoparticles have been synthesized using the ethanol reduction method, hydrogen reduction method, and other green methods. Nickel encapsulated by Pt (NiPt) has been synthesized using a green colloidal method Citation41. Platinum nanoparticles are very expensive as electrocatalysts so the remedy for this is to reduce the cost by the synthesis of NiPt bimetallic nanoparticles.
2.9. Aluminum oxide nanoparticles
Solvent-free methods as well as methods involving the use of water as solvent have been used to synthesize aluminum oxide nanoparticles. Aluminum oxide nanoparticles are synthesized in water as the solvent which makes it a green nanocatalyst Citation42. It is used as a catalyst for synthesis of 1,5-benzodiazepine and 1,5-benzothiazepine derivatives in mild conditions. The green aluminum oxide nanoparticles are used for the solvent-free synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones and result in higher yields with shorter reaction times Citation43.
2.10. Iron nanoparticles
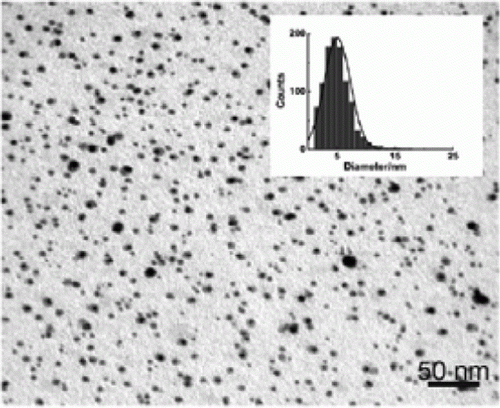
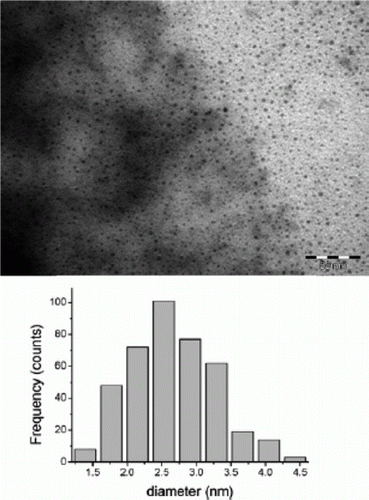
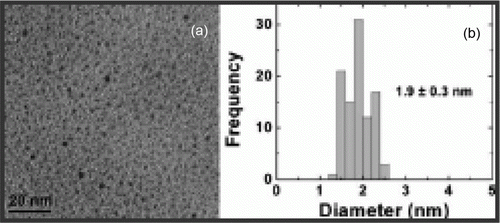
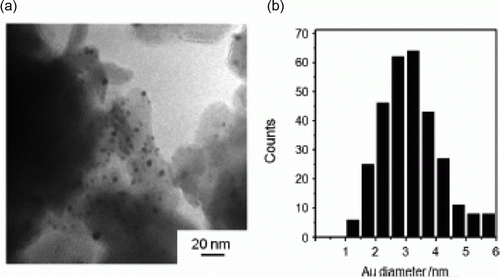
Iron nanoparticles have been synthesized using polymers as capping agents in water as green solvent as well as other types of green methods. A green synthesis of iron nanoparticles has been prepared by using tea polyphenols without the use of additional polymers and surfactants Citation44. The iron nanoparticles are used to catalyze the hydrogen peroxide for treatment of organic contamination. Iron nanoparticles have been used as environmentally benign catalysts for alkene and alkyne hydrogenations Citation45. shows the TEM image and size distribution histogram of the iron nanoparticles that has been synthesized as catalysts for environmentally benign alkene and alkyne hydrogenation reactions Citation45.
2.11 Rhodium nanoparticles
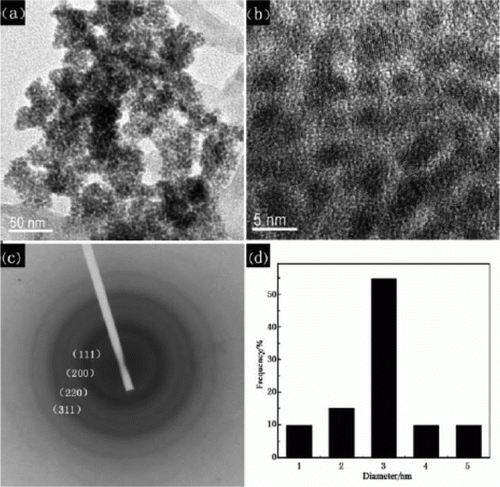
Rhodium nanoparticles can be synthesized using a variety of green methods such as the hydrogen reduction method in water as solvent, ethanol reduction method in an ethanol–water mixture in which the ethanol can be rotovaped, and several other green methods. Rhodium nanoparticles adsorbed onto titanium dioxide supports are synthesized using water as the solvent Citation46. They are also highly active catalysts for the arene hydrogenation reaction in water. The rhodium nanoparticles are stabilized using various types of ionic liquids which are green solvents Citation20. The rhodium nanoparticles have been used as catalysts for the hydrogenation of acetophenone. Hydrogenations of different nitrogen-, oxygen-, and sulfur-heterocyclic aromatic compounds have been catalyzed by using rhodium nanoparticles synthesized in water Citation47. shows TEM images of different sizes of rhodium nanoparticles synthesized using rhodium nanoparticles synthesized using water as the solvent Citation47. The reaction is conducted in mild reaction conditions, room temperature, and atmospheric pressure.
Figure 11. Rhodium nanoparticles synthesized in water and used as catalysts for hydrogenation of different heteroaromatic compounds (47). Reprinted.
Ionic liquids and supercritical carbon dioxide have been used for solventless rhodium nanoparticle catalyzed hydrogenations Citation20. The nanoparticles are stabilized by the ionic liquids, and supercritical carbon dioxide extraction was used for the removal of the metal precursor ligands in the catalyst synthesis step. The catalysts are used for the rhodium nanoparticle catalyzed hydrogenation of acetophenone.
2.12 Zinc oxide nanoparticles
A green synthesis of ZnO nanoparticles has been developed in which a new leucine-based diamine amphiphile was synthesized and self-assembled Citation48. In the presence of Zn2 + ions, the leucine-based diamine amphiphile assembled into nanofibers that efficiently formed ZnO nanoparticles upon heating in the presence of Zn(CH3COO)2.
3. Industrially important green reactions
3.1. Reduction reactions
The reduction of p-nitrophenol to p-aminophenol in the presence of nanosized nickel nanocatalyst has been reported to be a green and effective method Citation49. The reaction reached completion in much less time and can be reused effectively. This suggests that these catalysts are potentially very useful for this reduction reaction.
3.2. Oxidation reactions
Oxidation of benzyl alcohol to benzaldehyde by using gold nanoparticles supported on different substrates such as U3O8, MgO, Al2O3, or ZrO2 in the absence of solvent, which is environmentally friendly and green reaction Citation50. Gold nanoparticles loaded onto titania supports have been used as catalysts for oxidation of benzylic compounds into corresponding ketones without the use of any organic solvents under mild conditions Citation51. As an example, indan was oxidized to form 1-indanone and different parameters such as temperature and time have been investigated for the oxidation of indan.
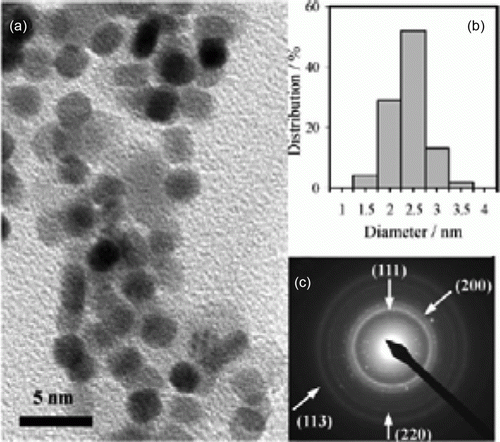
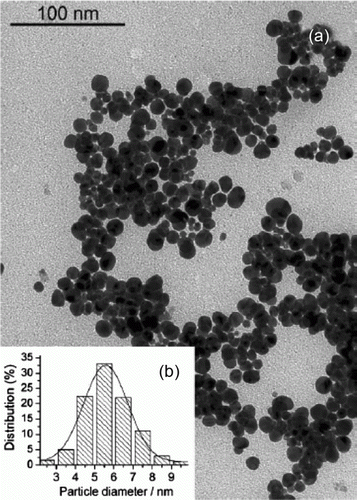
Hydrotalcite supported gold nanoparticles have been used as catalysts for highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid Citation52. This is a green synthesis in which the reaction takes place with water as the solvent and conducted in atmospheric pressure. shows an example TEM image and the size distribution of the gold nanoparticles supported onto hydrotalcite.
Figure 12. TEM image and size distribution of the gold nanoparticles supported on hydrotalcite (52). Reproduced by permission of the Royal Society of Chemistry.
The poly(ethylene glycol) is used as a catalyst phase and supercritical carbon dioxide as the mobile phase and represents a green approach for the oxidation of primary and secondary alcohols catalyzed using palladium nanoparticles as catalysts Citation53. shows TEM images of the palladium nanoparticles stabilized with PEG-1000 before and after oxidation of alcohols Citation53.
Figure 13. TEM image of the palladium nanoparticles formed using PEG-1000 as the stabilizer before oxidation of alcohols (a) and after oxidation (b) (53). Reproduced with permission from John Wiley and Sons.
Gold, silver, and copper nanoparticles supported on hydrotalcite have been used as catalysts for environmentally benign aerobic oxidation of alcohols Citation8. Gold nanoparticles are used to catalyze the oxidation of alcohols into its corresponding ketones Citation54. The overall protocol involves using green reagent, solvent, and catalyst making it a clean and green process. Magnesium oxide stabilized palladium nanoparticles have been used as catalysts for the selective oxidation of alcohols Citation55. Atmospheric oxygen is used as a green oxidant at room temperature. These catalysts can be used for transformation of various alcohols to their corresponding aldehydes and ketones.
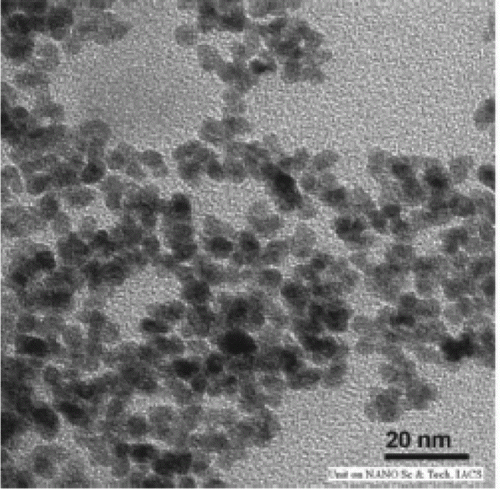
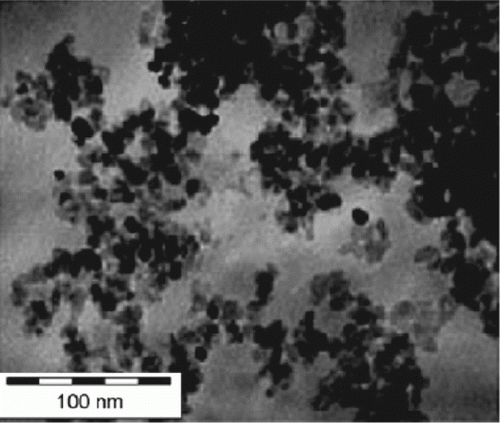
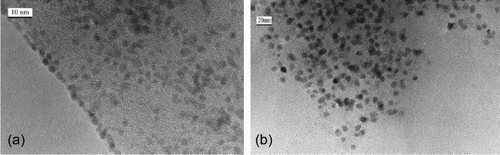
The catalytic process is green since gold nanoparticles are used as the catalyst and ionic liquids are used as the solvent for the methane oxidation reaction Citation56. Gold nanoparticles supported onto magnesium hydroxide support have been used as catalysts for the oxidation of biomass-derived polyhydroxyl compounds Citation57. This is an efficient and green route for biomass resource utility. Palladium nanoparticles are prepared by using neocuproine as the stabilizer and the hydrogen reduction method Citation58. The palladium nanoparticles are used to conduct aerobic oxidation of steroidal secondary alcohols to form the corresponding ketones. shows the TEM images before and after oxidation of the secondary alcohols to form ketones Citation58.
Figure 14. TEM images of palladium nanoparticles before and after oxidation of secondary alcohols (58). Reprinted with permission from Elsevier © 2010.
Hydroxyapatite supported gold nanoparticles are highly efficient and reusable catalysts for oxidation of silanes to silanols in water Citation59. This is the first report of this catalytic reaction being conducted in water. Fluorous silica gel supported is found to have excellent catalytic activity and selectivity for environmentally benign oxidation of alcohols to form the corresponding carbonyl compounds Citation60. Hydrogen peroxide is used as a green oxidant and results in only water being the byproduct. Gold nanoparticles intercalated into the walls of mesoporous silica were found to have high catalytic activity for the green alkane oxidation reaction Citation32. Gold nanoparticles supported on titania has been used as catalysts for the oxidation of benzylic compounds into corresponding ketones without any organic solvents and at 1 atm pressure Citation51.
3.3. Conversion of organosilanes to silanols
Selective hydrolytic oxidation of organohydrosilanes has been conducted using water as the solvent in the presence of platinum nanoparticles as catalyst Citation61. This type of synthesis was found to also be applicable for hydrosilanes having unsaturated functional groups to form corresponding silanols. Gold nanoparticles have been used as catalysts for oxidation of various alcohols to their corresponding carbonyl compounds Citation62. This is a green reaction since hydrogen peroxide is used as an environmentally benign oxidant and the solvent is organic-free. As a result, this provides a methodology of achieving the oxidation reaction while maintaining environmentally friendly conditions.
3.4. Suzuki cross-coupling reactions
The perfluoro-tagged palladium nanoparticles have been used as catalysts for the Suzuki cross-coupling reaction conducted in water as the solvent Citation63. The Suzuki cross-coupling reaction is catalyzed using Pd nanoparticles adsorbed onto diatomite Citation64. The reaction showed high reaction rates, high product yields, and green environmentally friendly reaction conditions. Palladium nanoparticles are prepared in water and uses 2-hydroxypropyl-α-cyclodextrin (α-HPCD) as both a reductant and stabilizer Citation65. These palladium nanoparticles are used as catalysts for the Suzuki cross-coupling reaction in water.
Palladium nanoparticles supported on grapheme showed excellent catalytic activity for the Suzuki reaction under environmentally friendly solvent system Citation66. shows TEM images of the palladium nanoparticles supported on graphite oxide Citation66. Palladium nanoparticles are synthesized in the presence of ionic liquids and have been used as catalysts for the Suzuki cross-coupling reaction in water Citation67.
3.5. Sonogashira cross-coupling reactions
Palladium nanoparticles are used to catalyze the Sonogashira cross-coupling reaction between aryl iodide and bromides with terminal alkynes Citation68. The reaction protocol involved the use of ethanol as the solvent, potassium carbonate as the base, and PVP capped palladium nanoparticles as the catalyst, so the protocol is environmentally friendly green reaction. The synthesis of 2-substituted benzo[b]furans was conducted in water and involves the Sonogashira coupling reaction between 2-iodophenols with phenylacetylenes followed by 5-endo-dig cyclization Citation69.
shows an example of a TEM image of palladium nanoparticles used as catalysts for the Sonogashira coupling reaction Citation69. Palladium nanoparticles are prepared in water and uses 2-hydroxypropyl-α-cyclodextrin (α-HPCD) as both a reductant and stabilizer Citation65. These palladium nanoparticles are used as catalysts for the Sonogashira cross-coupling reaction in water, making it an environmentally friendly green reaction.
3.6. Hydrogenations
Rhodium nanoparticles adsorbed onto titanium dioxide supports are synthesized using water as the solvent Citation46. They are also highly active catalysts for the arene hydrogenation reaction in water. shows HRTEM images of the titania support and the rhodium nanoparticles adsorbed onto titania Citation46. Solvent-free hydrogenations of naphthalene and cyclic polyenes have been conducted using RuPd and RuSn catalysts Citation7. The lack of need of a solvent is very highly desirable for green reactions.
3.7. Ullmann reaction
Palladium nanoparticles have been used as catalysts for the Ullmann reaction of an aryl chloride to form biaryls Citation70. The solvent used in the synthesis is a mixture of ionic liquid/supercritical carbon dioxide which is green and environmentally friendly solvents. Graphene oxide supported palladium nanoparticles have also been used as catalysts for the Ullmann reaction conducted in a mixture of ionic liquid/supercritical carbon dioxide Citation71. An Ullmann type homocoupling of bromoarenes and chloroarenes is catalyzed with palladium nanoparticles and the reaction is run using water as the solvent Citation72. shows TEM images of the palladium nanoparticles that are used as catalysts for the Ullmann type homocoupling reactions Citation72.
3.8. Hydrogenation reactions
Iron–platinum nanoparticles were found to be effective catalysts for hydrogenations conducted in aqueous solution Citation73. The FePt nanoparticles can be removed easily with an external magnet after the reaction is over. shows a TEM image, size distribution histogram, and electron diffraction patterns for the FePt nanoparticles used as catalysts for the hydrogenation reaction Citation73. Polymer capped palladium nanoparticles adsorbed onto SiO2 support has been used as effective catalysts for hydrogenation reactions such as 2,4-dimethyl-1,3-pentadiene to produce 2,4-dimethyl-2-pentene and allyl alcohol to produce 1-propanol using the environmentally benign supercritical carbon dioxide as the solvent Citation74. These nanocatalysts are green and contribute to the environmentally friendly general trend in nanocatalysis.
3.9. Heck cross-coupling reaction
Palladium nanoparticles are prepared in water and uses 2-hydroxypropyl-α-cyclodextrin (α-HPCD) as both a reductant and stabilizer Citation65. These palladium nanoparticles are used as catalysts for the Heck cross-coupling reaction in water. An environmentally benign Heck reaction using nickel nanoparticles as catalysts under hydrothermal conditions was found to be effective catalysts Citation75.
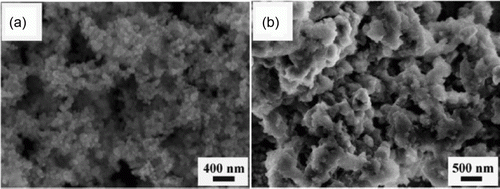
shows SEM images of the nickel nanoparticles before the Heck reaction and after six cycles of the Heck reaction Citation75. The Heck reaction catalyzed with Pd nanoparticles resulted in enhanced reaction rates, high product yields, and occurred with mild and environmentally friendly conditions Citation76. This shows that it is possible to achieve higher reaction rates and high product yields while using green conditions that are environmentally friendly.
3.10. Deoxygenation reaction
Gold nanoparticles stabilized with hydrotalcite have been used as catalysts for highly efficient and green catalytic dehydrogenation reaction of epoxides in water Citation77. Highly functionalized heterogeneous coinage metal nanoparticles (gold, silver, and copper) that are adsorbed onto hydrotalcite have been used as catalysts for environmentally benign organic transformations such as selective deoxygenation of epoxides and nitro aromatic compounds Citation8.
3.11. Alkynylation of aryl halides
The perfluoro-tagged palladium nanoparticles have been used as catalysts for the alkynylation of aryl halides conducted in water as the solvent Citation63. Multi-walled carbon nanotubes loaded with palladium nanoparticles have been used as catalysts for hydrogenation and alkynlation reactions in eco-friendly conditions Citation78.
shows a TEM image of the palladium nanoparticles adsorbed onto the multi-walled carbon nanotubes Citation78.
3.12. Arylations and diarylations
Iron and copper nanoparticles have been used as catalysts for a green microwave-assisted heterogeneous S-arylation protocol Citation79. The iron nanoparticles were used for the homo-coupling arylation reactions while the copper nanoparticles were used for the cross-coupling arylation reactions. Copper oxide nanospindles are shown to effectively catalyze the arylation heterocycle C–H bonds and uses environmentally friendly bases Citation80.
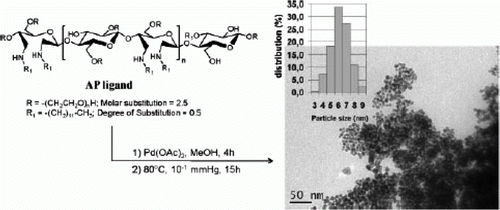
Glycerol is used as an inexpensive green solvent for diarylation of alkenes in the presence of palladium nanoparticles Citation81. The products of the reaction can be cleanly and selectively extracted in the presence of supercritical carbon dioxide. shows a TEM image of the palladium nanoparticles that are stabilized by using the AP ligand and these nanoparticles are used for diarylation of alkenes Citation81.
3.13. Acetylations
Zinc aluminate nanoparticles have been used as catalysts for acetylation of amines, alcohols, and phenols without the presence of a solvent. It is a clean, safe, and cost–effective method Citation82. Also, the use of the nanoparticles resulted in higher catalytic activity than the use of the corresponding bulk catalyst. shows a TEM image of the zinc aluminate nanoparticles that is used as a catalyst for the acetylation reactions Citation82.
3.14. Lactonization of diols
Gold, silver, and copper nanoparticles supported on hydrotalcite have been used as catalysts for environmentally benign lactonization of diols Citation8. Hydrotalcite supported copper nanoparticles are highly efficient heterogeneous nanocatalyst for oxidant-free lactonization of various diols Citation83. It is worth noting that these results have been obtained using environmentally friendly conditions.
3.15. Deoxygenation of epoxides
Gold, silver, and copper nanoparticles supported on hydrotalcite have been used as catalysts for environmentally benign deoxygenation of epoxides Citation8.
3.16. Oxidative coupling of alcohols
Gold nanoparticles supported on titanium dioxide are used as catalysts for the oxidative coupling of alcohols for the formation of imines Citation84. It was determined that the only byproduct that was formed is water and represents a green reaction protocol for the formation of imines.
3.17. Hydration of nitriles
The hydration of nitriles is conducted in water as solvent in the present of silver nanoparticles as catalysts Citation85. It can make a significant contribution for being an environmentally benign reaction. Magnetic nanoparticles supported onto ruthenium hydroxide have been used as catalysts for hydration of nitriles to amides in aqueous solution Citation86.
3.18. Esterification of alcohols
Methyl esters were prepared by a one step catalytic esterification of primary alcohols using oxygen as a green oxidant and gold nanoparticles supported onto silica Citation87. This process is greener than conventional methods since it avoids the use of environmentally unfriendly oxidants and avoids the use of strong acids or excess of reactants. shows a TEM image and size distribution histogram of the gold nanoparticles that are used as catalysts for the esterification of primary alcohols Citation87.
3.19. Additional organic synthesis reactions
ZnO nanoparticles have been used as catalysts for the synthesis of 4-aminopyrimidine-5-carbonitriles by a three-component reaction of malononitrile, aldehydes, and N-unsubstituted amidines Citation88. This reaction is conducted in water as the solvent, which makes the reaction a green one. Nanoflake shaped ZnO nanoparticles are used as catalysts for synthesis of quinoline derivatives under solvent-free conditions Citation89. The reaction conditions are environmentally benign, simple, and cost–effective. Magnetic iron oxide nanoparticles catalyze the reaction between 1,2-diamines and 1,2-dicarbonyl compounds in water and results in high yield of quinoxaline derivatives Citation90. Copper oxide nanoparticles are efficient, economic, and novel method for synthesis of quinoxaline, benzoxazine, and benzothiazine using ultrasonic irradiation Citation91. The green aluminum oxide nanoparticles are used for the solvent-free synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones and result in higher yields with shorter reaction times Citation43.
The reaction of indoles with aromatic or aliphatic aldehydes was conducted in solvent-free conditions using the nanotitania as a catalyst for the synthesis of bis(indolyl)methanes Citation92. Copper nanoparticles have been used for the synthesis of 1,2,3-triazole derivatives from benzyl halides or alkyl halides Citation93. The synthesis of seleno esters from acyl chlorides is catalyzed by using copper oxide nanoparticles and using ionic liquids as the solvent Citation94. Copper iodide nanoparticles have been used as catalysts for the synthesis of phenols, anilines, and thiophenols from aryl halides in the absence of ligands and organic solvents Citation95. Silver nanoparticles supported on mesoporous SBA-15 are catalysts for A3-coupling reactions of aldehydes, amines, and terminal alkynes using glycol as the green solvent Citation96. The Mannich reaction of secondary amines, aldehydes, and alkynes in water has been catalyzed in water using copper nanoparticles as catalysts Citation97. Nickel nanoparticles have been used as catalysts for the one-pot synthesis of polyhydroquinoline derivatives by the Hantzsch condensation under solvent-free conditions Citation98. Copper oxide nanoparticles are used as catalysts for the cross-coupling reaction between arylbromides and alkylbromides with diselenides conducted by using ionic liquids as the solvent Citation99. A wide variety of diaryl selenides are produced in good to excellent yields.
4. Summary and outlook
There been many different types of metal nanoparticles that have been used as catalysts for many reactions. In many cases, the metal nanoparticles are synthesized in aqueous solution in which water is the solvent, or is conducted in the presence of ionic liquids. There have also been cases where the nanoparticles are used as catalysts for different types of green reactions. Green reaction conditions include using water as the solvent, using solvent that is organic-free, conducting the reaction using ionic liquids, and running the reaction at atmospheric pressure. While there has been a lot of progress in applying the use of green chemistry to catalysis with nanoparticles, there is lot more room to further expand this field.
On the basis of a Scifinder Scholar search on catalysis with nanoparticles, 21,671 citations appear. When this sample set is refined by the word green, the number of citations drops tremendously to 526 citations. It can been seen that <2.5% of articles related to catalysis with nanoparticles have been conducted using green conditions or synthesized using green solvents, etc. As a result, there is much room for growth of applications of green chemistry to vast many catalytic organic and inorganic reactions as well as many types of organic synthesis reactions. The future of green chemistry remains very bright with many avenues of research that are open for research. The trend of using green chemistry in the catalysis with nanoparticles has been explosive in growth and developing more green nanocatalysts or modifying the reaction conditions to be greener would continue this trend in the future.
Acknowledgements
The author thank the University of Rhode Island start-up funds for funding of this work.
References
- Astruc , D. ; Blais , J.-C. ; Daniel , M.-C. ; Gatard , S. ; Nlate , S. ; Ruiz , J. C. R. Chim . 2003 , 6 ( 8–10 ), 1117 – 1127 .
- Barbaro , P. ; Dal Santo , V. ; Liguori , F. Dalton Trans . 39 ( 36 ), 8391 – 8402 .
- Beletskaya , I. ; Tyurin , V. Molecules 15 , 4792 – 4814 .
- Bhanage , B.M. ; Arai , M. Cat. Rev. Sci. Eng . 2001 , 43 ( 3 ), 315 – 344 .
- Dupont , J. ; Scholten , J.D. Chem. Soc. Rev . 39 ( 5 ), 1780 – 1804 .
- Fukuoka , A. ; Dhepe , P.L. Chem. Rec . 2009 , 9 ( 4 ), 224 – 235 .
- Johnson , B.F.G. ; Raynor , S.A. ; Brown , D.B. ; Shephard , D.S. ; Mashmeyer , T. ; Thomas , J.M. ; Hermans , S. ; Raja , R. ; Sankar , G. J. Mol. Catal. A: Chem . 2002 , 182 – 183 , 89 – 97 .
- Kaneda , K. ; Mitsudome , T. ; Mizugaki , T. ; Jitsukawa , K. Molecules 15 , 8988 – 9007 .
- Polshettiwar , V. ; Varma , R.S. Green Chem . 12 ( 5 ), 743 – 754 .
- Thomas , J.M. ; Raja , R. Aust. J. Chem . 2001 , 54 ( 9 & 10 ), 551 – 560 .
- Wasserscheid , P. Handb. Green Chem . 6 , 65 – 91 .
- Yan , N. ; Xiao , C. ; Kou , Y. Coord. Chem. Rev . 254 ( 9–10 ), 1179 – 1218 .
- Banerjee , S. ; Sereda , G. Tetrahedron Lett . 2009 , 50 ( 50 ), 6959 – 6962 .
- Banerjee , S. ; Das , J. ; Alvarez , R.P. ; Santra , S. N. J. Chem . 34 ( 2 ), 302 – 306 .
- Banerjee , S. ; Horn , A. ; Khatri , H. ; Sereda , G. Tetrahedron Lett . 52 ( 16 ), 1878 – 1881 .
- Lu , T. ; Blackburn , S. ; Dickinson , C. ; Rosseinsky , M.J. ; Hutchings , G. ; Axon , S. ; Leeke , G.A. Powder Technol . 2009 , 188 ( 3 ), 264 – 271 .
- Biboum , R.N. ; Keita , B. ; Franger , S. ; Nanseu Njiki , C.P. ; Zhang , G. ; Zhang , J. ; Liu , T. ; Mbomekalle , I.-M. ; Nadjo , L. Materials 3 , 741 – 754 .
- Chang , F. ; Kim , H. ; Lee , B. ; Park , S. ; Park , J. Tetrahedron Lett . 51 ( 32 ), 4250 – 4252 .
- He , F. ; Liu , J. ; Roberts , C.B. ; Zhao , D. Ind. Eng. Chemi. Res . 2009 , 48 ( 14 ), 6550 – 6557 .
- Jutz , F. ; Andanson , J.-M. ; Baiker , A. J. Catal . 2009 , 268 ( 2 ), 356 – 366 .
- Nadagouda , M.N. ; Polshettiwar , V. ; Varma , R.S. J. Mater. Chem . 2009 , 19 ( 14 ), 2026 – 2031 .
- Pacardo , D.B. ; Sethi , M. ; Jones , S.E. ; Naik , R.R. ; Knecht , M.R. ACS Nano 2009 , 3 ( 5 ), 1288 – 1296 .
- Sawant , A.D. ; Raut , D.G. ; Darvatkar , N.B. ; Desai , U.V. ; Salunkhe , M.M. Catal. Commun . 12 ( 4 ), 273 – 276 .
- Tao , R. ; Miao , S. ; Liu , Z. ; Xie , Y. ; Han , B. ; An , G. ; Ding , K. Green Chem . 2009 , 11 ( 1 ), 96 – 101 .
- Uozumi , Y. ; Yamada , Y.M.A. Chem. Rec . 2009 , 9 ( 1 ), 51 – 65 .
- Wang , Q. ; Cheng , H. ; Liu , R. ; Hao , J. ; Yu , Y. ; Zhao , F. Green Chem . 12 ( 8 ), 1417 – 1422 .
- Zhang , G. ; Zhou , H. ; Hu , J. ; Liu , M. ; Kuang , Y. Green Chem . 2009 , 11 ( 9 ), 1428 – 1432 .
- Engelbrekt , C. ; Soerensen , K.H. ; Luebcke , T. ; Zhang , J. ; Li , Q. ; Pan , C. ; Bjerrum , N.J. ; Ulstrup , J. Chem. Phys. Chem . 11 ( 13 ), 2844 – 2853 .
- Li , F. ; Li , F. ; Song , J. ; Song , J. ; Han , D. ; Niu , L. Electrochem. Commun . 2009 , 11 ( 2 ), 351 – 354 .
- Li , S. ; Yu , X. ; Zhang , G. ; Ma , Y. ; Yao , J. ; de Oliveira , P. Carbon 49 ( 6 ), 1906 – 1911 .
- Liu , S. ; Wang , J. ; Zeng , J. ; Ou , J. ; Li , Z. ; Liu , X. ; Yang , S. J. Power Sources 195 ( 15 ), 4628 – 4633 .
- Chen , L. ; Hu , J. ; Richards , R. J. Am. Chem. Soc , 2009 , 131 ( 3 ), 914 – 915 .
- Dash , P. ; Miller , S.M. ; Scott , R.W.J. J. Mol. Catal. A: Chem . 329 ( 1–2 ), 86 – 95 .
- Gupta , N. ; Singh , H.P. ; Sharma , R.K. Colloids Surf . A 367 ( 1–3 ), 102 – 107 .
- Quaresma , P. ; Soares , L. ; Contar , L. ; Miranda , A. ; Osorio , I. ; Carvalho , P.A. ; Franco , R. ; Pereira , E. Green Chem . 2009 , 11 ( 11 ), 1889 – 1893 .
- Saha , S. ; Pal , A. ; Kundu , S. ; Basu , S. ; Pal , T. Langmuir 26 ( 4 ), 2885 – 2893 .
- Zhan , G. ; Du , M. ; Huang , J. ; Li , Q. Catal. Commun . 12 ( 9 ), 830 – 833 .
- Frank , A.J. ; Rawski , J. ; Maly , K.E. ; Kitaev , V. Green Chem . 12 ( 9 ), 1615 – 1622 .
- Mohapatra , S. ; Kumar , R.K. ; Maji , T.K. Chem. Phys. Lett . 508 ( 1–3 ), 76 – 79 .
- Miedziak , P.J. ; Tang , Z. ; Davies , T.E. ; Enache , D.I. ; Bartley , J.K. ; Carley , A.F. ; Herzing , A.A. ; Kiely , C.J. ; Taylor , S.H. ; Hutchings , G.J. J. Mater. Chem . 2009 , 19 ( 45 ), 8619 – 8627 .
- Medina-Ramirez , I. ; Pan , X. ; Bashir , S. ; Liu , J. In Green Synthesis of Platinum-Encapsulated Nickel Nanocatalyst and its Nanostructure evaluation . Materials Research Society Symposium Proceedings 1213E, (Nanomaterials for Polymer Electrolyte Membrane Fuel Cells), Paper #: 1213-T10-12 .
- Hekmatshoar , R. ; Sadjadi , S. ; Shiri , S. ; Heravi , M.M. ; Beheshtiha , Y.S. Synth. Commun . 2009 , 39 ( 14 ), 2549 – 2559 .
- Kassaee , M.Z. ; Rostamizadeh , S. ; Shadjou , N. ; Motamedi , E. ; Esmaeelzadeh , M. J. Heterocycl. Chem . 47 ( 6 ), 1421 – 1424 .
- Hoag , G.E. ; Collins , J.B. ; Holcomb , J.L. ; Hoag , J.R. ; Nadagouda , M.N. ; Varma , R.S. J. Mater. Chem . 2009 , 19 ( 45 ), 8671 – 8677 .
- Phua , P.-H. ; Lefort , L. ; Boogers , J.A.F. ; Tristany , M. ; de Vries , J.G. Chem. Commun. (Camb. UK) 2009 , ( 25 ), 3747 – 3749 .
- Hubert , C. ; Denicourt-Nowicki , A. ; Beaunier , P. ; Roucoux , A. Green Chem . 12 ( 7 ), 1167 – 1170 .
- Mevellec , V. ; Roucoux , A. Inorg. Chim. Acta 2004 , 357 ( 10 ), 3099 – 3103 .
- Johnson , K.T. ; Gribb , T.E. ; Smoak , E.M. ; Banerjee , I.A. Chem. Commun. (Camb. UK) 46 ( 10 ), 1757 – 1759 .
- Abd El Maksod , I.H. ; Hegazy , E.Z. ; Kenawy , S.H. ; Saleh , T.S. Adv. Synth. Catal . 352 ( 7 ), 1169 – 1178 .
- Choudhary , V.R. ; Dhar , A. ; Jana , P. ; Jha , R. ; Uphade , B.S. Green Chem . 2005 , 7 ( 11 ), 768 – 770 .
- Dapurkar , S.E. ; Shervani , Z. ; Yokoyama , T. ; Ikushima , Y. ; Kawanami , H. Catal. Lett . 2009 , 130 ( 1–2 ), 42 – 47 .
- Gupta , N.K. ; Nishimura , S. ; Takagaki , A. ; Ebitani , K. Green Chem . 13 ( 4 ), 824 – 827 .
- Hou , Z. ; Theyssen , N. ; Brinkmann , A. ; Leitner , W. Angew. Chem. Int. Ed . 2005 , 44 ( 9 ), 1346 – 1349 .
- Kidwai , M. ; Bhardwaj , S. Appl. Catal. A: Gen . 387 ( 1–2 ), 1 – 4 .
- Layek , K. ; Maheswaran , H. ; Arundhathi , R. ; Kantam , M.L. ; Bhargava , S.K. Adv. Synth. Catal . 353 ( 4 ), 606 – 616 .
- Li , T. ; Wang , S.J. ; Yu , C.S. ; Ma , Y.C. ; Li , K.L. ; Lin , L.W. Appl. Catal. A: Gen . 398 ( 1–2 ), 150 – 154 .
- Ma , H. ; Nie , X. ; Cai , J.Y. ; Chen , C. ; Gao , J. ; Miao , H. ; Xu , J. Sci. China: Chem . 53 ( 7 ), 1497 – 1501 .
- Mifsud , M. ; Parkhomenko , K.V. ; Arends , I.W.C.E. ; Sheldon , R.A. Tetrahedron 66 ( 5 ), 1040 – 1044 .
- Mitsudome , T. ; Noujima , A. ; Mizugaki , T. ; Jitsukawa , K. ; Kaneda , K. Chem. Commun. (Camb. UK) 2009 , ( 35 ), 5302 – 5304 .
- Wang , L. ; Yi , W.-b. ; Cai , C. Chem. Sus. Chem . 3 ( 11 ), 1280 – 1284 .
- Chauhan , B.P.S. ; Sarkar , A. ; Chauhan , M. ; Roka , A. Appl. Organomet. Chem . 2009 , 23 ( 10 ), 385 – 390 .
- Ni , J. ; Yu , W.-J. ; He , L. ; Sun , H. ; Cao , Y. ; He , H.-Y. ; Fan , K.-N. Green Chem . 2009 , 11 ( 6 ), 756 – 759 .
- Bernini , R. ; Cacchi , S. ; Fabrizi , G. ; Forte , G. ; Petrucci , F. ; Prastaro , A. ; Niembro , S. ; Shafir , A. ; Vallribera , A. Green Chem . 12 ( 1 ), 150 – 158 .
- Hao , C. ; Zhao , X. Adv. Mater. Res . 113–116 ( Pt. 3 ), 1824 – 1827 .
- Senra , J.D. ; Malta , L.F.B. ; da Costa , M.E.H.M. ; Michel , R.C. ; Aguiar , L.C.S. ; Simas , A.B.C. ; Antunes , O.A.C. Adv. Synth. Catal . 2009 , 351 ( 14 + 15 ), 2411 – 2422 .
- Siamaki , A.R. ; Khder , A.E.R.S. ; Abdelsayed , V. ; El-Shall , M.S. ; Gupton , B.F. J. Catal . 279 ( 1 ), 1 – 11 .
- Taher , A. ; Kim , J.-B. ; Jung , J.-Y. ; Ahn , W.-S. ; Jin , M.-J. Synlett 2009 , ( 15 ), 2477 – 2482 .
- Li , P. ; Wang , L. ; Li , H. Tetrahedron 2005 , 61 ( 36 ), 8633 – 8640 .
- Saha , D. ; Dey , R. ; Ranu , B.C. Eur. J. Org. Chem . ( 31 ), 6067 – 6071 .
- Cheng , J. ; Tang , L. ; Xu , J. Adv. Synth. Catal . 352 ( 18 ), 3275 – 3286 .
- Cheng , J. ; Zhang , G. ; Du , J. ; Tang , L. ; Xu , J. ; Li , J. J. Mater. Chem . 21 ( 10 ), 3485 – 3494 .
- Monopoli , A. ; Calo , V. ; Ciminale , F. ; Cotugno , P. ; Angelici , C. ; Cioffi , N. ; Nacci , A. J. Org. Chem . 75 ( 11 ), 3908 – 3911 .
- Mori , K. ; Yoshioka , N. ; Kondo , Y. ; Takeuchi , T. ; Yamashita , H. Green Chem . 2009 , 11 ( 9 ), 1337 – 1342 .
- Wu , T. ; Jiang , T. ; Hu , B. ; Han , B. ; He , J. ; Zhou , X. Green Chem . 2009 , 11 ( 6 ), 798 – 803 .
- Zhang , W. ; Qi , H. ; Li , L. ; Wang , X. ; Chen , J. ; Peng , K. ; Wang , Z. Green Chem . 2009 , 11 ( 8 ), 1194 – 1200 .
- Zhang , Y. ; Ji , E. ; Chen , S. ; Tian , D. Adv. Mater. Res. (Zuerich, Switz.) 113–116 ( Pt. 3 ), 1675 – 1678 .
- Mitsudome , T. ; Noujima , A. ; Mikami , Y. ; Mizugaki , T. ; Jitsukawa , K. ; Kaneda , K. Chem. – Eur . J. 16 ( 39 ), 11818 – 11821 , S11818/1–S11818/7 .
- Olivier , J.-H. ; Camerel , F. ; Ziessel , R. ; Retailleau , P. ; Amadou , J. ; Pham-Huu , C. N. J. Chem . 2008 , 32 ( 6 ), 920 – 924 .
- Gonzalez-Arellano , C. ; Luque , R. ; Macquarrie , D.J. Chem. Commun. (Camb. UK) 2009 ( 11 ), 1410 – 1412 .
- Zhang , W. ; Zeng , Q. ; Zhang , X. ; Tian , Y. ; Yue , Y. ; Guo , Y. ; Wang , Z. J. Org. Chem . 76 ( 11 ), 4741 – 4745 .
- Delample , M. ; Villandier , N. ; Douliez , J.-P. ; Camy , S. ; Condoret , J.-S. ; Pouilloux , Y. ; Barrault , J. ; Jerome , F. Green Chem . 12 ( 5 ), 804 – 808 .
- Farhadi , S. ; Panahandehjoo , S. Appl. Catal. A: Gen . 382 ( 2 ), 293 – 302 .
- Mikami , Y. ; Ebata , K. ; Mitsudome , T. ; Mizugaki , T. ; Jitsukawa , K. ; Kaneda , K. Heterocycles 80 ( 2 ), 855 – 861 .
- Kegnaes , S. ; Mielby , J. ; Mentzel , U.V. ; Christensen , C.H. ; Riisager , A. Green Chem . 12 ( 8 ), 1437 – 1441 .
- Kim , A.Y. ; Bae , H.S. ; Park , S. ; Park , S. ; Park , K.H. Catal. Lett . 141 ( 5 ), 685 – 690 .
- Polshettiwar , V. ; Varma , R.S. Chem. – Eur. J. 2009 , 15 ( 7 ), 1582 – 1586 .
- Oliveira , R.L. ; Kiyohara , P.K. ; Rossi , L.M. Green Chem . 2009 , 11 ( 9 ), 1366 – 1370 .
- Hekmatshoar , R. ; Kenary , G.N. ; Sadjadi , S. ; Beheshtiha , Y.S. Synth. Commun . 40 ( 13 ), 2007 – 2013 .
- Hosseini-Sarvari , M. J. Iran. Chem. Soc . 8 ( Suppl ), S119 – S128 .
- Lue , H.-Y. ; Yang , S.-H. ; Deng , J. ; Zhang , Z.-H. Aust. J. Chem . 63 ( 8 ), 1290 – 1296 .
- Sadjadi , S. ; Sadjadi , S. ; Hekmatshoar , R. Ultrason. Sonochem . 17 ( 5 ), 764 – 767 .
- Rahimizadeh , M. ; Bakhtiarpoor , Z. ; Eshghi , H. ; Pordel , M. ; Rajabzadeh , G. Monatsh. Chem . 2009 , 140 ( 12 ), 1465 – 1469 .
- Sharghi , H. ; Khalifeh , R. ; Doroodmand , M.M. Adv. Synth. Catal . 2009 , 351 ( 1 + 2 ), 207 – 218 .
- Singh , D. ; Narayanaperumal , S. ; Gul , K. ; Godoi , M. ; Rodrigues , O.E.D. ; Braga , A.L. Green Chem . 12 ( 6 ), 957 – 960 .
- Xu , H.-J. ; Liang , Y.-F. ; Cai , Z.-Y. ; Qi , H.-X. ; Yang , C.-Y. ; Feng , Y.-S. J. Org. Chem . 76 ( 7 ), 2296 – 2300 .
- Yong , G.-P. ; Tian , D. ; Tong , H.-W. ; Liu , S.-M. J. Mol. Catal. A: Chem . 323 ( 1–2 ), 40 – 44 .
- Sharghi , H. ; Khalifeh , R. ; Moeini , F. ; Beyzavi , M.H. ; Beni , A.S. ; Doroodmand , M.M. J. Iran. Chem. Soc . 8 ( Suppl ), S89 – S103 .
- Sapkal , S.B. ; Shelke , K.F. ; Shingate , B.B. ; Shingare , M.S. Tetrahedron Lett . 2009 , 50 ( 15 ), 1754 – 1756 .
- Singh , D. ; Alberto , E.E. ; Rodrigues , O.E.D. ; Braga , A.L. Green Chem . 2009 , 11 ( 10 ), 1521 – 1524 .