Abstract
An efficient Henry reaction has been described for the synthesis of 3-hydroxy-3-(nitroalkyl)-2-oxindole by the reaction of isatins with nitroalkanes in N,N-dimethylformamide (DMF) under anhydrous condition. This method provides high yield of 3-hydroxy-2-oxindoles under mild reaction condition. Moreover, the procedure is environmentally benign in nature, efficient, and applicable to variety of isatins as well as nitroalkanes.
Introduction
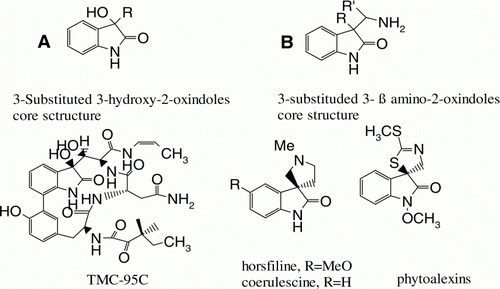
Substituted 3-hydroxy-2-oxindoles are important structural motif (, A) found in many biological active natural products Citation1–4 and pharmaceutical lead compounds Citation5–12. Particularly, chiral 3-substituted-3-hydroxyindolin-2-ones are important molecules in the field of medicinal chemistry Citation13–16 as an antibacterial, anti-inflammatory, laxative, growth hormone secretagogue agent, and new targets for cancer chemotherapy. The hydroxy indolines are also useful for the synthesis of chiral ligands which are used to obtain high enantioselectivities in numerous catalytic reactions Citation17. Additionally, 3-substituted-3-β amino-2-oxindol analogous structural motif is also found in several natural products Citation18–21 (, B). These molecules not only have an interesting molecular architecture but also have a densely functionalized core and exhibit significant bioactivities Citation22–28. Because of distinct biochemical properties and important medicinal values of 3-hydroxy indolines, an efficient protocol for the synthesis of these molecules is desirable.
Figure 1. Representative examples of natural products containing 3-hydroxy-2-oxindoles and 3-substituted 3-β amino-2-oxindoles structural motifs.
The Henry Reaction Citation29 is a base-catalyzed C-C bond-forming reaction between nitroalkanes and aldehydes or ketones. This reaction has been utilized Citation30 Citation31 for the synthesis of 3-hydroxy-3-(nitroalkyl)-oxindole by the reaction of nitoalkanes with isatins in presence of diethylamine Citation30 or alkali metal halides with electrochemical setup Citation31. Recently, catalyst-free reactions have been attracting more and more attention from synthetic chemists Citation32–39. In continuation of our research work Citation40–42 on synthesis of 3-hydroxy-2-oxindoles, we envisioned that the Henry reaction between isatin and nitroalkane might be readily proceed without use of conventional base catalyst under certain appropriate reaction conditions due to the fact that the highly reactive β-carbonyl group of isatin derivatives is much susceptible to nucleophilic attack Citation43 Citation44.
To the best of our knowledge, there is no precedent protocol of this work for the synthesis 3-hydroxy-3-(nitroalkyl)-oxindole in DMF under anhydrous condition. Herein, we wish to describe our preliminary results about DMF mediated efficient synthesis of 3-hydroxy-3 (nitroalkyl)-oxindole by the Henry reaction of isatin.
Results and discussion
Optimal reaction condition for the synthesis of 3-hydroxy-3-(nitromethyl)-2-oxindole (3a) was established by screening the reaction of nitromethane 2a with free isatin 1a (). As we envisioned that the reaction might proceed without use of conventional base catalyst, we began with the neat reaction of isatin with nitromethane as substrate and solvent. But the formation of desired product was not observed (, entry 1). Later, we attempted the reaction in polar aprotic solvents like dimethyl sulfoxide (DMSO) and DMF. The desired adduct 3a was obtained in very trace amount in DMSO (, entry 2) whereas the reaction in DMF gave encouraging results (33%), (, entry 3). Next we plan to examine the reaction in detail to increase the reaction rate and yield of reaction. The drastic increase in the reaction rate and yield (92%) of 3a was observed by adding 10 mg activated molecular sieves Citation45 (MS) 4 Å to the reaction system as an additive (, entry 4).
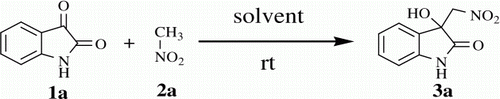
Table 1. Study towards the optimization of reaction condition for the synthesis of 3-hydroxy-3-(nitromethyl)-2-oxindole (3a).a
According to the recent study Citation46, hydration reduces the nucleophilicities of aliphatic nitroalkyl anions; hence we articulate that the decrease in nucleophilicity of aliphatic nitroalkyl anion can be detained in anhydrous condition. MS may seize the decrease in nucleophilicity of anion by absorbing the small amount of moisture present in the reaction system. Hence, we observe the drastic increase in the reaction rate and yield after addition of activated molecular sieve to the reaction system. With these observations, further we planned to study the reaction in completely anhydrous condition. We have attempted the reaction of isatin 1a with nitromethane 2a in freshly distilled dry DMF under nitrogen atmosphere Citation47. To our surprise, reaction proceeds smoothly under anhydrous condition to give desired adduct 3a with 95% yield in 72 min without any additional basic catalyst or additive (, entry 5).
Encouraged by these results, next we have studied the model reaction in different dry solvents under anhydrous condition. It is interesting to note that model reaction preceded very well in polar aprotic DMF solvent but worked poorly in other polar aprotic solvents like CH3CN, tetrahydrofuran (THF), and dichloromethane (DCM) (, entry 5–9). The exact reason for this is not clear, but we assume that the DMF acts like a poor base which form and stabilize nitromethyl anions in reaction media, which might have efficiently promoted this reaction in anhydrous conditions. Similarly, unsurprising results were obtained with polar protic solvents like ethanol, n-butanol and nonpolar solvents like CHCl3, toluene, dioxane, diethyl ether (, entry 10–15). This can be attributed to a less helpfulness of this solvent to form and stabilize the nitromethyl anion under catalyst-free condition. It is important to mention that the formations of any other possible by-products were not observed in present reaction condition. Accordingly, we have chosen the reaction of isatin 1a with nitromethane 2a in freshly distilled dry DMF under nitrogen atmosphere as an optimized condition for the synthesis of 3a, without any additional basic catalyst at room temperature.
With a set of optimized reaction conditions in our hand, we examined the scope of the reaction with respect to the different isatin electrophiles (, ). A wide range of free isatin derivatives bearing halogen atom (1b, c) underwent smooth reaction with nitromethane (2a) and resulted into corresponding products (3b, c) in very good yields (91%, 90%, respectively) within 75–90 min (, entry 2, 3). Encouraged by the aforementioned results we next studied the reactions of N-1 protected isatins (1d, e) with nitromethane 2a. It was found that in all cases the reactions preceded smoothly and provided their corresponding products in good yields (, entry 4, 5). It is worthy to mention that the N-hydroxymethylated adduct (3f) was also obtained in high yield (89%) in 90 min at room temperature with our optimized reaction condition (, entry 6).
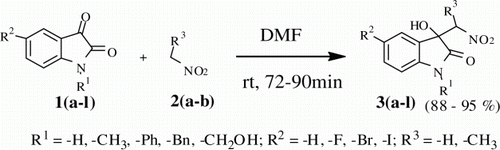
Further as a logical extension, we explored a series of isatin derivatives with nitroethane under the optimized reaction conditions (, entries 7–12). All isatin electophiles (1g–l) reacted well with nitroethane (2b) to give the respective adduct in very high yield (88–94%) and with moderate to high diasteroselectivity (63–91%, threo:erythro ratio, , entries 7–12). 5-Iodo isatin (1i) shown highest diasteroselectivity (threo:erythro ratio, 91:9; , entry 9). It is noteworthy that, such high diastereoselectivity was obtained with our optimized reaction condition without using any additional base catalyst or chiral mediator. The diasteromeric ratios of products were determined by 1H NMR analysis of crude product. As the reaction was performed without any chiral mediator, we proposed the thermodynamically more stable threo configuration for major isomer due to the possibility of formation of six member chair conformation by hydrogen bonding ()
Figure 2. Illustration for the possibility of formation of six member chair conformation by hydrogen bonding in threo configuration.
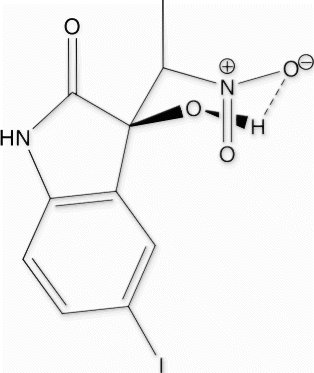
Table 2. Synthesis of 3-hydroxy-3-(nitroalkyl)-oxindole derivative by DMF mediated Henry reaction of isatins and nitroalkanes.a
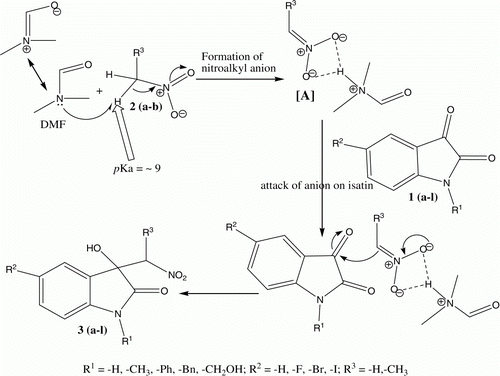
The tentative mechanistic pathway proposed for the DMF mediated Henry reaction of isatins is shown in . We reasoned that, DMF work as a solvent as well as a mild base which abstract acidic α-hydrogen of nitroalkane and efficiently form nitroalkyl anion [A]48. This nitroalkyl anion (azinate) attacks on highly reactive β-carbonyl group of isatin and form desired 3-hydroxy-3-(nitroalkyl)-2-oxindole product in high yield. We found that the reaction worked very well in DMF and gave very high yields of product within shorter reaction time as compared to the other solvents. The exact reason for this is not well understood, but we assume that, due to the distinctive basicity and polarity associated with DMF, it efficiently forms and stabilize nitroalkyl anion and hence resulted in to the high yields of products. However, may be due to the very less efficiency of other solvents to form and stabilize the nitromethyl anion is resulted in to the poor yields of product even after longer reaction time.
Experimental section
All reactions were conducted under an atmosphere of nitrogen (IOLAR Grade I). DMF was dried and freshly distilled over calcium hydride before use. Progress of the reactions was monitored by TLC on Merck Silica Gel 60 F-254 pre-coated. Evaporation of solvents was performed at reduced pressure on a BUCHI rotary evaporator. Column chromatography was carried out with silica gel grade 60–120 and 100–200 mesh. Melting points were measured on a BUCHI Melting Point machine. 1H NMR spectra were recorded at 300 MHz and 13C NMR spectra at 75 MHz in (CD3)2SO. J values were recorded in hertz and abbreviations used were: s, singlet; d, doublet; m, multiplet; dd, doublet of doublet; dt, doublet of triplet; and br, broad. Chemical shifts (δ) are reported relative to TMS (δ = 0.0) as an internal standard in ppm. IR spectra were recorded on Thermo Nicolet FT/IR-5700. Mass spectral data were obtained using MS (ESI), One or more of the following methods were used for visualization: UV absorption by fluorescence quenching; iodine staining; anisaldehyde stain (ethanol (135 mL)/H2SO4 (5 mL)/AcOH (1.5 mL)/p-anisaldehyde 3.7 mL). Ethyl acetate and hexane were the common eluents used.
Typical procedure
To a solution of isatins (1a–l; 1.0 mmol) in freshly distilled DMF (3 mL) was added nitroalkane (2a,b; 2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for stipulated time (). After the complete consumption of isatins as indicated by TLC, the reaction mixture was poured on 10 ml ice cold water to obtain the crude solid products. When reaction mixture does not give solid compound after pouring on ice cold water (in case of 3d, e, j, k), that time reaction mixture was extracted with ethyl acetate and crude products were obtained as yellow viscous liquid by evaporation of ethyl acetate under reduced pressure. The Crude products were sufficiently pure for spectroscopic analysis. However, crude products were further purified by silica gel column chromatography using ethyl acetate:hexane (1:3–1:1) as eluent to give the corresponding pure products.
All the synthesized compounds were characterized by 1H-NMR, 13C NMR, IR and Mass spectroscopic techniques and spectral data are given as following:
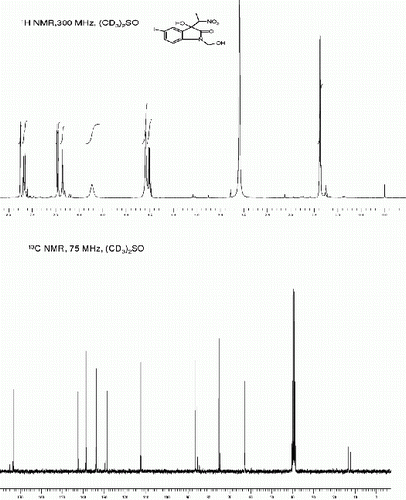
| 1. | 3-hydroxy-3-(nitromethyl)indolin-2-one (3a, , entry 1): Yield: 93%. Rf(50% EtOAc/hexanes) 0.48; white solid. M.p. 139 141°C[30, 31]; IR (KBR, cm −1 ): 3264, 3157, 2922, 1726, 1734, 1621, 1550, 1468, 1377, 1186, 755. 1 H NMR (300 MHz, (CD 3 ) 2 SO) [31]: δ 10.36(br s, 1H), 7.34(d, 1H, J=7.36 Hz), 7.22(t, 1H, J=7.74, Hz), 6.96 (t, 1H J=7.36 Hz), 6.87 (d, 1H, J=7.74 Hz) 6.58 (br s, 1H), 4.89 (d, 1H, J=12.46 Hz), 4.82 (d, 1H, J=12.46 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 176.5, 142.7, 130.6, 127.7, 124.7, 122.4, 110.8, 78.7, 73.4 ppm. MS(ESI): m/z=231 [M+Na]+. | ||||
| 2. | 5-Fluoro-3-hydroxy-3-(nitromethyl)indolin-2-one [42] (3b, , entry 2): Yield: 91%. Rf(50% EtOAc/hexanes) 0.40; Yellow solid. M.p. 161 163°C; IR (KBR, cm −1 ): 3358, 3271, 2924, 1726, 1612, 1555, 1470, 1374, 1182, 744. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 10.39(br s, 1H), 7.14(d, 1H, J=7.74 Hz), 6.94(dt, 1H, J=9.06, 2.26 Hz), 6.84-6.80(m, 1H), 6.69(br s, 1H), 4.90-4.82(m, 2H) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 179.9, 160.6, 157.4, 138.9, 129.6, 129.5, 117.5, 117.2, 113.2, 112.9, 112.1, 112.0, 78.8, 74.1 ppm. MS(ESI): m/z=249 [M+Na]+. | ||||
| 3. | 3-hydroxy-5-Iodo-3-(nitromethyl)indolin-2-one [42] (3c, , entry 3): Yield: 90%. Rf(50% EtOAc/hexanes) 0.39; White solid. M.p. 273 275°C; IR (KBR, cm −1 ): 3388, 3271, 2924, 1726, 1612, 1555, 1471, 1374, 1182, 822. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 10.46(br s, 1H), 7.64(d, 1H, J=1.51 Hz), 7.53(dd, 1H, J=8.30, 1.70 Hz), 6.70(d, 1H, J=8.30 Hz), 6.66(br s, 1H), 4.91-4.81 (m, 2H) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 175.2, 142.4, 138.6, 133.1, 130.5, 112.6, 84.2, 78.1, 72.6 ppm. MS(ESI): m/z=357 [M+Na]+. | ||||
| 4. | 3-Hydroxy-3-(nitromethyl)-1-phenylindolin-2-one [42] (3d, , entry 4): Yield: 91%. Rf(50% EtOAc/hexanes) 0.65; White solid. M.p. 99 101°C; IR (KBR, cm −1 ): 3328, 2933, 1709, 1617, 1559, 1467, 1389, 1090, 761. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 7.55-7.40(m, 6H), 7.29(t, 1H, J=7.5 Hz), 7.08(t, 1H, J=7.4 Hz), 6.91(br s, 1H), 6.75(d, 1H, J=7.7 Hz), 5.11-4.92(m, 2H) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 174.1, 143.7, 133.8, 129.7, 128.4, 128.1, 126.4, 125.4, 122.3, 109.1, 78.4, 72.7 ppm. MS(ESI): m/z=307 [M+Na]+. | ||||
| 5. | 1-Benzyl-5-bromo-3-hydroxy-3-(nitromethyl)indolin-2-one [42] (3e, , entry 5):Yield: 93%. Rf(50% EtOAc/hexanes) 0.43; Pale yellow solid. M.p. 121 123°C; IR (KBR, cm −1 ): 3334, 2921, 1711, 1621, 1561, 1457, 1391, 1097, 757. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 7.62(s, 1H), 7.41-7.19(m, 6H), 6.95(br s, 1H), 6.66(d, 1H, J=8.3 Hz), 5.05(s, 2H), 4.95(d, 1H, J=15.8 Hz), 4.85(d, 1H, J=15.8 Hz) ppm. 13 C NMR (50 MHz, (CD 3 ) 2 SO): δ 179.2, 147.6, 140.4, 138.1, 135, 134.7, 132.7, 132.4, 120, 116.6, 101.0, 83.0, 77.7, 48.7 ppm. MS(ESI): m/z=399 [M+Na]+. | ||||
| 6. | 3-Hydroxy-1-(hydroxymethyl)-5-iodo-3-(nitromethyl)indolin-2-one (3f, , entry 6): Yield: 89%. Rf(50% EtOAc/hexanes) 0.33; White solid. M.p. 267 269°C; IR (KBR, cm −1 ): 3249, 2945, 1711, 1608, 1549, 1476, 1370, 1033, 820. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 7.72(s, 1H), 7.65 (d, 1H, J=8.3 Hz), 6.97(d, 1H, J=8.3 Hz), 6.81(br s, 1H), 6.20(br s, 1H), 5.19-5.02(m, 2H), 4.91(s, 2H) ppm. 13 C NMR (75 MHz, CD 3 ) 2 SO): δ 173.1, 142.3, 138.4, 132.8, 129.5, 112.3, 82.1, 77.9, 72.2, 62.8 ppm. MS(ESI): m/z=387 [M+Na]+. | ||||
| 7. | 5-Bromo-3-hydroxy-3-(1-nitroethyl)indolin-2-one [42] (3g, , entry 7): Yield: 94%. Rf(50% EtOAc/hexanes) 0.40; Pale yellow solid. M.p. 167 169°C; IR (KBR, cm −1 ): 3356, 1729, 1628, 1572, 1558, 1487, 1371, 1161, 1091, 847. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro, 77:29* denotes minor diastereomer peaks): δ 10.54(br s, 1H), 10.48*(br s, 1H), 7.51(d, 1H, J=1.7 Hz), 7.37(d, 1H, J=8.1Hz), 6.8-6.7(m, 1H), 6.74(br s, 1H), 6.72*(br s, 1H), 6.7-6.6*(m, 1H), 5.01-4.59(m, 1H), 1.76*(d, 3H, J=7.0 Hz), 1.38(d, 1H, J=7.0 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 176.1, 175.8*, 142.1, 141.8*, 133.3, 132.7*, 130.7, 130.4*, 127.7, 127.2, 113.9, 113.4*, 112.4*, 112.1*, 86.2, 85.4*, 75.8, 75.9*, 13.7, 12.5* ppm. MS(ESI): m/z=323 [M+Na]+. | ||||
| 8. | 5-Fluoro-3-hydroxy-3-(1-nitroethyl)indolin-2-one [42] (3h, , entry 8): Yield: 89%. Rf(50% EtOAc/hexanes) 0.38; Pale yellow solid. M.p. 128 130°C; IR (KBR, cm −1 ): 3361, 3277, 2927, 1731, 1617, 1558, 1480, 1377, 1192, 749. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro, 67:33* denotes minor diastereomer peaks): δ 10.47(br s, 1H), 10.39*(br s 1H), 7.17(d, 1H, J=7.0 Hz), 7.07*(d, 1H, J=6.2 Hz), 7.01-6.96(m 1H), 6.84-6.81(m 1H), 6.78(br s, 1H), 6.78*(br s, 1H), 5.01-4.95(m, 1H), 1.72*(d, 3H, J=6.2 Hz), 1.35(d, 3H, J=6.2 Hz) ppm. 13 C NMR (75 MHz, CD 3 ) 2 SO): δ 176.28*, 175.61, 159.60, 159.51*, 156.42, 156.34*, 138.67*, 138.51, 128.81*, 128.77*, 128.41, 128.31, 116.54, 116.33*, 116.14, 116.01*, 113.71, 113.33, 112.74*, 112.41*, 111.01*, 110.91*, 110.85, 110.84, 86.38, 85.41*, 76.01, 75.21*, 13.64, 12.44 ppm. MS(ESI): m/z=263 [M+Na]+. | ||||
| 9. | 3-Hydroxy-5-iodo-3-(1-nitroethyl)indolin-2-one [42] (3i, , entry 9): Yield: 90%. Rf(50% EtOAc/hexanes) 0.37; White solid. M.p. 278 280°C; IR (KBR, cm −1 ): 3381, 3248, 2934, 1733, 1614, 1552, 1472, 1357, 1188, 816. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 91:9, * denotes minor diastereomer peaks): δ 10.55(br s, 1H), 10.49*(br s, 1H), 7.67(d, 1H, J=1.5 Hz), 7.55(dd, 1H, J=8.1, 1.5, Hz), 6.7(br s, 1H), 6.68(d, 1H, J=8.1 Hz), 4.99-4.92(m, 1H), 1.75*(d, 3H, J=7.0 Hz), 1.38(d, 3H, J=7.0 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 175.51*, 174.83, 142.32*, 142.27, 138.38, 133.15, 132.56*, 129.80*, 129.45, 112.36*, 112.30, 86.19, 85.18*, 84, 83.6*, 75.34, 74.64*, 13.5, 12.3* ppm. MS(ESI): m/z=371 [M+Na]+. | ||||
| 10. | 3-Hydroxy-1-methyl-3-(1-nitroethyl)indolin-2-one (3j, , entry 10): Yield: 92%. Rf(50% EtOAc/hexanes) 0.44; White solid. M.p. 140 142°C; IR (KBR, cm −1 ): 3316, 2939, 1703, 1616, 1551, 1468, 1385, 1355, 1113, 1061, 759. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 63:37, * denotes minor diastereomer peaks): δ 7.44*(s, 1H), 7.42*(s, 1H), 7.35(s, 1H), 7.33(s, 1H), 7.12-7.0(m, 1H), 6.88(d, 1H, J=3.5 Hz), 6.85*(d, 1H, J=3.2 Hz), 6.68*(br s, 1H), 6.63(br s, 1H), 5.05-4.98(m, 1H), 3.18*(s, 3H), 3.17(s, 3H), 1.76*(d, 3H, J=6.8 Hz), 1.37(d, 3H, J=6.8 Hz) ppm. 13 C NMR (75 MHz, CD 3 ) 2 SO): δ 174.83*, 174.4, 144.3*, 144.09, 130.43, 130.13*, 127.4*, 126.9, 124.24, 123.8*, 122.7, 122.3*, 108.9*, 108.57, 78.4, 77.8*, 72.75, 71.6*, 26.17, 25.3*, 13.9, 12.8* ppm. MS(ESI): m/z=259 [M+Na]+. | ||||
| 11. | 3-Hydroxy-3-(1-nitroethyl)-1-phenylindolin-2-one (3k, , entry 11): Yield: 89%. Rf(50% EtOAc/hexanes) 0.43; White solid. M.p 102 105°C. IR (KBR, cm −1 ): 3331, 2923, 1711, 1619, 1569, 1471, 1374, 1087, 769. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 72:28, * denotes minor diastereomer peaks): δ 7.56-7.37(m, 6H), 7.26(t, 1H, J=7.4 Hz), 7.19-7.15*(m, 1H), 7.10(t, 1H, J=7.5 Hz), 7.06-7.01*(m, 1H), 6.91(br s, 1H), 6.89*(br s, 1H), 6.74(d, 1H, J=7.4 Hz), 5.19-5.09 (m, 1H), 1.84*(d, 3H, J=6.9 Hz), 1.57(d, 3H, J=6.9 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 174.4*, 173.3, 143.9*, 143.6, 133.8*, 133.7, 130.0*, 129.4, 128.2*, 128.1, 128.03, 127.9*, 126.5*, 126.3, 125.2, 124.9*, 122.92, 122.79*, 109.18*, 109.05, 86.93, 85.7*, 74.7*, 74.6, 13.58, 12.2* ppm. MS(ESI): m/z=321 [M+Na]+. | ||||
| 12. | 3-Hydroxy-1-(hydroxymethyl)-5-iodo-3-(1-nitroethyl)indolin-2-one (3l, , entry 12): Yield: 88%. Rf(50% EtOAc/hexanes) 0.35; White solid. M.p. 151 153°C; IR (KBR, cm −1 ): 3341, 2982, 1731, 1604, 1537, 1480, 1333, 1194, 1029, 965, 810. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 88:12, * denotes minor diastereomer peaks) δ 7.75(d, 1H, J=7.5 Hz), 7.66(dd, 1H, J=8.3, 1.7 Hz), 7.61*(dd, 1H, J=8.3, 1.7 Hz), 6.95(d, 1H, J=8.1 Hz), 6.9(br s, 1H), 6.8*(br s, 1H), 6.2(br s, 1H), 5.1(s, 2H), 5.04-4.9(m, 1H), 1.76*(d, 1H, J=7.0 Hz), 1.37(d, 1H, J=7.0 Hz). 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 173.5*, 172.9, 142.3, 138.6, 133.6, 132.7*, 129.0*, 128.6, 112.4, 86.35, 85.6, 85.4, 85.3*, 75.1, 74.6*, 62.8, 13.52, 12.4*. MS(ESI): m/z=379 [M+1]+. | ||||
Conclusions
In summary, we have developed an efficient Henry reaction of variety of isatins with nitroalkanes in DMF at room temperature. Desired products, 3-hydroxy-3-(nitroalkyl)-oxindoles, were obtained in very good to excellent yields. Mild reaction condition, high yields and wide scope of substrates are the distinct features of our protocol. This protocol may find utility in the synthesis of biologically active oxindole analogous compounds. Further investigation to develop more efficient and convenient reaction condition from a green chemistry viewpoint is under way in our laboratory.
701337 supplementary material.doc
Download MS Word (3.1 MB)Acknowledgements
P.B.T., B.M.B. and V.M.B. thanks to CSIR New Delhi for the award of a fellowship and to Dr. J. S. Yadav, Director IICT, for his support and encouragement.
References
- Kamano , Y. ; Zhang , H.P. ; Ichihara , Y. ; Kizu , H. ; Komiyama , K. ; Pettit , G.R. Tetrahedron. Lett . 1995 , 36 , 2783 – 2784 .
- Kohno , J. ; Koguchi , Y. ; Nishio , M. ; Nakao , K. ; Kuroda , M. ; Shimizu , R. ; Ohnuki , T. ; Komatsubara , S. J. Org. Chem . 2000 , 65 , 990 – 995 .
- Gan , C.Y. ; Kam , T.S. Tetrahedron. Lett . 2009 , 50 , 1059 – 1061 .
- Tokunaga , T. ; Hume , W.E. ; Umezome , T. ; Okazaki , K. ; Ueki , Y. ; Kumagai , K. ; Hourai , S. ; Nagamine , J. ; Seki , H. ; Taiji , M. ; Noguchi , H. ; Nagata , R. J. Med. Chem . 2001 , 44 , 4641 – 4649 .
- Miah , S. ; Moody , C.J. ; Richards , I.C. ; Slawin , A.M.Z. J. Chem. Soc., Perkin Trans. 1 , 1997 , 16 , 2405 – 2412 .
- Frechard , A. ; Fabre , N. ; Pean , C. ; Montaut , S. ; Fauvel , M.T. ; Rollin , P. ; Fouraste , I. Tetrahedron Lett . 2001 , 42 , 9015 – 9017 .
- Jimenez , J.I. ; Huber , U. ; Moore , R.E. ; Patterson , G.M.L. J. Nat. Prod . 1999 , 62 , 569 – 572 .
- Lin , S. ; Yang , Z.Q. ; Kwok , B.H.B. ; Koldobskiy , M. ; Crews , C.M. ; Danishefsky , S.J. J. Am. Chem. Soc . 2004 , 126 , 6347 – 6355 .
- Suzuki , H. ; Morita , H. ; Shiro , M. ; Kobayashi , J. Tetrahedron . 2004 , 60 , 2489 – 2495 .
- Monde , K. ; Sasaki , K. ; Shirata , A. ; Takasugi , M. Phytochemistry . 1991 , 30 , 2915 – 2917 .
- Suchy , M. ; Kutschy , P. ; Monde , K. ; Goto , H. ; Harada , N. ; Takasugi , M. ; Dzurilla , M. ; Balentova , E. J. Org. Chem . 2001 , 66 , 3940 – 3947 .
- Goehring , R.R. ; Sachdeva , Y.P. ; Pisipati , J.S. ; Sleevi , M.C. ; Wolfe , J.F. J. Am. Chem. Soc . 1985 , 107 , 435 – 443 .
- Popp , F.D. J. Heterocycl. Chem . 1984 , 21 , 1367 – 1368 .
- Pajouhesh , H. ; Parsons , R. ; Popp , F.D. J. Pharm. Sci . 1983 , 72 , 318 – 321 .
- Tokunaga , T. ; Hume , W.E. ; Nagamine , J. ; Kawamura , T. ; Taiji , M. ; Nagata , R. Bioorg. Med. Chem. Lett . 2005 , 15 , 1789 – 1792 .
- Yong , S.R. ; Ung , A.T. ; Pyne , S.G. ; Skelton , B.W. ; White , A.H. Tetrahedron . 2007 , 63 , 5579 – 5586 .
- Berensa , U. ; Brown , J.M. ; Long , J. ; Seike , R. Tetrahedron: Asymmetry . 1996 , 7 , 285 – 292 .
- Garnick , R.L. ; LeQuesne , P.W. J. Am. Chem. Soc . 1978 , 100 , 4213 – 4219 .
- Sakai , S. ; Aimi , N. ; Yamaguchi , K. ; Yamanaka , E. ; Haginiwa , J. J. Chem. Soc. Perkin Trans. 1 , 1982 , 3 , 1257 – 1262 .
- Cui , C.B. ; Kakeya , H. ; Osada , H. Tetrahedron . 1996 , 52 , 12651 – 12666 ; Cui, C.B.; Kakeya, H.; Osada, H. J. Antibiot. 1996, 49, 832 835 .
- Pedras , M.S.C. ; Ahiahonu , P.W.K. J. Chem. Ecol . 2004 , 30 , 2163 – 2179 .
- Elderfield , R.C. ; Gilman , R.E. Phytochemistry . 1972 , 11 , 339 – 343 .
- Sakai , S. ; Aimi , N. ; Yamaguchi , K. ; Ohhira , H. ; Hori , K. ; Haginiwa , J. Tetrahedron Lett . 1975 , 16 , 715 – 718 .
- Aimi , N. ; Yamaguchi , K. ; Sakai , S. ; Haginiwa , J. ; Kubo , A. Chem. Pharm. Bull . 1978 , 26 , 3444 – 3449 .
- Ghedira , K. ; Zeches-Hanrot , M. ; Richard , B. ; Massiot , G. ; Le Men-Oliver , L. ; Sevener , T. ; Goh , S.H. Phytochemistry . 1988 , 27 , 3955 – 3961 .
- Jossang , A. ; Jossang , P. ; Hadi , H.A. ; Sevenet , T. ; Bodo , B. J. Org. Chem . 1991 , 56 , 6527 – 6530 .
- Wong , W.H. ; Lim , P.B. ; Chuah , C.H. Phytochemistry . 1996 , 41 , 313 .
- Anderton , N. ; Cockrum , P.A. ; Colegate , S.M. ; Edgar , J.A. ; Flower , K. ; Vit , I. ; Willing , R.I. Phytochemistry . 1998 , 48 , 437 – 439 .
- Henry , L. Compt. Rend . 1895 , 120 , 1265 .
- Conn , W.R. ; Lindwall , H.G. J. Am. Chem. Soc . 1936 , 58 , 1236 – 1239 .
- Michail , N.E. ; Alexey , I.I. ; Valentina , M.M. ; Fructuoso , B. ; Belen , B. Tetrahedron . 2008 , 64 , 5915 – 5919 .
- For some selected examples about reactions under catalyst-free condition see : Watahiki , T. ; Matsuzaki , M. ; Oriyama , T. Green Chem . 2003 , 5 , 82 – 84 .
- Watahiki , T. ; Ohba , S. ; Oriyama , T. Org. Lett . 2003 , 5 , 2679 – 2681 .
- Iwanami , K. ; Hinakubo , Y. ; Oriyama , T. Tetrahedron Lett . 2005 , 46 , 5881 – 5883 .
- Iwanami , K. ; Oriyama , T. Synlett . 2006 , 1 , 112 – 114 .
- Khatik , G.L. ; Kumar , R. ; Chakraborti , A.K. Org. Lett . 2006 , 8 , 2433 – 2436 .
- Oriyama , T. ; Aoyagi , M. ; Iwanami , K. Chem. Lett . 2007 , 36 , 612 – 613 .
- Zhang , M. ; Jiang , H.F. ; Liu , H.L. ; Zhu , Q.H. Org. Lett . 2007 , 9 , 4111 – 4113 .
- Chen , W.B. ; Liao , Y.H. ; Du , X.L. ; Zhang , X.M. ; Yuan , W.C. Green Chem . 2009 , 11 , 1465 – 1476 .
- Meshram , H.M. ; Ramesh , P. ; Reddy , B.C. ; Shreedhar , B. ; Yadav , J.S. Tetrahedron . 2011 , 67 , 3150 – 3155 .
- Meshram , H.M. ; Kumar , D.A. ; Reddy , B.C. ; Ramesh , P. Synth. Commun . 2010 , 40 , 39 – 45 .
- Meshram , H.M. ; Ramesh , P. ; Kumar , A.S. ; Swetha , A. Tetrahedron Lett . 2011 , 52 , 5862 – 5864 .
- Popp , F.D. Adv. Heterocycl. Chem . 1975 , 18 , 1 – 58 .
- da Silva , J.F.M. ; Garden , S.J. ; Pinto , A.C. J. Braz. Chem. Soc . 2001 , 12 , 273 – 324 .
- The powdered molecular sieves 4 Å were activated by placing the powder under vacuum and heating with the flame of gas burner .
- Bug , T. ; Tadeusz , L. ; Herbert , M. J. Org. Chem . 2004 , 69 , 7565 – 7576 .
- The solvents used in this work were all carefully dried and distilled freshly before use. DMF was dried and freshly distilled from calcium hydride before use. We have Added nearly 8 gm calcium hydride in to 100 ml DMF (SRL make, AR grade, min. 99% purity) and stirred for 5 hours under nitrogen atmosphere. Then calcium hydride was decanted and DMF was distilled under nitrogen at 60°C, by using approximately 30 mmHg vacuums. This distilled DMF was stored on powdered 4 Å molecular sieves and used as it is for the reactions .
- Marcelli , T. ; van der Haas , R.N.S. ; van Maarseveen , J.H. ; Hiemstra , H. Angew. Chem. Int. Ed . 2006 , 45 , 929 – 931 .
Supplementary information file
EXPERIMENTAL SECTION
General remarks:
All reactions were conducted under an atmosphere of nitrogen (IOLAR Grade I). DMF was dried and freshly distilled from calcium hydride before use. Progress of the reactions was monitored by TLC on Merck Silica Gel 60 F-254 pre-coated. Evaporation of solvents was performed at reduced pressure on a BUCHI rotary evaporator. Column chromatography was carried out with silica gel grade 60–120 and 100–200 mesh. Melting points were measured on a BUCHI Melting Point machine. 1H NMR spectra were recorded at 300 MHz and 13C NMR spectra at 75 MHz in (CD3)2SO. J values were recorded in hertz and abbreviations used were s—singlet, d—doublet, m—multiplet, dd—doublet of doublet, dt—doublet of triplet and br—broad. Chemical shifts (δ) are reported relative to TMS (δ = 0.0) as an internal standard in ppm. IR spectra were recorded on Thermo Nicolet FT/IR-5700. Mass spectral data were obtained using MS (ESI), One or more of the following methods were used for visualization: UV absorption by fluorescence quenching; iodine staining; anisaldehyde stain (ethanol (135 mL)/H2SO4 (5 mL)/AcOH (1.5 mL)/p-anisaldehyde 3.7 mL). Ethyl acetate and hexane were the common eluents used.
Typical procedure for DMF mediated Henry reaction of Isatins ( ): To a solution of isatins (1a-l) (1.0 mmol) in freshly distilled DMF (3 mL) was added nitroalkane (2a-b) (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for stipulated time (). After the complete consumption of isatins as indicated by TLC, the reaction mixture was poured on 10 ml ice cold water to obtain the crude products. (When reaction mixture does not give solid compound after pouring on ice cold water ((in case of 3d, 3e, 3j, 3k), that time reaction mixture was extracted with ethyl acetate and crude products were obtained as yellow viscous liquid by evaporation of ethyl acetate under reduced pressure). Crude products were sufficiently pure for spectroscopic analysis. However, crude products were further purified by silica gel column chromatography using ethyl acetate:hexane (1:3 to 1:1) as eluent to give the corresponding pure products. The inseparable diastereomers threo:erythro ratio of the product was determined by 1H NMR analysis of the crude reaction mixture. In 1H NMR spectrum, few peaks of minor isomer were well separated from the peaks of major isomer and few peaks were merged in major isomer peaks. 1H NMR peaks for minor isomer which were well separated from the peaks of major isomer are marked with asterisk (*) and written along with the peaks of major isomer in spectral data.
Detail experimental procedure and Spectraldata for compounds 3a-3l:
3-hydroxy-3-(nitromethyl)indolin-2-one (3a, , Entry 1): To a solution of isatin 1a (1.0 mmol) in freshly distilled DMF (3 mL) was added nitromethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 72 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3a as pale yellow solid. The Crude solid of 3a was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3a. Yield: 93%. Rf(50% EtOAc/hexanes) 0.48; white solid. M.p. 139 141°C [30, 31]; IR (KBR, cm −1 ): 3264, 3157, 2922, 1726, 1734, 1621, 1550, 1468, 1377, 1186, 755. 1 H NMR (300 MHz, (CD 3 ) 2 SO) [31]: δ 10.36(br s, 1H), 7.34(d, 1H, J=7.36 Hz), 7.22(t, 1H, J=7.74, Hz), 6.96 (t, 1H J=7.36 Hz), 6.87 (d, 1H, J=7.74 Hz) 6.58 (br s, 1H), 4.89 (d, 1H, J=12.46 Hz), 4.82 (d, 1H, J=12.46 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 176.5, 142.7, 130.6, 127.7, 124.7, 122.4, 110.8, 78.7, 73.4 ppm. MS(ESI): m/z=231 [M+Na]+.
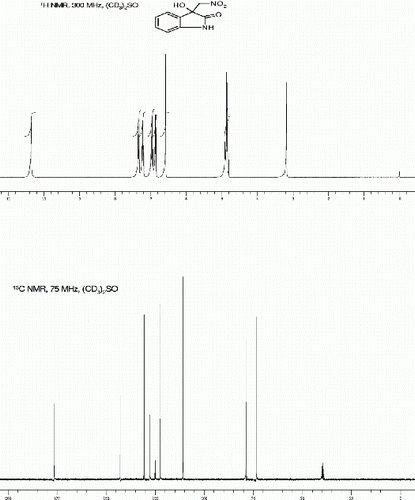
5-Fluoro-3-hydroxy-3-(nitromethyl)indolin-2-one [42] (3b, , Entry 2): To a solution of isatin 1b (1.0 mmol) in freshly distilled DMF (3 mL) was added nitromethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 75 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3b as pale yellow solid. The Crude solid of 3b was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3b. Yield: 91%. Rf(50% EtOAc/hexanes) 0.40; Yellow solid. M.p. 161 163°C; IR (KBR, cm −1 ): 3358, 3271, 2924, 1726, 1612, 1555, 1470, 1374, 1182, 744. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 10.39(br s, 1H), 7.14(d, 1H, J=7.74 Hz), 6.94(dt, 1H, J=9.06, 2.26 Hz), 6.84-6.80(m, 1H), 6.69(br s, 1H), 4.90-4.82(m, 2H) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 179.9, 160.6, 157.4, 138.9, 129.6, 129.5, 117.5, 117.2, 113.2, 112.9, 112.1, 112.0, 78.8, 74.1 ppm. MS(ESI): m/z=249 [M+Na]+.
3-hydroxy-5-Iodo-3-(nitromethyl)indolin-2-one [42] (3c, , Entry 3): To a solution of isatin 1c (1.0 mmol) in freshly distilled DMF (3 mL) was added nitromethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 90 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3c as off white solid. The Crude solid of 3c was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3c. Yield: 90%. Rf(50% EtOAc/hexanes) 0.39; White solid. M.p. 273 275°C; IR (KBR, cm −1 ): 3388, 3271, 2924, 1726, 1612, 1555, 1471, 1374, 1182, 822. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 10.46(br s, 1H), 7.64(d, 1H, J=1.51 Hz), 7.53(dd, 1H, J=8.30, 1.70 Hz), 6.70(d, 1H, J=8.30 Hz), 6.66(br s, 1H), 4.91-4.81 (m, 2H) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 175.2, 142.4, 138.6, 133.1, 130.5, 112.6, 84.2, 78.1, 72.6 ppm. MS(ESI): m/z=357 [M+Na]+.
3-Hydroxy-3-(nitromethyl)-1-phenylindolin-2-one [42] (3d, , Entry 4): To a solution of isatin 1d (1.0 mmol) in freshly distilled DMF (3 mL) was added nitromethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 85 min. After then, the reaction mixture was poured on 10 ml ice cold water and extracted with ethyl acetate (10x3). The Combine organic extract was dried on Na2SO4 and concentrate in Vacuo to provide crude 3d as pale yellow viscous liquid. The Crude 3d was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3d. Yield: 91%. Rf(50% EtOAc/hexanes) 0.65; White solid. M.p. 99 101°C; IR (KBR, cm −1 ): 3328, 2933, 1709, 1617, 1559, 1467, 1389, 1090, 761. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 7.55-7.40(m, 6H), 7.29(t, 1H, J=7.5 Hz), 7.08(t, 1H, J=7.4 Hz), 6.91(br s, 1H), 6.75(d, 1H, J=7.7 Hz), 5.11-4.92(m, 2H) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 174.1, 143.7, 133.8, 129.7, 128.4, 128.1, 126.4, 125.4, 122.3, 109.1, 78.4, 72.7 ppm. MS(ESI): m/z=307 [M+Na]+.
1-Benzyl-5-bromo-3-hydroxy-3-(nitromethyl)indolin-2-one [42] (3e, , Entry 5):
To a solution of isatin 1e (1.0 mmol) in freshly distilled DMF (3 mL) was added nitromethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 72 min. After then, the reaction mixture was poured on 10 ml ice cold water and extracted with ethyl acetate (10x3). The Combine organic extract was dried on Na2SO4 and concentrate in Vacuo to provide crude 3e as pale yellow viscous liquid. The Crude 3e was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3e. Yield: 93%. Rf(50% EtOAc/hexanes) 0.43; Pale yellow solid. M.p. 121 123°C; IR (KBR, cm −1 ): 3334, 2921, 1711, 1621, 1561, 1457, 1391, 1097, 757. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 7.62(s, 1H), 7.41-7.19(m, 6H), 6.95(br s, 1H), 6.66(d, 1H, J=8.3 Hz), 5.05(s, 2H), 4.95(d, 1H, J=15.8 Hz), 4.85(d, 1H, J=15.8 Hz) ppm. 13 C NMR (50 MHz, (CD 3 ) 2 SO): δ 179.2, 147.6, 140.4, 138.1, 135, 134.7, 132.7, 132.4, 120, 116.6, 101.0, 83.0, 77.7, 48.7 ppm. MS(ESI): m/z=399 [M+Na]+.
3-Hydroxy-1-(hydroxymethyl)-5-iodo-3-(nitromethyl)indolin-2-one (3f, , Entry 6): To a solution of isatin 1f (1.0 mmol) in freshly distilled DMF (3 mL) was added nitromethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 90 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3f as off white solid. The Crude solid of 3f was further purified by silica gel column chromatography using ethyl acetate:hexane (1:1) as eluent to give the pure title compound 3c. Yield: 89%. Rf(50% EtOAc/hexanes) 0.33; White solid. M.p. 267 269°C; IR (KBR, cm −1 ): 3249, 2945, 1711, 1608, 1549, 1476, 1370, 1033, 820. 1 H NMR (300 MHz, (CD 3 ) 2 SO): δ 7.72(s, 1H), 7.65 (d, 1H, J=8.3 Hz), 6.97(d, 1H, J=8.3 Hz), 6.81(br s, 1H), 6.20(br s, 1H), 5.19-5.02(m, 2H), 4.91(s, 2H) ppm. 13 C NMR (75 MHz, CD 3 ) 2 SO): δ 173.1, 142.3, 138.4, 132.8, 129.5, 112.3, 82.1, 77.9, 72.2, 62.8 ppm. MS(ESI): m/z=387 [M+Na]+.
5-Bromo-3-hydroxy-3-(1-nitroethyl)indolin-2-one [42] (3g, , Entry 7): To a solution of isatin 1g (1.0 mmol) in freshly distilled DMF (3 mL) was added nitroethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 86 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3g as yellow solid. The Crude solid of 3g was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3g. Yield: 94%. Rf(50% EtOAc/hexanes) 0.40; Pale yellow solid. M.p. 167 169°C; IR (KBR, cm −1 ): 3356, 1729, 1628, 1572, 1558, 1487, 1371, 1161, 1091, 847. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro, 77:29* denotes minor diastereomer peaks): δ 10.54(br s, 1H), 10.48*(br s, 1H), 7.51(d, 1H, J=1.7 Hz), 7.37(d, 1H, J=8.1Hz), 6.8-6.7(m, 1H), 6.74(br s, 1H), 6.72*(br s, 1H), 6.7-6.6*(m, 1H), 5.01-4.59(m, 1H), 1.76*(d, 3H, J=7.0 Hz), 1.38(d, 1H, J=7.0 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 176.1, 175.8*, 142.1, 141.8*, 133.3, 132.7*, 130.7, 130.4*, 127.7, 127.2, 113.9, 113.4*, 112.4*, 112.1*, 86.2, 85.4*, 75.8, 75.9*, 13.7, 12.5* ppm. MS(ESI): m/z=323 [M+Na]+.
5-Fluoro-3-hydroxy-3-(1-nitroethyl)indolin-2-one [42] (3h, , Entry 8): To a solution of isatin 1h (1.0 mmol) in freshly distilled DMF (3 mL) was added nitroethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 90 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3h as pale yellow solid. The Crude solid of 3h was further purified by silica gel column chromatography using ethyl acetate:hexane (1:2) as eluent to give the pure title compound 3h. Yield: 89%. Rf(50% EtOAc/hexanes) 0.38; Pale yellow solid. M.p. 128 130°C; IR (KBR, cm −1 ): 3361, 3277, 2927, 1731, 1617, 1558, 1480, 1377, 1192, 749. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 67:33, * denotes minor diastereomer peaks): δ 10.47(br s, 1H), 10.39*(br s 1H), 7.17(d, 1H, J=7.0 Hz), 7.07*(d, 1H, J=6.2 Hz), 7.01-6.96(m 1H), 6.84-6.81(m 1H), 6.78(br s, 1H), 6.78*(br s, 1H), 5.01-4.95(m, 1H), 1.72*(d, 3H, J=6.2 Hz), 1.35(d, 3H, J=6.2 Hz) ppm. 13 C NMR (75 MHz, CD 3 ) 2 SO): δ 176.28*, 175.61, 159.60, 159.51*, 156.42, 156.34*, 138.67*, 138.51, 128.81*, 128.77*, 128.41, 128.31, 116.54, 116.33*, 116.14, 116.01*, 113.71, 113.33, 112.74*, 112.41*, 111.01*, 110.91*, 110.85, 110.84, 86.38, 85.41*, 76.01, 75.21*, 13.64, 12.44 ppm. MS(ESI): m/z=263 [M+Na]+.
3-Hydroxy-5-iodo-3-(1-nitroethyl)indolin-2-one [42] (3i, , Entry 9): To a solution of isatin 1i (1.0 mmol) in freshly distilled DMF (3 mL) was added nitroethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 86 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3i as off white solid. The Crude solid of 3i was further purified by silica gel column chromatography using ethyl acetate:hexane (1:2) as eluent to give the pure title compound 3i. Yield: 90%. Rf(50% EtOAc/hexanes) 0.37; White solid. M.p. 278 280°C; IR (KBR, cm −1 ): 3381, 3248, 2934, 1733, 1614, 1552, 1472, 1357, 1188, 816. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 91:9, * denotes minor diastereomer peaks): δ 10.55(br s, 1H), 10.49*(br s, 1H), 7.67(d, 1H, J=1.5 Hz), 7.55(dd, 1H, J=8.1, 1.5, Hz), 6.7(br s, 1H), 6.68(d, 1H, J=8.1 Hz), 4.99-4.92(m, 1H), 1.75*(d, 3H, J=7.0 Hz), 1.38(d, 3H, J=7.0 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 175.51*, 174.83, 142.32*, 142.27, 138.38, 133.15, 132.56*, 129.80*, 129.45, 112.36*, 112.30, 86.19, 85.18*, 84, 83.6*, 75.34, 74.64*, 13.5, 12.3* ppm. MS(ESI): m/z=371 [M+Na]+.
3-Hydroxy-1-methyl-3-(1-nitroethyl)indolin-2-one (3j, , Entry 10): To a solution of isatin 1j (1.0 mmol) in freshly distilled DMF (3 mL) was added nitroethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 72 min. After then, the reaction mixture was poured on 10 ml ice cold water and extracted with ethyl acetate (10x3). The Combine organic extract was dried on Na2SO4 and concentrate in Vacuo to provide crude 3j as pale yellow viscous liquid. The Crude 3j was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3j. Yield: 92%. Rf(50% EtOAc/hexanes) 0.44; White solid. M.p. 140 142°C; IR (KBR, cm −1 ): 3316, 2939, 1703, 1616, 1551, 1468, 1385, 1355, 1113, 1061, 759. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 63:37, * denotes minor diastereomer peaks): δ 7.44*(s, 1H), 7.42*(s, 1H), 7.35(s, 1H), 7.33(s, 1H), 7.12-7.0(m, 1H), 6.88(d, 1H, J=3.5 Hz), 6.85*(d, 1H, J=3.2 Hz), 6.68*(br s, 1H), 6.63(br s, 1H), 5.05-4.98(m, 1H), 3.18*(s, 3H), 3.17(s, 3H), 1.76*(d, 3H, J=6.8 Hz), 1.37(d, 3H, J=6.8 Hz) ppm. 13 C NMR (75 MHz, CD 3 ) 2 SO): δ 174.83*, 174.4, 144.3*, 144.09, 130.43, 130.13*, 127.4*, 126.9, 124.24, 123.8*, 122.7, 122.3*, 108.9*, 108.57, 78.4, 77.8*, 72.75, 71.6*, 26.17, 25.3*, 13.9, 12.8* ppm. MS(ESI): m/z=259 [M+Na]+.
3-Hydroxy-3-(1-nitroethyl)-1-phenylindolin-2-one (3k, , Entry 11): To a solution of isatin 1k (1.0 mmol) in freshly distilled DMF (3 mL) was added nitroethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 90 min. After then, the reaction mixture was poured on 10 ml ice cold water and extracted with ethyl acetate (10x3). The Combine organic extract was dried on Na2SO4 and concentrate in Vacuo to provide crude 3k as pale yellow viscous liquid. The Crude 3k was further purified by silica gel column chromatography using ethyl acetate:hexane (1:3) as eluent to give the pure title compound 3k. Yield: 89%. Rf(50% EtOAc/hexanes) 0.43; White solid. M.p 102 105°C. IR (KBR, cm −1 ): 3331, 2923, 1711, 1619, 1569, 1471, 1374, 1087, 769. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 72:28, * denotes minor diastereomer peaks): δ 7.56-7.37(m, 6H), 7.26(t, 1H, J=7.4 Hz), 7.19-7.15*(m, 1H), 7.10(t, 1H, J=7.5 Hz), 7.06-7.01*(m, 1H), 6.91(br s, 1H), 6.89*(br s, 1H), 6.74(d, 1H, J=7.4 Hz), 5.19-5.09 (m, 1H), 1.84*(d, 3H, J=6.9 Hz), 1.57(d, 3H, J=6.9 Hz) ppm. 13 C NMR (75 MHz, (CD 3 ) 2 SO): δ 174.4*, 173.3, 143.9*, 143.6, 133.8*, 133.7, 130.0*, 129.4, 128.2*, 128.1, 128.03, 127.9*, 126.5*, 126.3, 125.2, 124.9*, 122.92, 122.79*, 109.18*, 109.05, 86.93, 85.7*, 74.7*, 74.6, 13.58, 12.2* ppm. MS(ESI): m/z=321 [M+Na]+.
3-Hydroxy-1-(hydroxymethyl)-5-iodo-3-(1-nitroethyl)indolin-2-one (3l, , entry 12): To a solution of isatin 1l (1.0 mmol) in freshly distilled DMF (3 mL) was added nitroethane (2 mmol) under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 90 min. After then, the reaction mixture was poured on 10 ml ice cold water to obtain the crude 3l as off white solid. The Crude solid of 3l was further purified by silica gel column chromatography using ethyl acetate:hexane (1:1) as eluent to give the pure title compound 3l. Yield: 88%. Rf(50% EtOAc/hexanes) 0.35; White solid. M.p. 151 153°C; IR (KBR, cm −1 ): 3341, 2982, 1731, 1604, 1537, 1480, 1333, 1194, 1029, 965, 810. 1 H NMR (300 MHz, (CD 3 ) 2 SO) (diastereomeric ratio, threo:erythro 88:12, * denotes minor diastereomer peaks) δ 7.75(d, 1H, J=7.5 Hz), 7.66(dd, 1H, J=8.3, 1.7 Hz), 7.61*(dd, 1H, J=8.3, 1.7 Hz), 6.95(d, 1H, J=8.1 Hz), 6.9(br s, 1H), 6.8*(br s, 1H), 6.2(br s, 1H), 5.1(s, 2H), 5.04-4.9(m, 1H), 1.76*(d, 1H, J=7.0 Hz), 1.37(d, 1H, J=7.0 Hz). 13 CNMR (75 MHz, (CD 3 ) 2 SO): δ 173.5*, 172.9, 142.3, 138.6, 133.6, 132.7*, 129.0*, 128.6, 112.4, 86.35, 85.6, 85.4, 85.3*, 75.1, 74.6*, 62.8, 13.52, 12.4*. MS(ESI): m/z=379 [M+1]+.
1 H NMR and 13 C NMR Spectrum of compound 3a-3l
3-Hydroxy-3-(nitromethyl)indolin-2-one (3a, , Entry 1)
5-fluoro-3-Hydroxy-3-(nitromethyl)indolin-2-one (3b, , entry 2)
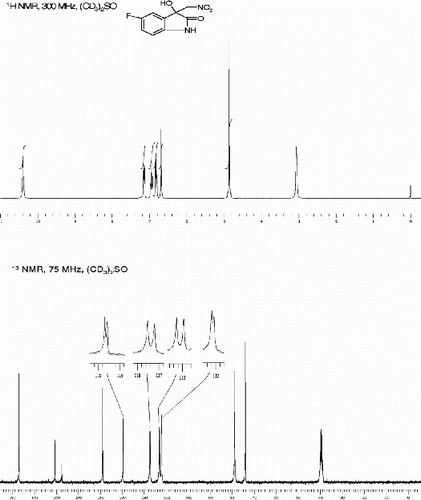
3-hydroxy-5-iodo-3-(nitromethyl)indolin-2-one (3c, , entry 3)
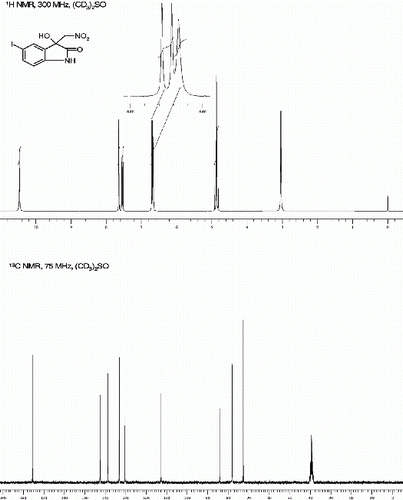
3-hydroxy-3-(nitromethyl) -1- phenylindolin-2-one (3d, , Entry 4)
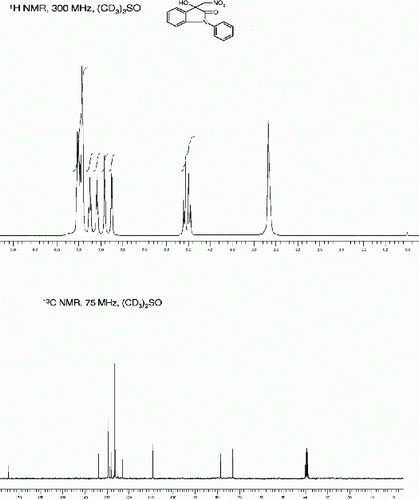
1-benzyl-5-bromo-3-hydroxy-3-(nitromethyl)indolin-2-one (3e, , Entry 5)
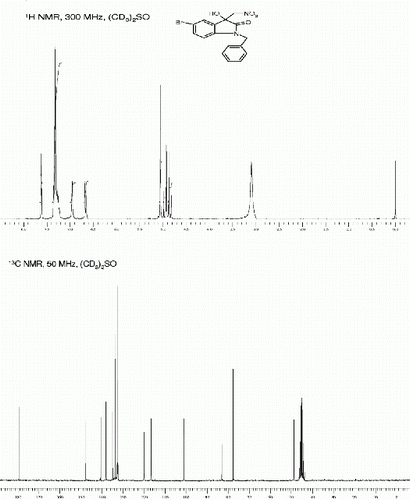
3-hydroxy-1-(hydroxymethyl)-5-iodo-3-(nitromethyl)indolin-2-one (3f, , entry 6)
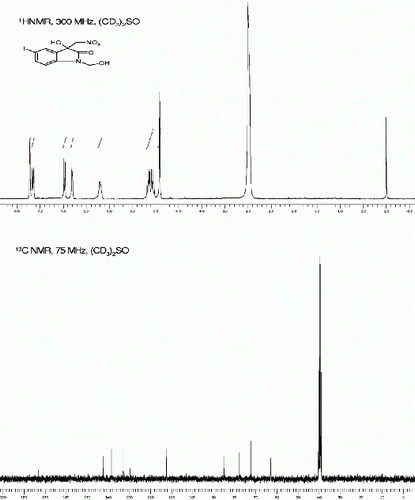
5-bromo-3-hydroxy-3-(1-nitroethyl)indolin-2-one, (3g, , entry 7)
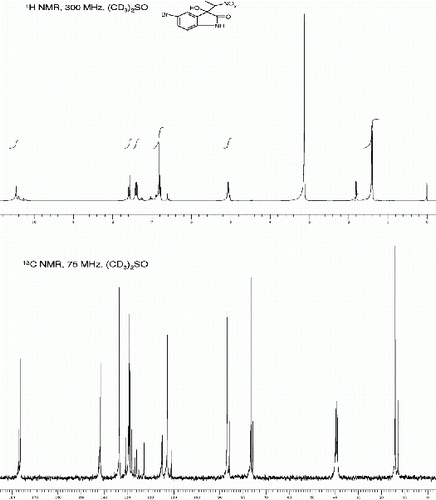
5-fluoro-3-hydroxy-3-(1-nitroethyl)indolin-2-one(3h, , entry 8)
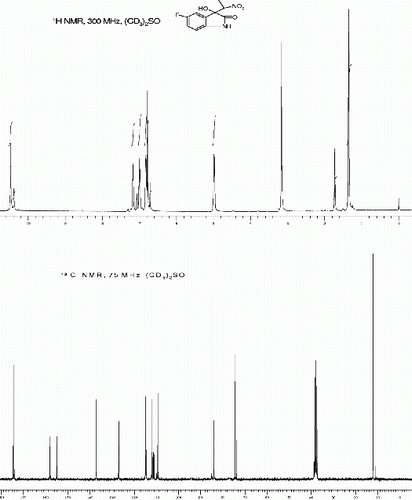
3-hydroxy-5-iodo-3-(1-nitroethyl)indolin-2-one, (3i, , Entry 9)
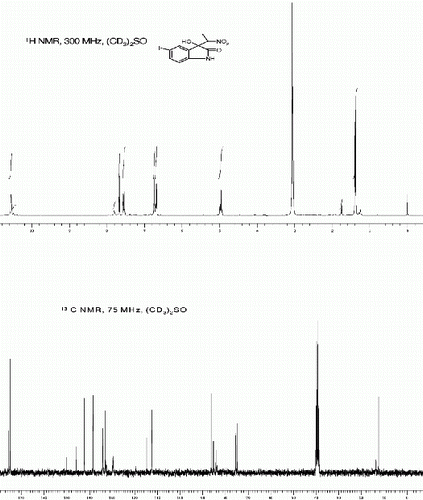
3-hydroxy-1-methyl-3-(1-nitroethyl)indolin-2-one (3j, , entry 10)
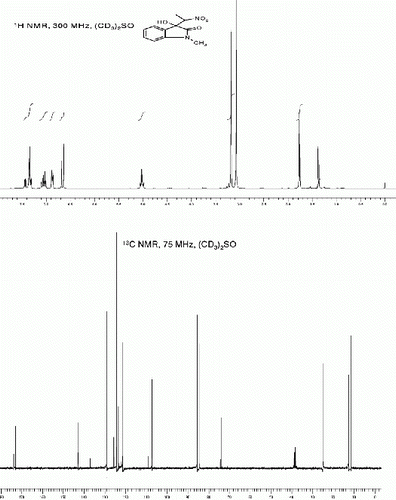
3-hydroxy-3-(1-nitroethyl)-1-phenylindolin-2-one (3k, , Entry 11)
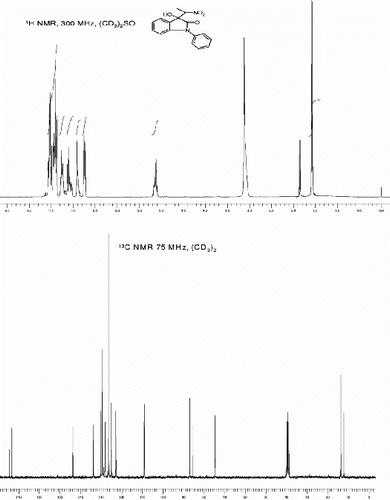
3-hydroxy-1-(hydroxymethyl)-5-iodo-3-(1-nitroethyl)indolin-2-one, (3l, , Entry 12)