Abstract
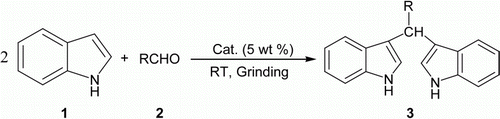
An efficient, chemoselective method for the preparation of bis(indol-3-yl)methanes from aldehydes and indoles using phosphate-impregnated titania as the reusable catalyst under solvent-free grinding conditions is reported. The desired products were obtained in excellent yields with tolerability to functional groups such as −OMe, −Me, −Cl, −NO2, and −OH in a simple and environmentally benign procedure.
Introduction
Indole is one of the most profoundly studied, ubiquitous, and π-electron excessive nitrogen heterocyclic systems Citation1 having interesting biological properties that received particular interest in the mid-1950s, when alkaloid reserpine Citation2 was introduced as one of the first drugs for the treatment of central nervous system (CNS) disorders such as anxiety and mental disorder. Substituted indoles are referred to as “privileged structures” since they are capable of binding to many receptors with high affinity. Indole-containing compounds have been reported to exhibit anti-apoptotic properties Citation3. Compared to benzene, indole shows enhanced reactivity in the electrophilic aromatic substitution reaction. C-3 position of Indole is about 1013 times more reactive than benzene Citation4 Citation5.
Unsubstituted indoles respond to electrophilic substitution reaction with aldehyde or ketone in the presence of Lewis or Brønsted acids Citation6 Citation7 via reactive C-3 position resulting bis(indol-3-yl)methanes (BIMs). Various catalysts, e.g. FeF3 Citation8, ZrCl4 Citation9, InCl3 Citation10, In(OTf)3 Citation11, poly(vinyl sulfonic acid) Citation12, [bimim][MeSO4] Citation13, Cu1.5PMo12O40 Citation14, SiO2–AlCl3 Citation15, NH4Cl Citation16, Zn(OTf)2 Citation17, ultrasound Citation18, cellulose sulfuric acid Citation19, supported-SO3H Citation20, etc. have been reported for the synthesis of BIMs. There are reports describing the preparation of BIMs in water Citation21. Very recently, new β-lactam compounds containing a bis(indolyl) framework were synthesized Citation22.
Naturally occurring or synthetic BIMs and its privileged structures are important intermediates in organic synthesis Citation23 and are particularly important in pharmaceutical chemistry as they exhibit various pharmacological activities and are important metabolites Citation24–27. BIM derivatives as highly selective colorimetric and fluorescent molecular chemosensors for Cu2+ cation has been reported Citation28. Although a number of different methods have been reported for the preparation of BIMs, these methods have their merits in some way as claimed, but, on the other way, they suffer from certain drawbacks; e.g. longer reaction time, harsh reaction conditions, expensive use of reagent/catalyst, sensitivity to moisture and air, incompatibility of functional groups, environmental incompatibility, many Lewis acids are prone to undergo decomposition in the presence of nitrogen containing reactants and this requires the use of excess and sometimes stoichiometric amount of Lewis acid catalyst Citation29. Hence, there is still a need to search for better catalysts in terms of toxicity, handling, availability, economic viability, and operational simplicity. In view of the recent trend in catalytic process which comes under the purview of green chemistry, investigations for new and less hazardous catalysts have become a priority in synthetic organic chemistry. Previously, our collaborator, Chaudhari et al. reported the synthesis of phosphate-impregnated titania solid acid catalyst and its use in the nitration Citation30, sulfoxidation Citation31, and aza-Michael reaction Citation32. Inspired by those works, in this communication, we want to report an efficient green synthesis of BIMs 3 () from indole 1 and aldehydes 2 catalyzed by solid acid, phosphate-impregnated titania catalyst. To mention, in recent years, the use of solid acid catalysts has received paramount attention and interest in different areas of organic synthesis because of their several inherent useful properties such as environmental compatibility, reusability, greater selectivity, simplicity of handling, lesser toxicity, noncorrosiveness, cheap, and ease of isolation. Also, generation of wastes and byproducts can be minimized or avoided by using solid acid catalyst, thereby developing cleaner synthetic routes Citation33,Citation34.
Results and discussion
First, 4-chlorobenzaldehyde (2b) was chosen as a model substrate for the reaction with indole 1. Compound 2b (1 mmol, 0.140 g) and indole 1 (2 mmol, 0.234 g) were mixed and grounded in a mortar with a pestle in the presence of 5 wt% (0.0187 g) of the catalyst at room temperature for 1 min without any solvent. It is our mandates in the laboratory that whenever, we start a new reaction, we always first check it in the absence of a solvent following green chemistry practice. When we checked the TLC after 1 min for monitoring the progress of the reaction, interestingly, we were surprised to observe that the reaction was indeed over by that time providing BIM 3b. It has been observed that by increasing the catalyst amount from 1 to 5 wt%, the yield of the product also increased from 20 to 99% within 1 min. Further increasing the amount of catalyst did not have any observable effect on the yield of the product. However, using less than 5 wt% of the catalyst and for longer time (beyond 10 min) there was an enhancement of 2–4% only in the overall yield of the product (). Hence, 5 wt% was chosen as the optimum catalyst amount for this reaction. Having established the optimum reaction conditions, structurally diversed carbonyl compounds were treated with indole 1 under the same reaction conditions in order to investigate the reaction scope and generality, and the results are summarized in (entries 2a–t). The methodology is found to be quite general as variety of substituted aromatic aldehydes, aliphatic aldehydes, and heterocyclic aldehydes (entries 2a–p, ) reacted efficiently with indole 1 to give the BIMs 3a–p in excellent yields quickly than any other reported methods. Many of the pharmacologically relevant substitution patterns on the aromatic ring could be introduced with high efficiency using this procedure. Furthermore, unsaturated aldehyde, cinnamaldehyde (entry 2g, ) gave the corresponding BIM 3g without polymerization under the above reaction conditions in 98% yield within 1.5 min. The heterocyclic aldehydes like 2-furaldehyde, which was known as acid-sensitive species, was proved to be applicable under the reaction conditions (entry 2i, ), indicating the usefulness of our methodology. Furthermore, thiophene-2-carboxaldehyde (entry 2h, ) also worked well without forming any side products. Notably, the reaction conditions are mild enough and work nicely without damaging moieties such as −OMe, −Me, −Cl, −NO2, and −OH. No solvent was used for the reaction and it was carried out at room temperature under grinding conditions. In absence of the catalyst the reaction did not proceed, thereby indicating the necessity of a catalyst.
Table 1. Effect of the catalyst amount investigated for the .
Table 2. Synthesis of BIMs 3 using phosphate-impregnated titania catalyst.
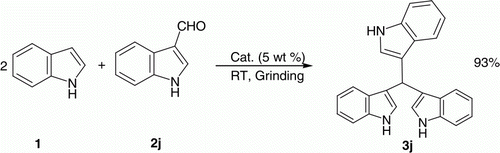
Interestingly, indol-3-carbaldehyde (entry 2j, ) reacting with two equivalents of indole 1 under similar conditions afforded tri-indolylmethane 3j in 93% yield ().
To note, aliphatic aldehydes (entries 2k–n, ) took some what longer time than the aromatic ones. However, ketones (entries 2q–t, ) did not react at all with indole 1 under the reaction conditions, demonstrating the chemoselective nature of the methodology. For example, when an equimolar mixture of benzaldehyde (entry 2a, ) and acetophenone (entry 2q, ) were allowed to react with indole 1 in the presence of the catalyst, only phenyl-3,3′-bis(indolyl)methane was obtained, while acetophenone 1q remained as such and was recovered. When we carried out the reaction with 6-oxo-6-phenylhexanal (entry 2p, ) only the aldehydic part of the molecule reacted leaving aside the ketonic group intact, hence demonstrated the chemoselective nature of the method further. As expected, the electron-rich aldehydes should react faster than the aldehydes with electron withdrawing group. However, we did not observe any such difference; this may be due to the short reaction time with the exception, p-hydroxybenzaldehyde (entry 2f, ) that required longer time (9.5 min).
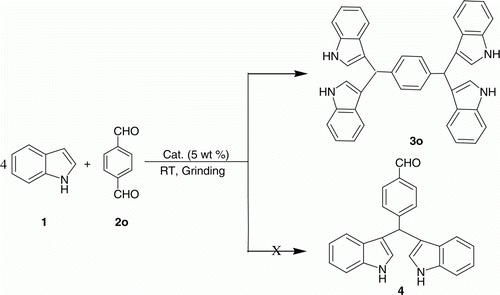
Encouraged by this result, we extended the reaction to dialdehyde. Accordingly, the reaction of terephthaldehyde (entry 2o, ) with indole 1 was investigated under similar conditions (). To our delight, terephthaldehyde 2o (1 mmol) reacted smoothly with indole 1 (4 mmol) to provide 1,4-bis(di(1H-indol-3-yl)methyl)benzene 3o in 93% yield, which indicated that both the two aldehydic groups of terephthaldehyde reacted. Formation of 4-bis((1H-indol-3-yl)methyl)benzene-1-carbaldehyde 4 () was not observed.
The generality of the reaction is also investigated for the substituted indole. Indole derivatives also react with the aldehyde to give the corresponding bis-indolyl methane () supporting the generality of the methodology.
Table 3. Reaction between 4-chlorobenzaldehyde and indole derivatives.
The IR spectra of BIMs 3 show characteristic IR absorptions within 3437–3480 cm−1 (N–H). In addition, the 1H NMR spectrum of 3b displays characteristic signals within δ 7–8 ppm (br s, NH, exchangeable with D2O).
To compare our catalyst with the reported one, the efficiency of the various reported catalysts for the condensation of indole with benzaldehyde is shown in . Among those catalysts, our catalyst is found to be superior in terms of yield and reaction time.
Table 4. Comparison of catalytic condensation of indole with benzaldehyde.
The reusability of the catalyst was examined for the reaction of 4-chlorobenzaldehyde 2b and indole 1 as model substrates vide . The result showed that the solid catalyst, phosphate-impregnated titania could be reused for at least four times without reducing the catalytic property of the catalyst appreciably (); thereafter, the yield started decreasing because of the leaching of the catalyst.
Table 5. Efficacy of recycled catalyst.
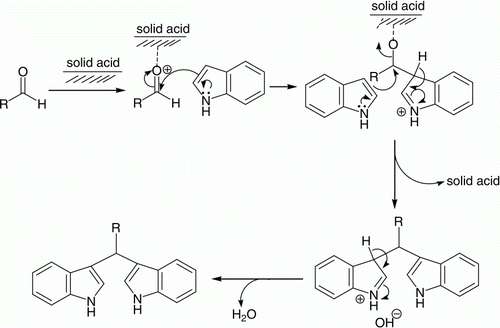
A plausible mechanism for the reaction is shown in . The interaction of the external surface of the solid catalyst, phosphate-impregnated titania with the aldehyde probably occurs, that increases the electrophilicity of the carbonyl carbon and which in turn facilitates the participation of indole molecules. The interaction may also occur via the external acidic sites of the catalyst.
In conclusion, we have developed a highly efficient electrophilic substitution reaction of indole with various aromatic, aliphatic as well as heterocyclic aldehydes using phosphate-impregnated titania as a recyclable catalyst. The procedure offers several advantages including improved yield of products with no by-products formation, simple experimental procedure with an economic and environmentally friendly solvent-free conditions, cleaner reaction profile, short reaction time, and use of inexpensive catalyst; hence, it is a useful addition to the existing methods for the synthesis of BIMs. Interestingly, the experimental procedure for these reactions is remarkably simple without requiring dry solvents, inert atmosphere, and reflux conditions, which makes it a useful and attractive process for the rapid synthesis of substituted BIMs.
Experimental
Melting points were determined on a Büchi 504 apparatus and are uncorrected and were compared with the literature value and gave good resemblance. IR spectra were recorded in KBr pallets on a Nicolet (Impact 410) FT-IR spectrophotometer. 1H (400 MHz) and 13C NMR (100 MHz) spectra were recorded on an ECS 400 MHz NMR (JEOL) spectrophotometer. TMS served as the internal standard. GC–MS was carried out on gas chromatograph (Perkin Elmer, Claurus 600) with Thermal Conductivity Detector (TCD) detector, Elite wax column. The progress of the reactions was followed by TLC, using silica gel 60 F254 plates (Merck).
Typical procedure for the synthesis of BIM 3b
A mixture of indole 1 (2 mmol, 0.234 g), 4-chlorobenzaldehyde 2a (1 mmol, 0.140 g), and catalyst, phosphate-impregnated titania (0.0167 g, 5 wt% based on 1) under solvent-free conditions was grounded in a mortar with pestle at room temperature for an appropriate time (1 min). After completion of the reaction, as indicated by TLC, the reaction mixture was extracted with ethyl acetate (2×10 mL) and filtered to separate the catalyst. The combined organic layers were dried over anhydrous Na2SO4 and solvent was removed under reduced pressure in a rotary evaporator to furnish a crude product that was further purified by column chromatography on silica gel [Eluent: EtOAc/Pet. Ether (1:2)] to give the pure product (99% yield).
Typical procedure for the synthesis of tetra-indolyl(terephthalyl)dimethane 3o
A mixture of indole 1 (4 mmol, 0.468 g), terephthaldehyde 2o (1 mmol, 0.134 g), and the catalyst (0.0234 g, 5 wt% based on 1) under solvent-free conditions was grounded in a mortar with pestle at room temperature for an appropriate time (1 min). After completion of the reaction, as indicated by TLC, the reaction mixture was extracted with ethyl acetate (2×10 mL) and filtered to separate the catalyst. The combined organic layers were dried over anhydrous Na2SO4 and solvent was removed under reduced pressure in a rotary evaporator to furnish a crude product that was further purified by column chromatography on silica gel (Eluent: EtOAc/Pet. Ether [1:2]) to give the pure product (93% yield).
All products were identified and characterized by 1H NMR, 13C NMR, IR, LCMS, GC–MS, and melting point determination to be consistent with the literature result. The yields of the products reported here are isolated yields.
Physical and spectral data for 3b
Yield 99%, solid, melting point 104–105 °C; IR (KBr) (υmax/cm−1) 3402, 3050, 2986, 1615, 1600, 1455, 1112. 1H NMR (400 MHz, CDCl3) δH 5.83 (s, 1H), 6.68 (d, J=2.24 Hz, 2H), 7.10–7.45 (m, ArH, 12H), 7.81 (br s, 2H, NH, exchangeable with D2O); 13C NMR (100 MHz, CDCl3) δc 31.6, 110.9, 111.9, 118.4, 119.5, 121.2, 124.0, 126.3, 127.1, 128.5, 128.6, 137.0, 145.2 ppm. MS, m/z 322 (M+). Elemental analyses: Calculated, C, 85.72; H, 5.89; N, 8.72%. Found: C, 85.73; H, 5.90; N, 8.74%.
Physical and spectral data for 3r
Yield 90%, solid, melting point 122–124 °C; IR (KBr) (υmax/cm−1) 3410, 2927, 1624, 1466, 1176, 1088. 1H NMR (400 MHz, DMSO-d 6) δH 5.58 (s, 1H), 6.66 (d, J=2.28 Hz, 2H), 7.1–7.3 (m, 10H), 8.51 (s, 2H), 10.4 (s, 2H); 13C NMR (100 MHz, DMSO-d 6) δC 30.3, 103.6, 111.7, 112.2, 117.1, 124.5, 127.6, 128.4, 130.62, 130.69, 131.6, 144.4, 150.47 ppm. MS, m/z 386 (M+). Elemental analyses: Calculated C, 54.76; H, 40.47; N, 4.76%. Found C, 54.74; H, 40.48; N, 4.78%.
Physical and spectral data for 3s
Yield 96%, solid, melting point 191–192°C; IR (KBr) (υmax/cm−1) 3423, 2926, 1448, 1089. 1H NMR (400 MHz, DMSO-d 6) δH 5.73 (s, 1H), 6.62 (s, 2H), 7.24–7.25 (m, 10H), 8.11 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δC 39.38, 112.74, 112.85, 118.6, 122.21, 124.84, 125.18, 128.56, 128.66, 129.95, 132.29, 135.44, 141.73 ppm. MS m/z 514 (M+). Elemental analyses: Calculated, C, 57.5; N, 5; H, 37.5%. Found: C, 57.6; N, 5.04; H, 37.8%.
Synthesis and characterization of the catalyst
Synthesis of the phosphate-impregnated titania catalyst was carried out as per report Citation28. Here, we have followed the same procedure for the preparation of the catalyst as reported: titania and phosphoric acid (88%) were mixed in the molar ratio of 1:1 in a silica boat followed by heating at 200–220 °C on a hot sand bath under stirring until the mass solidified. Heating was then withdrawn and temperature was allowed to come down to approximately 100 °C, then the catalyst was transferred to a vacuum desiccator. Finally the catalyst was properly stored in an airtight sample vial. After preparation, the catalyst was characterized Citation28 by IR, XRD technique and was compared with the reported data.
Reusability of the catalyst
After filtration, the catalyst was recharged by heating at 200–220 °C for 30 min. which was reused for the reaction.
Acknowledgements
Ashim Jyoti Thakur thanks Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial support to the project CSIR (01(2147)/07/EMR-II) and Prof. MK Chaudhari, Vice Chancellor, Tezpur University for helpful discussions. Dhrubajyoti Talukdar thanks Tezpur University for the institutional fellowship.
References
- Bandini , M. ; Eichholzer , A. Angew. Chem. Int. Ed. Engl . 2009 , 48 , 9608 .
- Chen , F.R. ; Huang , J. Chem. Rev . 2005 , 105 , 4671 .
- Kuo , C.C. ; Hsieh , H.P. ; Pan , W.Y. ; Chen , C.P. ; Liou , J.P. ; Lee , S.J. ; Chang , Y.L. ; Chen , L.T. ; Chen , C.T. ; Chang , J.Y. Cancer Res . 2004 , 64 , 4621 .
- Yagil , G. Tetrahedron 1967 , 23 , 2855 .
- Zeng , M. ; You , S.L. Synlett 2010 , 9 , 1289 .
- Olah , G.A. ; Krisnamurthi , R. ; Prakash , G.K.S. In Comprehensive Organic Synthesis , 1st ed ; Trost , B.M. , Fleming , I. ; Pergamon : Oxford , 1991 . 3 , pp 293 – 335 .
- Roberts , R.M. ; Khalaf , A.A. Friedel-Craft Alkylation Chemistry. A Century of Discovery ; Marcel Dekker : New York , 1984 .
- Kamble , V.T. ; Bandgar , B.P. ; Suryawanshi , S.B. ; Bavikar , S.N. Aust. J. Chem . 2006 , 59 , 837 .
- Nagawade , R.R. ; Shinde , D.B. Acta Chim. Solv . 2006 , 53 , 210 .
- Babu , G. ; Sridhar , N. ; Perumal , P.T. Synth. Commun . 2000 , 30 , 1609 .
- Nagarajan , R. ; Perumal , P.T. Tetrahedron 2002 , 58 , 1229 .
- Ekbote , S.S. ; Deshmukh , K.M. ; Qureshi , Z.S. ; Bhange , B.M. Green Chem. Lett. Rev . 2011 , 4 , 117 .
- Chakraborti , A.K. ; Roy , S.R. ; Kumar , D. Green Chem . 2008 , 10 , 1111 .
- Seyedi , N. ; Khabazzadeh , H. ; Saidi , K. Mol. Divers . 2009 , 13 , 337 .
- Boroujeni , K.P. ; Parvanak , K. Chin. Chem. Lett . 2011 , 22 , 939 .
- Naskar , S. ; Hazara , A. ; Paria , P. ; Sahu , K.B. ; Benerjee , S. ; Nirup , B.J. Chem. Res . 2008 , 10 , 568 .
- Praveen , C. ; Kumar , P.D. ; Muralidharan , D. ; Perumal , P.T. Bio. Med. Chem. Lett . 2010 , 20 , 7292 .
- Li , J. ; Sun , M.X. ; He , G.Y. ; Xu , X. Ultrason. Sonocem . 2011 , 18 , 412 .
- Sadaphal , S.A. ; Sonar , S.S. ; Ware , M.N. ; Shingare , M.S. Green Chem. Lett. Rev . 2008 , 1 , 191 .
- Karam , A. ; Alonso , J.C. ; Gereganova , T.I. ; Ferreira , P. ; Bion , N. ; Barrault , J. ; Jèrôme , F. Chem. Comm . 2009 , 45 , 7000 .
- Yu , L. ; Chen , D. ; Li , J. ; Wang , P.G. J. Org. Chem . 1997 , 62 , 3575 .
- Galletti , P. ; Quintavalla , A. ; Ventrici , C. ; Giannini , G. ; Cabrib , W. ; Giacomini , D. New J. Chem . 2010 , 34 , 2861 .
- Shiri , M. ; Zolfigol , M.A. ; Kruger , H.G. ; Tanbakouchian , Z. Chem. Rev . 2010 , 110 , 2250 .
- Xia , M. ; Wang , S. ; Yuan , W.B. Synth. Commun . 2004 , 34 , 3175 .
- Damodiran , M. ; Muralidharan , D. ; Perumal , P.T. Bioorg. Med. Chem. Lett . 2009 , 19 , 3611 .
- Gribble , G.W. J. Chem. Soc. Perkin Trans. 1 , 2000 , 7 , 1045 .
- Karamyan , A.J.K. ; Hamann , M.T. Chem. Rev . 2010 , 110 , 4489 .
- Martinez , R. ; Espinosa , A. ; Tarraga , A. ; Molina , P. Tetrahedron 2008 , 64 , 2184 .
- Kobayashi , S. ; Araki , M. ; Yasuda , M. Tetrahedron Lett . 1995 , 36 , 5773 .
- Bharadwaj , S.K. ; Hussain , S. ; Kar , M. ; Chaudhuri , M.K. Appl. Catal. A Gen . 2008 , 343 , 62 .
- Bharadwaj , S.K. ; Sharma , S.S. ; Hussain , S. ; Chaudhuri , M.K. Tetrahedron Lett . 2009 , 50 , 3767 .
- Nath , J. ; Chaudhuri , M.K. Catal. Lett . 2009 , 133 , 388 .
- Harmer , M.A. In Handbook of Green Chemistry and Technology ; Clark , J. , MacQuarrie , D. ; Blackwell Science Ltd .: London , 2002 ; pp 86 – 117 .
- Wilson , K. ; Clark , J.H. Pure Appl. Chem . 2000 , 72 , 1313 .