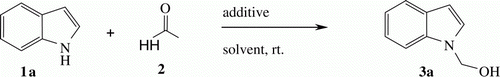Abstract
The synthesis of hemiaminal of indole is described by the reaction of indole with formaldehyde in the presence of TBAF in water at ambient temperature. The procedure is very efficient, mild, convenient, and environmentally benign with high yield of product. Moreover, the reuse of reaction media makes the procedure an attractive alternative to earlier methods.
Introduction
Indole and its myriad of derivatives continue to capture the attention of synthetic chemist because of their profound biological activity Citation1. Some hemiaminals of indole and related N-heterocycles possess anti-tumor activity Citation2 Citation3. Additionally, these compounds have been utilized as labile precursor for in-situ generation of formaldehyde Citation4–6 and a self-cleavable linker for drug molecules to improve their bioavailability Citation7 Citation8. In the view of their potential applications in pharmaceuticals, the development of an efficient method for the synthesis of hemiaminals of indoles is desirable.
The conventional methods for the synthesis of hemiaminals involve the addition of formaldehyde or paraformaldehyde to the amines under strong basic condition which give lower- to-moderate yield (34–70%) even at higher temperature (100°C) Citation2 Citation3 Citation9–15. Alternatively, the deprotection of N-[2-(trimethylsilyl)ethoxy]methylamine (SEMNR2) or benzyloxymethylamine (BOM-NR2) occasionally gives hemiaminals as byproducts Citation16–23. The two-step synthesis of hemiaminal of indole is also reported by the reduction of carbamates Citation24.
Organic solvents are widely used for organic reactions and have been the cause of concern because of their environmental hazards. Because of the growing awareness of the environmental obligations, there is a considerable incentive to find catalytic system with reusable reaction media that are efficient and environmental friendly. Recently organic reactions in aqueous media Citation25–29 have attracted much attention in synthetic organic chemistry, not only because water is one of the most abundant, cheapest, and environmentally friendly solvents, but also because water exhibits unique reactivity and selectivity which is different from those obtained in the conventional organic solvents. Thus, the use of water instead of organic solvents has gained importance as an essential component of the development of sustainable chemistry Citation30. Tetrabutylammonium fluoride has been widely recognized as a convenient source of naked fluoride ion, and it has been extensively used Citation31–46 in organic synthesis for various transformations like desilylation Citation34–37 and Aldol condensation Citation43–46.
In continuation of our research program Citation47–55, for the development of environmentally benign methodologies, we envisioned to explore the catalytic activity of TBAF for the synthesis of hemiaminal of indole. Herein, we wish to report the synthesis of hemiaminals by the reaction of indoles with formaldehyde utilizing TBAF in water as reusable reaction media at ambient temperature.
Results and discussion
The initial reaction of indole 1a (1 mmol) with formaldehyde 2 (1 ml, 37% aqueous solution) in water (5 ml) at room temperature gave only trace amount of desired adduct 3a even after longer reaction time (12 hours). However, the addition of catalytic amount (10 mol%) of TBAF to the above reaction mixture accelerated the rate of reaction, and desired hemiaminal was obtained with high yield (94%) within 2 hours. This may be presumed that the naked fluoride ions from TBAF certainly catalyzed the reactions. To search for the better fluoride ion source, we screened different fluoride ion sources under the similar reaction condition (; ).
Table 1. Optimization of reaction condition for the synthesis of Hemiaminal 3aa.
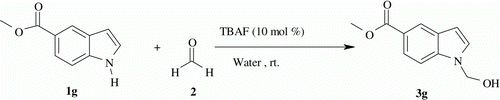
It was found that the fluoride ion of TBAF in water greatly enhanced the efficiency of reaction. This may be attributed to the hydrophobic interaction of water which induces a favorable aggregation of the polar components in water. It is important to note that the present method is suitable for a selective N-hydroxymethylation of indole. For example, the reaction of indole with formaldehyde (aq.) in the presence of TBAF gave exclusively N-hydroxymethylated indole. Though the C-3 position of indole is nucleophilic in nature Citation56 Citation57, we have not observed the formation of any C-3 hydroxymethylated product with TBAF in water. After numerous attempts, we have established that 10 mol% of TBAF is suitable for optimum conversion. Further, with a set of optimized reaction conditions in our hand, we examined the scope of the reaction with respect to the different substituted indoles. Various indoles with electron-donating or electron-withdrawing group reacted smoothly to afford the desired products in good to excellent yields, and the results are summarized in . During investigation it was observed that electron withdrawing group facilitated the reaction. For example, indole with ester (, Entry 7, 11) or nitro (, Entry 8) substituents underwent hydroxymethylation very efficiently and gave high yield of desired product. Other substituted indoles also reacted analogously to provide expected product in good yield. The efficiency of the procedure is strengthened by examining the reaction of fused heterocyclic system. We observed that azaindole also reacted with formaldehyde in standard condition to give corresponding product in good yield (, Entry 13).
Table 2. Synthesis of hemiaminals of indole derivatives by using TBAF in water as reusable reaction mediaa.
Further, we have studied the reusability Citation58 of reaction media. After completion of the reaction, the product was isolated simply by extraction with EtOAc-Hexane (1:1). The remaining aqueous layer containing TBAF was directly used for the next cycle. The reusability of reaction media was studied up to fifth cycle without any substantial loss in catalytic activity of medium. However, during the fifth cycle, the reaction requires longer time for completion (; ). We believe that the efficiency and reusability of reaction media makes the procedure more eco-friendly.
Table 3. Study on the reuse of reaction media for the synthesis of hemiaminala.
Although the synthesis of hemiaminals has been reported in water medium with reflux under strong basic condition Citation2 Citation6, the present method has provided a useful alternative over conventional methods for the same transformation, as our method has distinct advantages such as, mild reaction condition, shorter reaction time, high yield and reusable reaction media. Moreover, the functionalities such as ester, aldehyde, ketones, double bond, and ether remains unaffected in the present reaction condition. It is also noteworthy that the present method provides access to the synthesis of new indole derivatives which are not prepared earlier.
Experimental section
All chemicals were purchased from Fluka and S. D. Fine Chemicals. TLC: precoated silica gel plates (60 F 254 , 0.2 mm layer; E. Merk. M.p.: Barnstead Electrothermal 9300 apparatus; uncorrected. IR spectra: Thermo Nicolet Nexus 670 Spectrometer; ν in cm−1. 1H–, 13C-NMR Spectra: Varian 200, Bruker 300 and 50, 75 MHz spectrometer, respectively; in CDCl3 or (D6) DMSO; δ in ppm relative to TMS as internal standard, J in Hz. Mass spectra: VG Autospec; in m/z.
Typical procedure
Indoles (1 mmol) and formaldehyde (1 ml, 37% aqueous solution) were combined with 5 ml water in a closed round bottomed flask equipped with a stir bar. Tetrabutylammonium fluoride (10 mol%) was added to above reaction mixture and stirred at room temperature for stipulated time (). After completion of reaction, as indicated by TLC, the reaction mixture was extracted with a mixture of ethyl acetate:hexane ((1:1), 3×6 ml). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The resulting crude product was further purified by column chromatography using ethyl acetate:hexane (1:3) as eluent to afford the pure hemiaminal of indoles.
All the synthesized compounds were characterized by 1H-NMR, 13C-NMR, IR, and mass spectroscopic techniques. Spectral data for selected new compounds are given below.
| 1. | Compound 3c (, Entry 3): Brown solid, melting point 83–85 °C; 1H NMR (300 MHz, CDCl3): δ 7.47(d, J=7.9 Hz,1H), 7.36(s, 1H), 7.31(d, J=7.9 Hz, 1H), 7.22–7.09(m, 2H), 5.47 (s, 2H), 4.58 (brs, 1H); 13C NMR (75 MHz, CDCl3): δ 135.40, 131.28, 130.88, 123.31, 121.34, 121.20, 109.59, 69.47, 57.85; IR (KBr) ν=3410.64, 3053.03, 2901.54, 1460.77, 1308.83, 1199.21, 1013.73, 822.41, 742.46 cm–1. MS (ESI) m/z 296(M + Na)+. | ||||
| 2. | Compound 3g (, Entry 7): white solid, melting point 150–152°C; 1H NMR (300 MHz, CDCl3): δ 8.3 (d, J=1.5 Hz,1H), 7.85(dd, J=1.5, 8.3 Hz, 1H), 7.40(d, J=8.3 Hz, 1H), 7.19(d, J=3.0 Hz, 1H), 6.56 (d, J=3.0 Hz, 1H), 5.61 (s, 2H), 3.90 (s, 3H), 2.82 (brs, 1H); 13C NMR (75 MHz, (CD3)2SO): δ 167.65, 138.39, 130.74, 128.67, 123.40, 122.56, 121.43, 110.81, 103.13, 69.26, 52.17; IR (KBr) ν=3408.93, 3095.47, 2953.01, 1690.03, 1610.23, 1301.81, 1199.33, 1033.65, 751.47 cm−1. MS (ESI) m/z 228(M + Na)+. | ||||
| 3. | Compound 3i (, Entry 9): white solid, melting point 116–118°C; 1H NMR (300 MHz, CDCl3): δ 7.54 (d, J=1.7 Hz, 1H), 7.34(d, J=8.6 Hz, 1H), 7.17(d, J=1.7 Hz, 1H), 7.13(d, J=3.2 Hz, 1H), 6.42 (d, J=3.2 Hz, 1H), 5.56 (s, 2H), 2.42 (brs, 1H); 13C NMR (75 MHz, CDCl3): δ 133.30, 131.74, 128.22, 124.25, 122.65, 120.63, 110.52, 102.69, 70.08; IR (KBr) ν=3413.54, 3043.13, 2911.34,1469.67, 1311.91, 1187.91, 1014.53, 812.41, 741.16 cm−1. MS (ESI) m/z 204(M + Na)+. | ||||
| 4. | Compound 3j (, Entry 10): white solid, melting point 105–107°C; 1H NMR (300 MHz, CDCl3): δ 7.71 (d, J=1.5 Hz,1H), 7.29–7.28 (m, 2H), 7.11(d, J=3.0 Hz, 1H), 6.42 (d, J=3.0 Hz, 1H), 5.54 (s, 2H), 2.53 (brs, 1H); 13C NMR (75 MHz, CDCl3): δ 134.82, 129.91, 126.12, 124.52, 122.42, 119.23, 112.54, 104.39, 69.23; IR (KBr) ν=3411.44, 3033.03, 2901.24,1459.57, 1301.81, 1177.81, 1004.43, 849.41, 747.17 cm−1. MS (ESI) m/z 249(M + Na)+. | ||||
| 5. | Compound 3k (, Entry 11): white solid, melting point 98–100°C; 1H NMR (300 MHz, CDCl3): δ 8.12 (d, J=1.5 Hz,1H), 7.73(dd, J=1.5, 8.3 Hz, 1H), 7.56(d, J=8.3 Hz, 1H), 7.30(d, J=3.7 Hz, 1H), 6.50 (d, J=3.7 Hz, 1H), 5.61 (s, 2H), 3.83 (s, 3H), 3.71 (brs, 1H); 13C NMR (75 MHz,CDCl3): δ 168.28, 135.05, 132.95, 130.73, 123.41, 121.23, 120.53, 111.99, 103.08, 69.47, 51.93; IR (KBr) ν=3408.93, 3095.47, 2953.01, 1690.03, 1610.23, 1301.81, 1199.33, 1033.65, 751.47 cm−1. MS (ESI) m/z 228 (M + Na)+. | ||||
| 6. | Compound 3m (, Entry 13): pale yellow solid, melting point 95–98°C; 1H NMR (300 MHz, (CD3)2SO): δ 8.22 (dd, J=0.9, 4.5 Hz, 1H), 7.85 (dd, J=1.1, 7.7 Hz, 1H), 7.43(d, J =3.4 Hz, 1H), 7.05–7.01 (m, 1H), 6.42 (d, J=3.4 Hz, 1H), 6.28 (t, J=6.6 Hz, 1H [for OH]), 5.51 (d, J=6.6 Hz, 2H); 13C NMR (75 MHz, (CD3)2SO): δ 146.91, 142.41, 128.72, 128.54, 120.39, 116.06, 99.99, 66.35; IR (KBr) ν=3399.64, 3073.03, 2899.54,1480.71, 1328.73, 1179.91, 1011.71, 743.44 cm−1. MS (EI) m/z 148 (M)+. | ||||
Conclusion
In conclusion, we have developed highly efficient procedure for the synthesis of hemiaminal of indoles by utilizing TBAF in water as reusable reaction media. This method is bestowed with advantages such as simple, mild, high-yielding, and environmentally benign nature. The present protocol was successfully applied to a variety of substituted indoles to obtain their respective hemiaminals in good to excellent yield. The important features such as mild reaction condition, aqueous reaction media, and reusability of reaction media may contribute to the green chemistry.
Acknowledgements
P.B.T and B.M.B. thank CSIR New Delhi for the award of a fellowship and they also Dr J. S. Yadav, Director IICT, for his support and encouragement.
References
- Sundberg , R.J. The Chemistry of Indoles ; Academic press : New York , London , 1970 .
- Liou , J.P. ; Wu , C.Y. ; Hsieh , H.P. ; Chang , C.Y. ; Chen , C.M. ; Kuo , C.C. ; Chang , J.Y. J. Med. Chem . 2007 , 50 , 4548 – 4552 .
- Kemnitzer , W. ; Drewe , J. ; Jiang , S. ; Zhang , H. ; Crogan-Grundy , C. ; Labreque , D. ; Bubenick , M. ; Attardo , G. ; Denis , R. ; Lamothe , S. ; Gourdeau , H. ; Tseng , B. ; Kasibhatla , S. ; Cai , S.X. J. Med. Chem . 2008 , 51 , 417 – 423 .
- Smith , M.B. ; March , J. March's Advanced Organic Chemistry ; Wiley-Interscience : New York , 2007 ; 1281 – 1283 .
- Chudek , J.A. ; Foster , R. ; Young , D. J. Chem. Soc., Perkin Trans . 1985 , 2 , 1285 – 1289 .
- Deguest , G. ; Bischoff , L. ; Fruit , C. ; Marsais , F. Org. Lett . 2007 , 9 , 1165 – 1167 .
- Zhu , Z. ; Chen , H.G. ; Goel , O.P. ; Chan , H. ; Stilgenbauer , L.A. ; Stewart , B.H. Bioorg. Med. Chem. Lett . 2000 , 10 , 1121 – 1124 .
- Bundgaard , H. In Design of Prodrugs ; Bundgaard , H. ; Springer : Amsterdam , 1985 ; 1 – 92 .
- McFarland , J.M. ; Joshi , N.S. ; Francis , M.B. J. Am. Chem. Soc . 2008 , 130 , 7639 – 7644 .
- Sui , Y. ; Liu , L. ; Zhao , J.L. ; Wang , D. ; Chen , Y.J. Tetrahedron Lett . 2007 , 48 , 3779 – 3782 .
- Kren , V. ; Fiserova , A. ; Weignerova , L. ; Sibor , I. ; Halada , P. ; Prikrylova , V. ; Sedmera , P. ; Pospisil , M. Bioorg. Med. Chem . 2002 , 10 , 415 – 424 .
- Williams , D.M. ; Brown , D.M. J. Chem. Soc., Perkin Trans . 1995 , 1 , 1225 – 1231 .
- Tupper , D.E. ; Pullar , I.A. ; Clemens , J.A. ; Risius , F.C. ; Timms , G.H. ; Wedley , S. J. Med. Chem . 1993 , 36 , 912 – 918 .
- Mompon , B. ; Vassal , T. ; Poirier , P. J. Nat. Prod . 1985 , 48 , 273 – 278 .
- Taggart , M.S. ; Richter , G.H. J. Am. Chem. Soc . 1934 , 56 , 1385 – 1386 .
- Evans , G.B. ; Furneaux , R. ; Hutchison , T.L. ; Kezar , H.S. ; Morris , P.E. Jr. ; Schramm , V.L. ; Tyler , P.C. J. Org. Chem . 2001 , 66 , 5723 – 5730 .
- Hagiwara , H. ; Choshi , T. ; Nobuhiro , J. ; Fujimoto , H. ; Hibino , S. Chem. Pharm. Bull . 2001 , 49 , 881 – 886 .
- Macor , J.E. ; Forman , J.T. ; Post , R.J. ; Ryan , K. Tetrahedron Lett . 1997 , 38 , 1673 – 1676 .
- de Leon , C.Y. ; Ganem , B. Tetrahedron 1997 , 53 , 7731 – 7752 .
- Delgado , A. ; Clardy , J. J. Org. Chem . 1993 , 58 , 2862 – 2866 .
- Dhanak , D. ; Reese , C.B. J. Chem. Soc., Perkin Trans . 1986 , 1 , 2181 – 2186 .
- Figueroa-Perez , S. ; Bennabi , S. ; Schirok , H. ; Thutewohl , M. Tetrahedron Lett . 2006 , 47 , 2069 – 2072 .
- Garg , N.K. ; Caspi , D.D. ; Stoltz , B.M. J. Am. Chem. Soc . 2005 , 127 , 5970 – 5978 .
- He-Chu , H. ; Duen-Ren , H. Tetrahedron Lett . 2009 , 50 , 7169 – 7171 .
- Lindstrom , U.M. ; Organic Reactions in Water ; Blackwell : Oxford , 2007 .
- HerrerIas , C.I. ; Yao , X. ; Li , Z. ; Li , C.J. Chem. Rev . 2007 , 107 , 2546 – 2562 .
- Li , C.J. Chem. Rev . 2005 , 105 , 3095 – 3165 .
- Li , C.J. ; Chen , L. Chem. Soc. Rev . 2006 , 35 , 68 – 82 .
- Lindstrom , U.M. Chem. Rev . 2002 , 102 , 2751 – 2772 .
- Yadav , J.S. ; Swami , T. ; Reddy , B.V.S. ; Krishna Rao , D. J. Mol. Catal. A: Chem . 2007 , 274 , 116 – 119 .
- Mori , A. ; Kawashima , J. ; Shimada , T. ; Suguro , M. ; Hirabayashi , K. ; Nishihara , Y. Org. Lett . 2000 , 2 , 2935 – 2937 .
- Barluenga , J. ; Andina , F. ; Fernandez-Rodriguez , M.A. ; Garcia-Garcia , P. ; Merino , I. ; Aquilar , E. J. Org. Chem . 2004 , 69 , 7352 – 7354 .
- Crouch , R.D. Tetrahedron 2004 , 60 , 5833 – 5871 .
- Capperucci , A. ; Degl'Innocenti , A. ; Leriverend , C. ; Metzner , P. J. Org. Chem . 1996 , 61 , 7174 – 7177 .
- Sirol , S. ; Courmarcel , J. ; Mostefai , N. ; Riant , O. Org. Lett . 2001 , 3 , 4111 – 4113 .
- Denmark , S.E. ; Sweis , R.F. J. Am. Chem. Soc . 2001 , 123 , 6439 – 6440 .
- Mattson , A.E. ; Zuhl , A.M. ; Reynolds , T.E. ; Scheidt , K.A. J. Am. Chem. Soc . 2006 , 128 , 4932 – 4933 .
- Albanese , D. ; Landini , D. ; Penso , M. J. Org. Chem . 1998 , 63 , 9587 – 9589 .
- Shellhamer , D.F. ; Horney , M.J. ; Pettus , B.J. ; Pettus , T.L. ; Stringer , J.M. ; Heasley , V.L. ; Syvert , R.G. ; Dobrolsky , J.M. Jr. J. Org. Chem . 1999 , 64 , 1094 – 1098 .
- Fan , R.H. ; Zhou , Y.G. ; Zhang , W.X. ; Hou , X.L. ; Dai , L.X. J. Org. Chem . 2004 , 69 , 335 – 338 .
- Yin , J. ; Zarkowsky , D.S. ; Thomas , D.W. ; Zhao , M.M. ; Huffman , M.A. Org. Lett . 2004 , 6 , 1465 – 1468 .
- Sun , H. ; DiMagno , S.G. J. Am. Chem. Soc . 2005 , 127 , 2050 – 2051 .
- Clark , J.H. Chem. Rev . 1980 , 80 , 429 – 452 .
- Pearson , A.J. ; Roush , W.R. Activating Agents and Protecting Groups ; J. Wiley and Sons : Chichester , 1999 .
- Ooi , T. ; Maruoka , K. Acc. Chem. Res . 2004 , 37 , 526 – 533 .
- Ménand , M. ; Dalla , V. Synlett 2005 , 1 , 95 – 98 .
- Varma , R.S. ; Saini , R.K. ; Meshram , H.M. Tetrahedron Lett . 1997 , 38 , 6525 – 6528 .
- Meshram , H.M. ; Srinivas , D. ; Yadav , J.S. Tetrahedron Lett . 1997 , 38 , 8743 – 8744 .
- Meshram , H.M. ; Reddy , G.S. ; Yadav , J.S. Tetrahedron Lett . 1997 , 38 , 8891 – 8894 .
- Meshram , H.M. ; Reddy , G.S. ; Reddy , M.M. ; Yadav , J.S. Tetrahedron Lett . 1998 , 39 , 4103 – 4106 .
- Meshram , H.M. ; Reddy , P.N. ; Sadashiv , K. and Yadav , J.S. Tetrahedron Lett . 2005 , 46 , 623 – 626 .
- Meshram , H.M. ; Reddy , P.N. ; Sadashiv , K. And Yadav , J.S. Tetrahedron. Lett . 2006 , 47 , 991 – 995 .
- Meshram , H.M. ; Reddy , B.C. ; Goud , P.R. Synth. Commun . 2009 , 39 , 2297 – 2303 .
- Meshram , H.M. ; Kumar , G.S. ; Ramesh P. ; Reddy , B.C. Tetrahedron Lett . 2010 , 51 , 2580 – 2585 .
- Meshram , H.M. ; Ramesh P. ; Kumar , G.S. ; Reddy , B.C. Tetrahedron Lett . 2010 , 51 , 4313 – 4316 .
- Pratihar , S. ; Roy , S. J. Org. Chem . 2010 , 75 , 4957 – 4963 .
- Lakhdar , S. ; Westermaier , M. ; Terrier , F. ; Goumont , R. ; Boubaker , T. ; Ofial , A.R. ; Mayr , H. J. Org. Chem . 2006 , 71 , 9088 – 9095 .
- Shijay , G. ; Chen , H.T. ; Ching-Fa , Y. Synlett 2009 , 6 , 949 – 954 .