Abstract
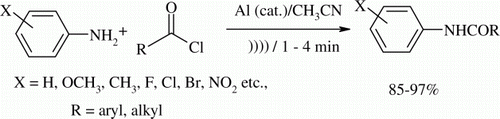
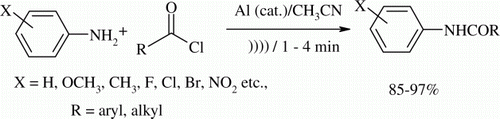
Substituted aryl amines undergo smooth acylation with different acyl chlorides to give anilides under sonic condition (35 kHz, 25°C) in the presence of catalytic amounts of aluminum metal powder in acetonitrile as solvent. All the reactions go to completion within 4 min and give the products in high yields (85–97%).
Introduction
Sonochemistry can be defined as a study of chemical reactions under the influence of ultrasound. In the year 1927, the acceleration of a conventional reaction was reported by Richards and Loomis under sonic condition Citation1. Increasing interest for sonochemical reactions is due to the positive chemical and mechanical effects when ultrasound waves propagate in a liquid medium Citation2. Compared to traditional methods, reactions under the influence of ultrasound are more convenient and hence a large number of organic reactions have been reported in the past three decades Citation3 Citation4. Ultrasound is beneficial in one or more ways such as (i) for accelerating a chemical reaction; (ii) to use less purified or cruder reagents; (iii) for initiating reactions without any additives or catalysts; (iv) for avoiding forcing conditions such as high temperatures and high pressures and (v) for simplifying the reaction by reducing the number of steps. Under sonic condition, the yields are high and the reactions occur in shorter reaction durations.
The conversion of aryl amines into anilides is an important reaction in organic synthesis and the amide function is widely distributed in organic and biological molecules. Amides and anilides are also important in pharmaceutical, agrochemical industries, and are used as protecting groups in organic synthesis Citation5 Citation6. Synthetic methods for preparing these compounds involve direct introduction of acyl group into amino group of amines and anilines Citation7–9. The most common methods include either conversion of carboxylic acid to a more reactive functional group such as an acyl chloride, a mixed anhydride, an acyl azide, an active ester or via an in situ activation of carboxyl group. Some reagents which have been used to convert amines or anilines into amides or anilides, respectively, are: carbodiimides Citation10, TiCl4 Citation11, Sn[NH(TMS)2]2 Citation12, ArB(OH)2 Citation13, activated phosphate Citation14, and triphenylphosphine/trichloroacetonitrile Citation15. Some reports describe the preparation of primary, secondary, and tertiary amides Citation16 Citation17. Other reactions reported under microwave irradiation include use of catalysts such as imidazole Citation18, Zeolite-HY Citation19, p-toluenesulfonic acid Citation20, and TaCl5-silica gel Citation21. Yadev et al. have demonstrated the preparation of amides by the reaction of acid chlorides with amines in the presence of zinc dust under normal condition. The reaction generally requires about 30 min to 3 hr for completion depending on the nature of the substrates used Citation22 Citation23). Synthesis of anilides is generally carried out either at high temperatures or requires longer duration. Other disadvantages include use of expensive reagents, or unsatisfactory yield of the products. Hence, we feel that there is still scope for investigation toward milder reaction conditions, shorter reaction time and better yields which can possibly be achieved using ultrasound as energy source for acylation of aryl amines.
In continuation of our work on the use of ultrasound for accelerating the rate of the chemical reactions Citation24–29, we wish to report a mild and efficient acylation of various substituted aryl amines to provide anilides within 4 min in high yields by irradiating at 35 kHz in a sonic bath maintained at 25°C. Different substituted aroyl or acyl chlorides are found to react efficiently in the presence of catalytic amount of aluminum metal powder in MeCN as shown in .
Results and discussion
Selection of suitable solvent is important for the successful ultrasound-assisted synthesis. Initially, in search for an optimal solvent, the reaction of 4-toludine (10 mmol) with benzoyl chloride (10 mmol) was selected as the model reaction, and the reaction was studied in ether, EtOH, MeCN and THF under ultrasonic irradiation using Al powder (5 mmol). The results of this study are presented in . As shown in , the reactions using MeCN as solvent resulted in higher yield of the amide 3j and the reaction went to completion in shorter reaction duration than that in other solvents. Hence, MeCN was used as the solvent for all further ultrasound-assisted reactions.
Table 1. Solvent optimization for the synthesis of 3j under sonic condition at 25°C using 5 mmol of aluminum powder.
The influence of the amount of aluminum metal powder was then investigated with regard to the model reaction, and the results indicate that, the reaction did not proceed to a great extent even after 30 min without aluminum metal. When 5 mmol of Al was added, the reaction proceeded rapidly, and the yield was 93% after 80 sec. There was no obvious increase in the yield when more or less amount of aluminum metal was used. The results of this study are presented in .
Table 2. Amount of Al optimization for the synthesis of 3j under ultrasonic irradiation at 25°C in 10 mL MeCN.
In order to have a meaningful comparison, the synthesis of 3j was performed under both ultrasound irradiation and under mechanical stirring at 25°C. The reaction was efficiently promoted by ultrasound and the reaction time was strikingly shortened to 80 sec from the 30 min required under mechanical stirring, and the yield was increased from 20 to 93% (, entry j). Therefore, ultrasound irradiation exhibited advantages over normal stirring by significantly reducing the reaction time and dramatically improving the product yield.
Table 3. Reaction of aryl amines with different acid chlorides.
We found that the ultrasonic reaction to be simple, as different acyl chlorides including aroyl chlorides having electron-releasing or electron-withdrawing groups reacted satisfactorily to give anilides in high yield within 4 min (). This reaction condition did not affect the functional groups such as nitro, methoxy, and halogen. The protocol has strengthened the utility of various substituted acid chlorides in amide formation reaction, which allows application in peptide and protein synthesis Citation30. The anilides prepared, the yields, and time taken for completion of the reaction are presented in .
The synthesized anilides were characterized by FTIR, 1H NMR and GC–MS spectral analysis. The appearance of single peak at 3227–3350 cm−1 (NH) and a strong peak at 1624–1657 cm−1 (C = O) for secondary amide and the disappearance of two peaks at 3180–3390 cm−1 (NH2) in the IR spectra indicated the formation of the corresponding anilides. In the 1H NMR spectra, the aromatic protons were observed between δ7 and 8 ppm. The signal of NH of the amide group was observed at δ10.4 ppm. The molecular ion peaks (M+) were in total agreement with the molecular weight.
We have compared the present method with the reported methods, and it is found that the present method is efficient and simple for the preparation of anilides from aniline and acid chlorides. The comparison of the results for the preparation of 3j is presented in .
Table 4. Comparison of catalytic activity of present method with reported methods.
The rapid increase in the rate of the reaction under sonic condition is due to acoustic cavitation. Acoustic cavitation is the driving force for all the sonochemical reactions, which is nothing but the formation of small cavities in a fluid produced by ultrasound that undergoes highly energetic collapse Citation35 Citation36. More precisely, the dissolved gases present in liquids on sonication under proper conditions can undergo a violent collapse, which generates localized “hot spots” with a transient high temperature and pressures, inducing molecular fragmentation, and highly reactive species are locally produced, which are responsible for the chemical effects of ultrasound in homogeneous as well as heterogeneous conditions. In some cases, ultrasonic irradiation can probably provide more efficient stirring Citation37. All these factors can cause the present reaction to take place rapidly and efficiently under sonic condition.
Experimental
All starting materials were commercial products. All solid amines were used without further purification; liquid amines were distilled before use. Reactions were monitored on TLC by comparing with authentic samples; yields refer to yield of the isolated products. Melting points were measured on a Raaga, Chennai, Indian-made melting point apparatus. NMR spectra were obtained on a 400 MHz Bruker AMX spectrometer in CDCl3 using TMS as a standard. GC–Mass spectra were obtained using a Shimadzu GC–MS QP 5050A instrument. IR spectra were recorded using Shimadzu FT-IR-8400s spectrophotometer as KBr pellets. All acyl chlorides were obtained according to the methods reported in the literature, which were distilled before use Citation38–40. All the reactions were studied using SIDILU, Indian-made sonic bath functioning at 35 kHz (constant frequency) and maintained at 25°C without mechanical stirring.
General procedure for the synthesis of anilides under sonic condition at 35 kHz
Benzoyl chloride (1.410 g, 10 mmol), 4-toludine (1.07 g, 10 mmol), aluminum metal powder (0.135 g, 5 mmol) in MeCN (10 mL) were placed in a 50-mL round bottom flask. The contents were sonicated in a sonic bath functioning at 35 kHz (constant frequency) and maintained at 25°C by circulating water. At the end of the reaction (80 s, monitored by TLC, 20% EtOAc, pet-ether [60–80°C]), the reaction mixture was filtered through a celite pad, washed with diethyl ether (3×20 mL). The combined ether filterate was washed successively with sat. NaHCO3, and water and the ethereal extract were dried over anhydrous MgSO4 and the solvent distilled out. The product after drying under vacuum was identified to be 4-methyl-benzanilide (3j, , 1.96 g, 93%).
Conclusion
We have developed an efficient method for the synthesis of anilides from aryl amines and acyl chlorides under sonic condition at 25°C. In addition to efficiency and simplicity, this protocol follows Green Chemistry norms and provides a very fast and low-cost procedure for the synthesis of a wide range of anilides in the presence of catalytic amounts of aluminum metal powder.
References
- Richards , W.T. ; Loomis , A.L. J. Am. Chem. Soc . 1927 , 49 , 3086 – 3100 .
- Mason , T.J. ; Peters , D. Practical Sonochemistry , 2nd ed .; Ellis Horwood : London , 2002 .
- Nagaraja , D. ; Pasha , M.A. Tetrahedron Lett . 1999 , 40 , 7855 – 7856 .
- Luche , J.L. Synthetic Organic Sonochemistry , Plenum Press : New York , 1998 ; 10 – 15 .
- Pasha , M.A. ; Nanjundaswamy , H.M. J. Chem. Res (S) . 2004 , 750 – 752 .
- Hartley , D. ; Kidd , H. The Agrochemicals Hand Book , 2nd ed .; Royal Society of Chemistry : Nottingham , 1987 .
- Grieco , P.A. ; Clark , D.S. ; Withers , G.P. J. Org. Chem . 1979 , 44 , 2945 – 2947 .
- Kluger , R. ; Hunt , J.C. J. Am. Chem. Soc . 1989 , 111 , 3325 – 3328 .
- Wamser , C.C. ; Yates , J.A. J. Org. Chem . 1989 , 54 , 150 – 154 .
- Lawrence , R.H. ; Biller , S.A. ; Fryszman , O.M. ; Poss , M.A. Synthesis 1997 , 553 – 558 .
- Wilson , J.D. ; Hobbs , C.F. ; Weingarten , H. J. Org. Chem . 1970 , 35 , 1542 – 1545 .
- Burnell-Curty , C. ; Roskamp , E.J. Tetrahedron Lett . 1993 , 34 , 5193 – 5196 .
- Ishihara , K. ; Ohara , S. ; Yamamoto , H. J. Org. Chem . 1996 , 61 , 4196 – 4197 .
- Yasuhara , T. ; Nagaka , Y. ; Tomioka , K. J. Chem. Soc. Perkin Trans. 1 , 2000 , 2901 – 2901 .
- Jang , D.O. ; Park , D.J. ; Kim , J. Tetrahedron Lett . 1999 , 40 , 5323 – 5326 .
- Varma , R.S. ; Naicker , K.P. Tetrahedron Lett . 1999 , 40 , 6177 – 6180 .
- Khadikar , B.M. ; Madyar , V.R. Synth. Commun . 2002 , 32 , 1731 – 1734 .
- Nezhad , A.K. ; Babak , M. ; Rad , M.N.S. Tetrahedron Lett . 2003 , 44 , 7325 – 7328 .
- Gadhwal , S. ; Dutta , M.P. ; Boruah , A. ; Prajapati , D. ; Sandhu , J.S. Indian J. Chem . 1998 , 37B , 725 – 727 .
- Hajipour , A.R. ; Ghasemi , M. Indian J. Chem . 2001 , 40B , 504 – 507 .
- Chandrasekhar , S. ; Takhi , M. ; Uma , G. Tetrahedron Lett . 1997 , 38 , 8089 – 8092 .
- Meshram , H.M. ; Reddy , G.S. ; Reddy , M.M. ; Yadav , J.S. Tetrahedron Lett . 1998 , 39 , 4103 – 4106 .
- Meshram , H.M. ; Reddy , G.S. ; Reddy , M.M. ; Yadav , J.S. Tetrahedron Lett . 1998 , 39 , 4107 – 4110 .
- Datta , B. ; Pasha , M.A. Ultrason. Sonochem . 2011 , 18 2 , 562 – 566 .
- Pasha , M.A. ; Jayashankara , V.P. J. Chem. Res (S) . 2004 , 282 – 283 .
- Pasha , M.A. ; Jayashankara , V.P. Ultrason. Sonochem . 2005 , 12 , 433 – 435 .
- Rama , K. ; Pasha , M.A. Ultrason. Sonochem . 2005 , 12 , 437 – 440 .
- Pasha , M.A. ; Jayashankara , V.P. Ultrason. Sonochem . 2006 , 13 , 42 – 46 .
- Pasha , M.A. ; Myint , Y.Y. Ultrason. Sonochem . 2006 , 13 , 175 – 179 .
- Greene , T.W. ; Wuts , P.G.M. Protective Groups in Organic Synthesis , 3rd ed .; Wiley & Sons : New York , 1999 .
- Zarichi , M.A.K. ; Bahadoran , A. J. Appl. Poly. Sci . 2011 , 119 , 2345 – 2349 .
- Amir , E.W. ; Jiangnan , P. ; Mark , T.H. Tetrahedron Lett . 2009 , 50 27 , 3901 – 3904 .
- Katrizky , A.R. ; Cai , C. ; Singh , S.K. J. Org. Chem . 2006 , 71 , 3375 – 3380 .
- Chiriac , C.I. ; Tanasa , F. ; Onciu , M. Rev. Roum. Chim . 2006 , 51 4 , 269 – 272 .
- Gogate , P.R. ; Pandit , A.B. Adv. Environ. Res . 2003 , 7 , 283 – 299 .
- Mason , T.J. Ultrason. Sonochem . 2003 , 10 , 175 – 179 .
- Dahlem , O. ; Reisse , J. ; Halloin , V. Chem. Eng. Sci . 1999 , 54 , 2829 – 2838 .
- Vogel , A.I. A Text Book of Practical Organic Chemistry , 5th ed .; Longman Group Ltd : London , 1989 , 692 – 693 , pp. 1073–1074 .
- Mann , F.G. ; Saunders , B.C. Practical Organic Chemistry , 4th ed .; Longman Group Ltd : London , 1970 , 238 – 241 .
- Pasha , M.A. ; Jayashankara , V.P. Indian J. Chem . 2004 , 43B , 2464 – 2466 .