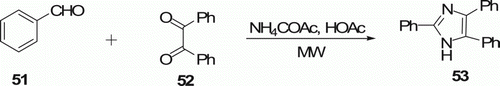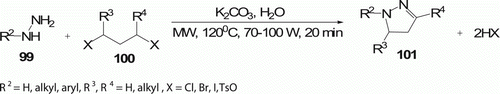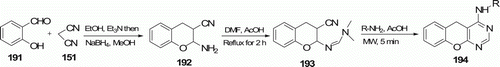Abstract
In the recent years, microwave (MW) radiation is widely used as a source of heating in organic synthesis. Since the discovery of the MW heating approach, MW-assisted reaction has emerged as a new green-method in organic synthesis as it provides spectacular accelerations, higher yields under milder reaction conditions, and higher product purities, and it reduces pollution of the environment through the use of solvent-free reaction protocols. N-containing heterocycles hold a special place among pharmaceutically significant natural products and synthetic compounds needed for any developed human society. Therefore, organic chemists have been engaged in extensive efforts to produce these compounds following various greener techniques, primarily to circumvent growing environmental concerns. In this review, we discuss only the MW-assisted synthesis of N-containing heterocyclic compounds.
1. Introduction
Heterocycles form by far the largest of the classical divisions of organic chemistry. Heterocycles are essential building blocks that are frequently used in the pharmaceutical; more than 95% of pharmaceuticals contain at least one heterocyclic fragment Citation1. They also have application in a wide variety of industries including cosmetics, reprography, information storage, plastics, solvents, antioxidants, and vulcanization accelerators Citation2 Citation3.
Amongst the heterocycles, N-based heterocycles have been the object of considerable focus, because N-containing heterocycles are structural components of many bioactive natural products such as vitamins, hormones, antibiotics, alkaloids, glycosides, and many more compounds which are of significance for human and animal health Citation4. Many natural drugs such as quinine, papaverine, emetine, theophylline, atropine, codeine, morphine, and reserpine are N-containing heterocycles Citation1 Citation5. Therefore, N-containing heterocycles are especially considered “privileged” structures for the synthesis and development of new drugs Citation6 Citation7. Traditional methods of organic synthesis are orders of magnitude too slow to satisfy the demand for these compounds Citation8 Citation9. It is therefore easy to understand why the development of new methods for the synthesis of complex heterocyclic compounds continues to drive the field of synthetic organic chemistry.
Green or sustainable chemistry has now become a subject of intensive research [Citation10–13]. The concept of “green chemistry” emerged in the early 1990s Citation12 and is now widely adopted to meet the fundamental scientific challenges to protect the human health and environment while simultaneously achieving commercial viability Citation3 Citation14. Nonclassical methods following the principles of green chemistry Citation9 reduce or even eliminate the generation of hazardous substances [Citation12–15] as well as eliminate the use of conventional volatile organic solvents. There have been tremendous successes in the synthesis of numerous numbers of heterocyclic compounds and in the development of new processes under clean, environmentally benign methodologies that are sustainable for the long term.
Microwave (MW) is one of the potential green chemistry techniques used during the recent years Citation16. The ability of MW-assisted organic synthesis to rapidly synthesize organic compounds is of significant benefit for library generation. Moreover, it allows modifications in selectivity (chemo-, regio-, and stereo-selectivity) and solvent-, catalyst-free conditions Citation17. This review outlines the use of MW, highlights the importance of a number of N-containing heterocycles, and summarizes some advances in their synthesis under MW irradiation through recent selected examples.
2. MW irradiation
A nonclassical heating technique using microwaves (MWs), termed “Bunsen burner of the 21st century”, is rapidly becoming popular and is dramatically reducing reaction times Citation14. The rate of acceleration observed in MW irradiation is due to material–wave interactions leading to dielectric heating. MW consists of an electric and magnetic field and thus represents electromagnetic energy. This energy can act as a nonionizing radiation that causes molecular motions of ions and rotation of the dipoles, but does not affect molecular structure Citation14. The applied MW field causes the molecules, on average, to temporarily spend slightly more time orienting themselves in the direction of the electric field rather than in other directions. When the field is removed, thermal agitation returns the molecules to a disordered state in the relaxation time and thermal energy is released. Hence this phenomenon relies on the ability of a substance (solvent or reactant) to absorb MWs and convert them into heat Citation18. This internal heating is much more homogeneous than the classical heating.
Synthesis of various N-containing heterocycles utilizing MW irradiation are now discussed under the following headings.
2.1. Pyrroles
Pyrroles are heterocycles of great importance because pyrrole is a basic substructure of numerous biologically active alkaloids and pharmaceutical products Citation19 Citation20. Classically, pyrroles were synthesized by various methods viz. Knorr pyrrole synthesis Citation21, Paal–Knorr synthesis Citation22, Hantzsch pyrrole synthesis Citation23, etc.
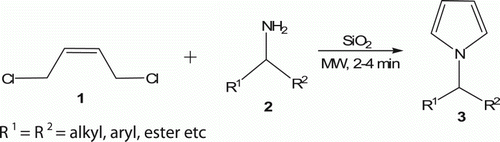
Aydogan et al. Citation19 carried out the reaction of cis-1,4-dichloro-2-butene 1 with various amine compounds 2, amino alcohols, and amino acid esters without solvent under MW irradiation on silica gel for 2–4 min, and N-substituted homochiral pyrrole derivatives 3 (11 examples) were obtained in good yield (49–69%) instead of the expected 3-pyrrolines ().
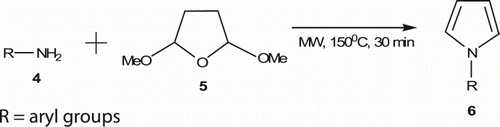
Heating an aniline 4 with 2,5-dimethoxytetrahydrofuran 5 in water (0.64 M) in a MW reactor at 150°C for 30 min resulted in the formation of the corresponding pyrroles 6 (10 examples) in water in 81–99% yield (). Wilson et al. Citation20 reported this simplified approach to the uncatalyzed Paal–Knorr condensation.
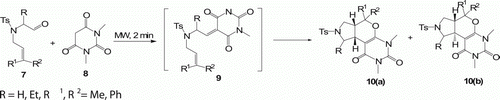
Jayagobi et al. Citation24 have reported the synthesis of pyrano[4,5-c]pyrroles 10(a) and 10(b) by one-pot intramolecular Knoevenagel-Hetero Diels-Alder reaction of alkenyl aldehyde 7 with barbituric acid 8 in excellent yield (80%) under MW heating () in 2 min in toluene. Traditional method in refluxing toluene in the presence of ethylene diaminediacetate (EDDA) provides 67% yield in 6 h.
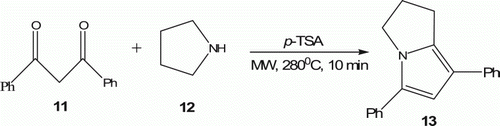
Deb et al. Citation25 have reported synthesis of ring-fused pyrrole 13 in a single operation by the reaction of 1,3-diketone 11 and cyclic aniline 12 under MW irradiation at 280°C in the presence of 0.5 equivalent of p-toluenesulfoic acid (p-TSA) with 53% yield () in 10 min.
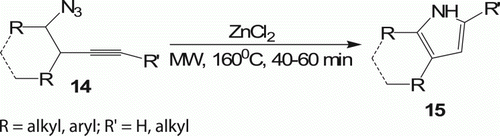
Ligand-free 5-endo-dig cyclization of homopropargyl azide 14 in the presence of 20 mol% ZnCl2 (1.0 M in ether) in CH2Cl2 at 105°C provided pyrroles 15 (eight examples) in high to moderate yield (91–41%) Citation26 in 40–60 min (). Conventional heating also furnished the same product but at 75°C for 16 h.
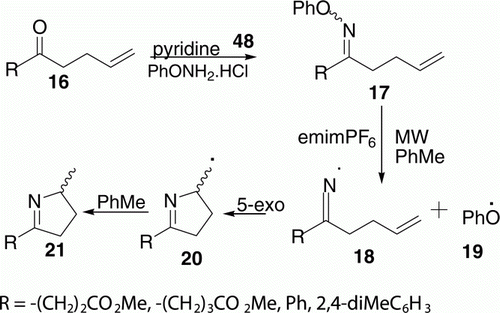
Portela-Cubillo et al. Citation27 investigated iminyl radical generation and cyclization using the set of functionalized O-phenyl oxime ethers 17 promoted by MWs to produce dihydropyrrole 21 (four examples) in 68–82% yield (). Reaction took place at 160°C for 15 min with one equivalent of ionic liquid 1-ethyl-3-methyl-1H-imidazol-3-ium hexafluorophosphate (emimPF6). However, it was difficult to furnish the conventional thermolyses of O-phenyl oxime ethers as reaction times had to be long, the products were not cleanly formed and yields were disappointingly low.
2.2. Indole and its derivatives
Indole nuclei are broadly found in a large amount of synthetic and natural products. Indole is a popular component of fragrances and the precursor to many pharmaceuticals Citation28. Classically, indoles have been synthesized by various methods Citation29 Citation30. The use of more environmental friendly protocols for the synthesis of indole derivatives has been reported which are mentioned below.
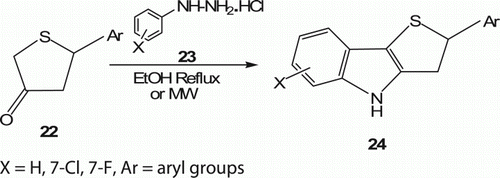
A series of novel 2-aryl-3,4-dihydro-2H-thieno[3,2-b]indoles 24 (22 examples) have been synthesized by Karthikeyan and his co-workers Citation29 regioselectively in excellent yields (85–98%) under MW irradiation () at 90°C in 3–6 min. 24 were also obtained under conventional condition in refluxing ethanol but completed in 30–70 min.
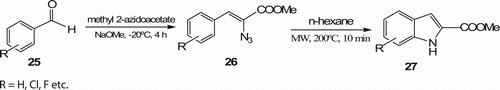
The synthesis of indoles 27 (six examples) which begins with the intermediates 26 prepared from the corresponding commercially available aldehydes 25 () was accomplished by Lehmann et al. Citation30. Formation of indoles took place with 99.9% yield at 200 W, 200°C, and 15-min irradiation time. The existing methods for the synthesis of these compounds afford only relatively low to modest yields (53–79%) in several hours.
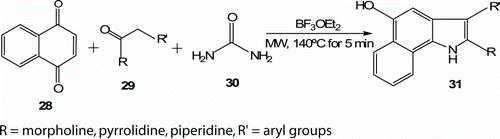
Borthakur et al. Citation31 recently described this formation of various 5-hydroxybenzo[g]indole 31 (12 examples) at 140°C for 5 min in 80% yield ().
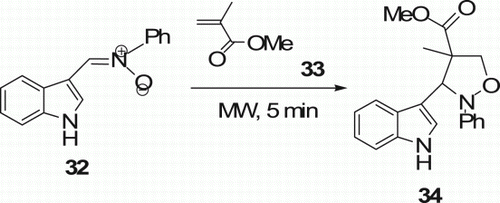
Bhella et al. Citation32 recently reported monomode MW-assisted regio- and stereo-selective 1,3-dipolar cycloadditions of C-(3-indolyl)-N-phenylnitrone 32 with olefinic dipolarophiles 33 to afford the formation of isoxazolidines 34 (six examples) in high yields (87%) in 5 min ().
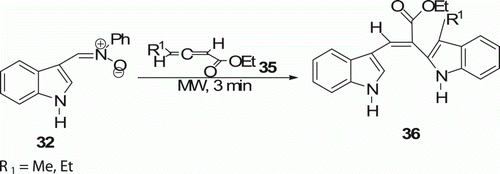
The same group Citation32 extended their investigations on 1,3-dipolar cycloadditions of nitrone 32 to allenic esters 35 (two examples) under MW irradiation in solvent-free conditions which afforded potentially biologically active bis-indole derivatives 36 in high yields (84–90%) in 3 min ().
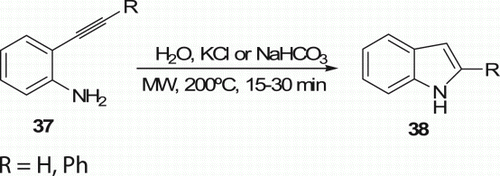
Carpita et al. Citation33 reported differently substituted indoles 38 (22 examples) via MW-assisted cycloisomerization in water of 2-alkynylanilines 37 which is promoted by catalytic amounts of neutral or basic salts or by stoichiometric weak organic bases in good to high yields (45–66%) without any added metal catalyst in 15–30 min, and did not take place by applying conventional heating (). Earlier, the same group Citation34 produced similar indoles in moderate to good yields (16–77%) from same reactants 37 under MW irradiation in water without any added metal catalyst, acid, or base in 0.25–1.5 h.
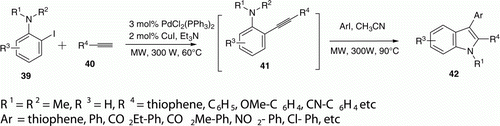
A MW-assisted, one-pot, three-component coupling reaction for the synthesis of 2,3-disubstituted indoles 42 has been developed by Chen et al. Citation35 (). This reaction was carried out in two steps under standard Sonogashira coupling conditions which afforded a variety of 2,3-disubstituted indoles (24 examples) in 40–80 min in moderate to excellent yields (60–91%).
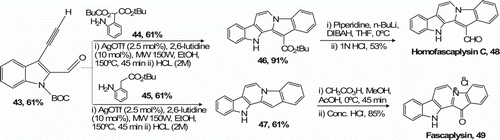
Waldmann et al. Citation36 described a silver-catalyzed and MW-assisted one-pot cascade reaction sequence that gives access to different alkaloid-inspired polycyclic scaffold classes including fascaplysin-type alkaloids 49 (52%) and analogs thereof 48 (53%; ).
2.3 Imidazoles
Imidazole derivatives have attracted considerable attention in recent years as these are endowed with a wide range of pharmaceutical activities Citation6 Citation37. The application of imidazoles as 1,3-disubstituted imidazole salts as ionic liquids are also well known Citation37. Classically, imidazoles have been synthesized by various methods [Citation38–41].
The MW-mediated preparation of lophine (2,4,5-triphenylimidazole) 53 has been described by Crouch et al. Citation42 as shown in . When the homogenous mixture of equal molar quantities of benzaldehyde 51, benzil 52, glacial acetic acid, and ammonia was irradiated to raise the temperature from room temperature to 120°C over 10 min and then to 125°C over five more minutes, compound 53 was obtained in 90%. The existing methodology requires gram quantities of reagent, large quantities of solvent, and reaction times of greater than 1 h.
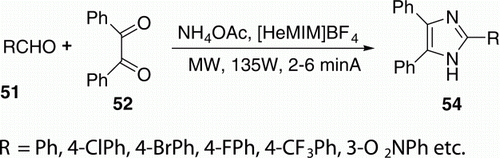
Later, Xia et al. Citation43 reported a MW-assisted three-component synthesis of various 2,4,5-trisubstituted imidazoles 54 (14 examples) with good to excellent yields (74–93%) in ionic liquid 1-methyl-3-heptyl-imidazolium tetrafluoroborate ([HeMIM]BF4), without solvent and additional acid (). The reaction time was dramatically reduced from several hours in conventional heating to just a few minutes under MW irradiation.
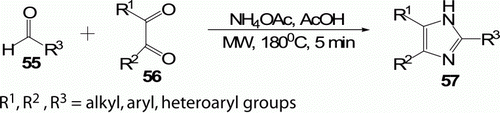
Wolkenberg et al. Citation44 described a simple, high-yielding (80–99%) synthesis of 2,4,5-trisubstituted imidazoles 57 from 1,2-diketones 56 and aldehydes 55 in the presence of NH4OAc under MW irradiation at 180°C for 5 min (). Classical methods require harsh reaction conditions (150–200°C, 4–6 h) and suffer from low yields (40–90%), mixtures of products, and lack of generality. These compounds have utility in expedient preparations of the imidazolium alkaloid lepidiline B6 and the platelet aggregation inhibitor trifenagrel.
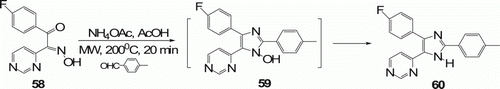
Sparks and his co-workers Citation45 have discovered an efficient MW-assisted, one-pot, two-step synthesis of 2,4,5-triarylimidazoles 60 (15 examples) from keto-oximes 58 and aldehydes in moderate (17–37%) to good yields (40–63%) via cyclization to the N-hydroxyimidazole (). The novel thermally induced N–O reductive bond cleavage step takes place upon MW irradiation at 200°C for 20 min, whereas conventional methods require 2 days.
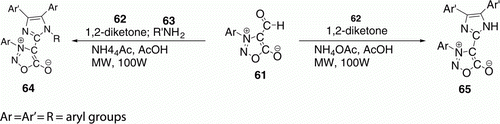
4,5-Diaryl-2-sydnonyl-1-substituted imidazoles 65 (16 examples) have been prepared by Shih et al. Citation46 by the one-pot condensation of 3-(4-ethoxyphenyl)-4-formylsydnone 61, benzil derivatives 62, and ammonium acetate, under MW irradiation (). The use of MW heating reduces reaction time (52–85% yield in 30–90 min) compared to classical heating at 90–110°C (46–77% yields in 1–3 days). When primary amines 63 added, similar treatment produced 64 (eight examples) in 2–3 h with 45–60% yields. Conventional heating also affords 64, but in 2–3 days with 20–34% yield.
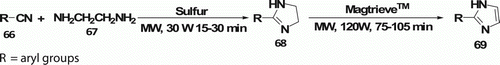
2-Imidazolines 69 have been synthesized by Hoz et al. Citation47. MW-assisted cyclization of nitriles 66 with ethylenediamine 67 which aromatize in toluene and MagtrieveTM (oxidant) and further afforded imidazoles (five examples) in 75–105 min (). The use of conventional heating with MnO2 requires longer reaction times (24–48 h) to obtain good results (76–93%).
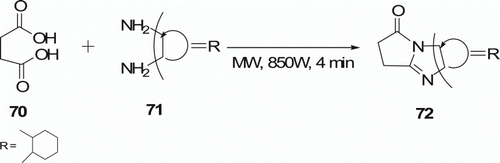
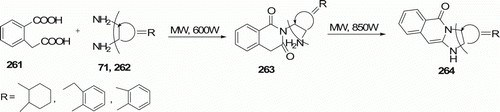
Equimolar ratio of succinic acid 70 and cyclohexane-1,2-diamine 71 were mixed together thoroughly and then subjected to MW irradiation at 850 W for 4 min to furnish the product octahydro-1H-pyrrolo-[1,2-a]benzimidazol-1-one 72 in quantitative yield (). This one-step process for the synthesis of tricyclic heterocyclic molecules (three examples) was reported by Sondhi et al. Citation48.
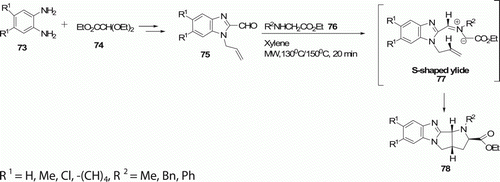
The parent pyrrolidino[2′,3′:3,4]pyrrolidino[1,2-a]benzimidazole-2-carboxylates 78 (11 examples) have been synthesized by Meng et al. Citation49 utilizing a MW-assisted condensation of carbaldehyde 75 with a secondary amino ester 76 and the subsequent 1,3-dipolar cycloaddition of the S-shaped ylide 77 (). The reaction was carried out in xylene at 130 or 150°C for 20 min to afford the polycyclic pyrrolidine compounds 78 in (52–93%) yield. Azomethine ylide cycloadditions run under classical reaction conditions require longer reaction times and afford products in lower yields Citation49.
2.4. Pyrazoles
Pyrazole and its derivatives have displayed broad spectrum of pharmacological and biological activities. In particular, pyrazolo[3,4-b]pyridines are useful for treatment of a wide variety of stress-related illnesses. Well-known methods for pyrazole synthesis are Pechmann pyrazole synthesis and Knorr pyrazole synthesis Citation50 Citation51.
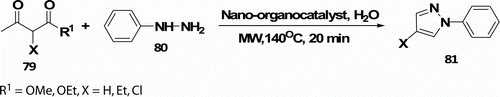
Polshettiwar et al. Citation52 reported MW-assisted synthesis of pyrazole derivatives 81 (eight examples) using nano-organocatalyst in water at 140°C in 20 min in high yield (84–96%; ).
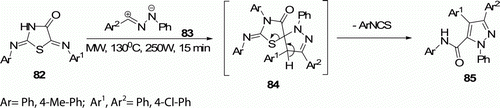
Hatem et al. Citation53 reported the synthesis of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides 85 by 1,3-dipolar cycloaddition of nitrilimines 83 with 5-arylidene-2-arylimino-4-thiazolidinones 82 under solvent-free and MW-assisted conditions at 130°C for 15 min ().
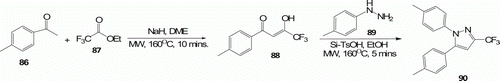
Paul et al. Citation54 carried out reaction of 4-methylacetophenone 86 with ethyl trifluoroacetate 87 applying MW heating which afforded enol ketone 88 in 10 min at 160°C with high yield (95%). In contrast, the highest yielding (88%) non-MW conditions took 5 days. In the next step, 88 reacted with 4-methylphenylhydrazine 89 to deliver various 1,5-diarylpyrazoles 90 (nine examples) under MW irradiation at 160°C with 95% yield in the presence of silica-supported toluenesulfonic acid (Si-TsOH) in ethanol in 5 min. Whereas, under thermal conditions (100°C), 84 obtained in 7 h with 84% yield ().
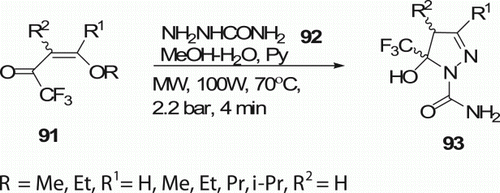
Sauzem et al. Citation55 have synthesized 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles 93 (10 examples) from one-pot cyclocondensation reaction of 4-alkoxy-1,1,1-trifluoromethyl-3-alken-2-ones 91 with the help of MW-assisted synthesis at 70°C in 4 min with high yield (82–96%; ). Some of these compounds were effective on neurogenic pain. Conventional method entails moderate yields and a long process (ca. 24 h).
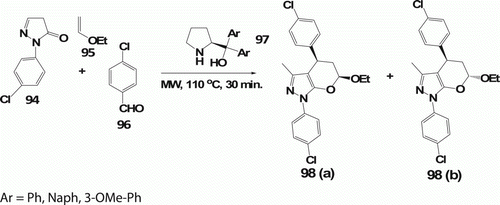
Radi et al. Citation56 developed multi-component MW-assisted organocatalytic domino Knoevenagel-hetero Diels-Alder reaction (DKHDA) applied for the synthesis of 2,3-dihydropyran[2,3-c]pyrazoles 98 (22 examples). When a mixture of pyrazolone 94, aldehyde 96, and ethylvinyl ether 95 was irradiated at 110°C for 30 min in the presence of the diaryl-prolinol catalyst 97 and t-BuOH as the solvent, the desired compounds 98a and 98b were obtained in 56% and 12%, respectively, and compared to the conventional heating at 80°C for 48 h for similar compounds ().
Ju et al. Citation57 described the double alkylation of unprotected hydrazines 99 by alkyl dihalides 100 via cyclocondensation under MW irradiation. These reactions took place in aqueous media in the presence of a mild base with MW power of 70–100 W, at 120°C for 20 min to provide a series of pyrazoles 101 (11 examples) with high yield (60–80%) in a simple SN2-like sequential heterocyclization experimental protocol which has never been fully realized under conventional reaction conditions ().
2.5. Pyrazolines
Pyrazoline moieties have a wide range of applications in agricultural pesticides and luminescent and fluorescent target molecules Citation58. Chemists use pyrazolines in bioactive moieties to synthesize new compounds possessing biological activities.
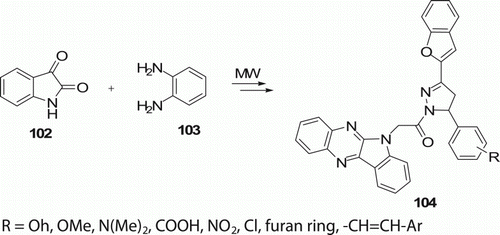
Manna et al. Citation59 have synthesized new 2-[1-(5,8-dihydro quinoxalino[2,3-b]indoloacetyl)-3-(1-benzofuran-2-yl)-4,5-dihydro-1H-pyrazol-5-yl] phenyl derivatives 104 (14 examples) using MW-assisted route with an excellent yield (84–98%) in 20–30 min (). In ordinary synthetic route (reflux), 28–72% yield was obtained in 8–9.5 h.
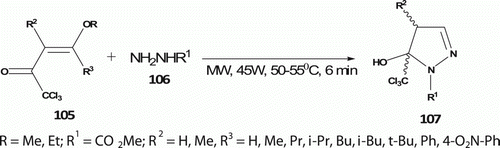
Martins et al. Citation60 reported the synthesis of novel 4,5-dihydro-1H-pyrazole 107 (12 examples) from cyclocondensation reaction of enones 105 with hydrazine methyl carboxylate 106 under solvent-free conditions () at 50–55°C in 6 min with 70–98% yield. Conventional heating gave only moderate yields (70–79%) in 24 h.
2.6. Pyrazolones
Several heterocyclic compounds containing pyrazolone moiety were found to be useful intermediates for medical drugs. They have a wide range of approved biological and pharmaceutical activities Citation61. Among other activities, pyrazolones act as appropriate precursors for the preparation of herbicides, liquid crystals, and dyes. They are traditionally synthesized by treatment of β-ketoesters with substituted hydrazines under acidic conditions at elevated temperature Citation62.
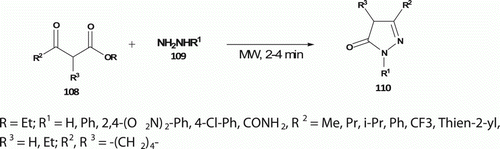
Pal et al. Citation63 have also reported the synthesis of a series of pyrazolones 110 (12 examples) from the cyclocondensation reaction of hydrazines 109 with β-ketoesters 108 under solvent-free conditions using MW heating (). Compared to conventional method (in refluxing MeOH for 10 h), MW-assisted reaction completes in 2–4 min in high yield (62–89%).
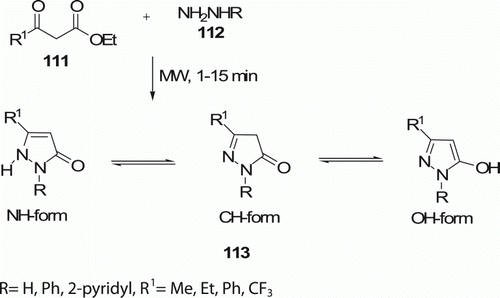
Mutairi et al. Citation62 have irradiated the mixture of hydrazine derivatives 112 and β-keto esters 111 with MW (300 W) for 1–15 min under solvent-free condition which produced pyrazolones 113 (12 examples) in moderate to good yields (40–91%; ). 113 are also produced under conventional condition (stirring in EtOH, at room temperature [RT] for 1–5 h).
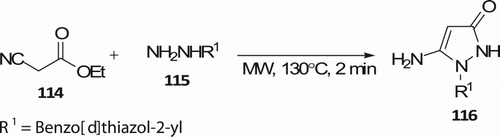
Deshmukh et al. Citation64 reported a MW-assisted process to synthesize 5-aminopyrazolone 116 under solvent-free conditions () with 88% yields at 130°C in 2 min. The same conversion carried out under conventional thermal heating required 4 h and furnished the products in 80% yield Citation64.
2.7. Triazole
1,2,3-Triazoles are indispensable structural motifs of compounds with increasing importance in the field of pharmaceuticals, crop protection, and material sciences Citation65. Conventional Huisgen's 1,3-dipolar cycloaddition technique proved to be highly versatile in giving substituted 1,2,3-triazoles by the reaction between organic azides and substituted alkynes Citation66.
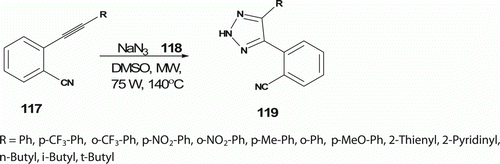
Tsai et al. Citation67 have synthesized 4,5-disubstituted-2H-1,2,3-triazoles 119 (14 examples) via cycloaddition reactions of azides 118 with terminal alkynes 117 following the MW procedure (75 W) in DMSO at 130°C in 4–90 min with 60–99% yields (). In contrast, the conventional method to get 113 requires 6–10 days.
Yang et al. Citation68 have described a convenient and efficient one-pot synthesis of C-carbamoyl-1,2,3-triazoles 123 (14 examples) under MW irradiation with 72–93% yield (), whereas, conventional method requires long reaction time and needs purification of the intermediates.
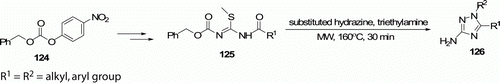
Meng et al. Citation69 have reported regioselective MW-assisted synthesis of N1-substituted 3-amino-1,2,4-triazoles 126 (10 examples) in 34–70% yields ().
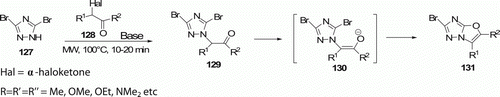
Ball et al. Citation70 developed a rapid synthesis of unprecedented Citation1 Citation3oxazolo[3,2-b]Citation1 Citation2 Citation4triazoles 131 (eight examples) using MW irradiation by tandem alkylation/cyclization reaction of 3-bromo-1,2,4-triazoles 127 and α-haloketones 128, via intermediate enolate 130 at 100°C in 10–20 min in the presence of a base with 30–81% yields ().
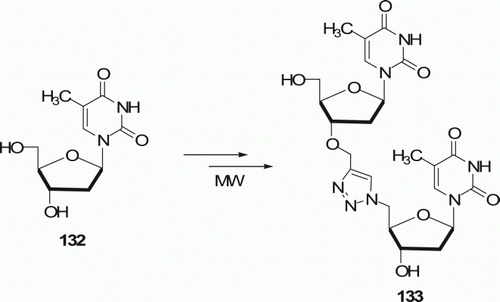
Synthesis of a triazole-linked 3′–5′ thymidine dimer 133 was reported by Lucas et al. Citation71 making use of 1,3-dipolar cycloaddition under MW irradiation in 1–3 min with 62–84% yield at 80°C (). MW-assisted route is faster than the conventional method, which requires heating at 80°C for 5 h to achieve the desired product.
2.8. Pyridine
Pyridines have a wide range of applications in medicinal chemistry. Pyridine derivatives have been used as herbicides and for enrichment of cereals. Some bifunctional pyridines are used as nonlinear optical materials, electrical materials, chelating agents in metal–ligand chemistry, and as fluorescent liquid crystals Citation72. Despite the numerous synthetic methodologies available in the literature Citation72, novel methods for pyridines synthesis are still in demand.
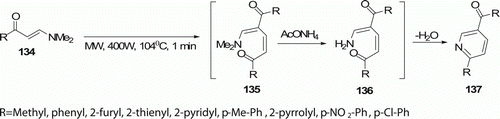
Heating enaminones 134 with excess of ammonium acetate (as an ionic liquid), at 110°C for 20 min or irradiating under MW for 1 min at 105°C (400 W), yielded the pyridines 137 (five examples). This work was reported by Khadijah et al. Citation73 (). Yield obtained following the MW procedure is higher (65–77%) than the heating method (50–62%).
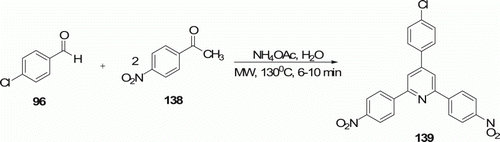
A one-pot effective Kröhnke condensation reaction was carried out by Tu et al. Citation74 in an aqueous medium using MW irradiation (6–10 min) and ammonium acetate for the synthesis of substituted pyridines 139 (18 examples) at 130°C with 90–96% yield (). Heating in oil bath requires much more time (2.5–4 h) with 76–84% yield.
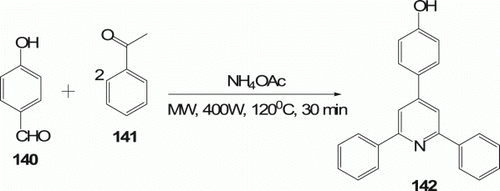
A facile solvent- and catalyst-free method for the synthesis of a series of new hydroxylated 2,4,6-trisubstituted pyridines 142 (15 examples) was obtained in high yield (83%) under MW irradiation (400 W) at 120°C for 30 min via a three-component condensation of 4-hydroxybenzaldehyde 140, acetophenone 141, and NH4OAc (). This work was reported by Yin and his co-workers Citation75.
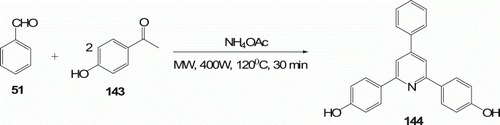
But, treatment of hydroxyl-substituted aryl ketone (4-acetylphenol) 143 (five examples) with benzaldehyde 51 under the same conditions afforded bis-hydroxyl compound 144 in 82% yields ().
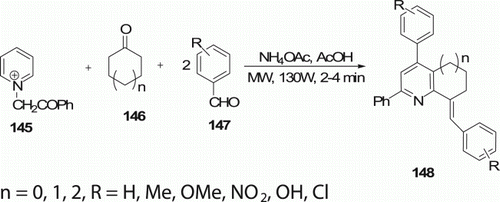
Yan et al. Citation76 described a modified Kröhnke procedure of annulated pyridine derivatives 148 (10 examples) in one-pot reactions of N-phenacylpyridinium bromide 145 with aromatic aldehydes 147 and cyclic ketones 146 in the presence of ammonium acetate and acetic acid under MW irradiation in 2–4 min with 70–80% yield (). 148 compounds were produced in 84% yields by heating a mixture of five reaction components at 90°C for 3 h.
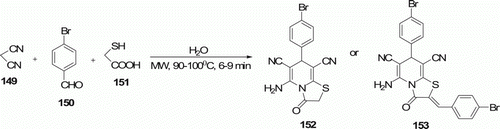
The chemoselective synthesis of thiazolo[3,2-a]pyridine derivatives 152 and 153 was achieved by Shi et al. Citation77 in water via MW-assisted three-component reactions of malononitrile 149, aromatic aldehydes 150, and 2-mercaptoacetic acid 151 with molar ratios of 2:1:1.5 and 2:2.2:1, respectively (). 130 was obtained at 90°C with 80–89% yield in 6–9 min and 131 was produced at 100°C with 82–89% yield in 6–7 min.
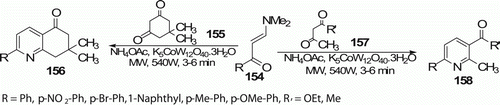
A highly efficient, MW-assisted, regioselective one-pot three component synthesis of trisubstituted pyridines 158 (eight examples) and 7,7-dimethyl-2-aryl-5,6,7,8-tetrahydroquinoline-5-ones 156 (five examples) from enaminones 154 in the presence of potassium dodecatungstocobaltate trihydrate, K5CoW12O40.3H2O (as a reusable heterogeneous catalyst) within 3–6 min with an excellent yield (up to 98%) under solvent-free conditions was reported by Kantevari et al. Citation78 ().
2.9. Quinoline
Quinoline derivatives occur in various natural products, especially in alkaloids and are often used for the design of many synthetic compounds with diverse pharmacological properties Citation79. A number of methods of quinoline synthesis viz. Skraup, Doebner-von Miller, Friedlander, Pfitzinger, Conrad-Limpach, Combes have been known since the late 1800s Citation80.
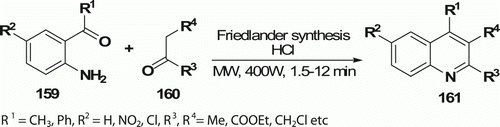
Muscia et al. Citation81 synthesized a series of substituted quinolines 161 (10 examples) in good yields (50–89%) via the Friedländer reaction by the reaction of 2-aminoacetophenone 159 or benzophenones with a variety of ketones 160 and keto esters and a catalytic amount of hydrochloric acid within a time period of 1.5–12 min following the MW procedure (). Conventional synthesis requires prolong heating (6 h) at 100°C.
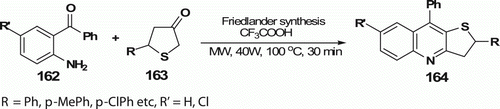
Balamurugan et al. Citation82 also have reported regioselective 2,9-diaryl-2,3-dihydrothieno[3,2-b]quinolines 164 (18 examples) via the Friedländer reaction (). A mixture of 5-aryldihydro-3(2H)-thiophenone 163, 2-aminoaryl ketone 162, and trifluoroacetic acid was irradiated in an MW oven at 100°C (40 W) for 30 min. This reaction proceeded more rapidly and afforded better yields (60–98%) than the thermal reaction at 100°C (60–85% yields in 2–3 h).
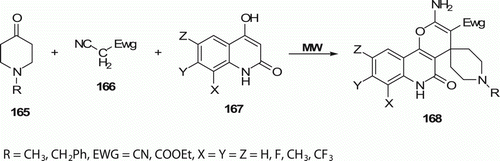
Fluorine-containing spiro[piperidine-4,4′-pyrano[3,2-c]quinolines] 168 (14 examples) were synthesized by Dandia et al. Citation83 through a rapid one-pot multi-component reaction under MW irradiation () with an excellent yield (80–96%) and short reaction time (3–10 min), whereas conventional heating needs 5–12 h to get 68–76% yield.
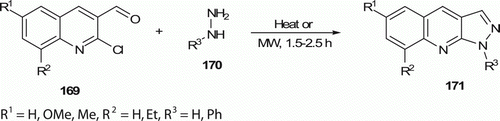
Mali et al. Citation84 has recently developed a one-pot water-mediated synthetic route to produce quinoline derivatives 171 (eight examples) using both thermal and MW energy resources (). MW-assisted route offers high yields (87–93%), short reaction times (1.5–2.5 h) compared to conventional heating which affords 85–91% yield within 7–10 h.
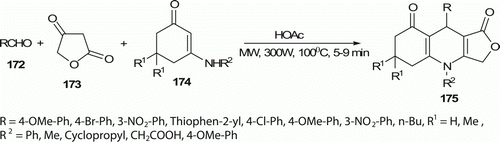
Tu et al. Citation85 synthesized N-substituted furo[3,4-b]quinoline derivatives 175 (28 examples) via a three-component reaction of an aldehyde 172, an enamineone 174, and tetronic acid 173 in glacial acetic acid without any catalyst (). Following the MW procedure at 100°C, the reaction time was strikingly shortened to 5–9 min from 3 to 5 h required under traditional heating conditions, and the yields were increased to 90–98% from 37% to 56%.
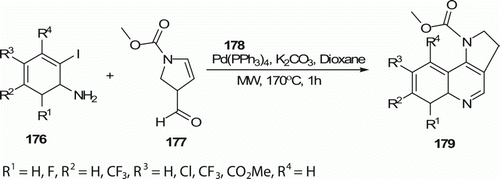
2,3-Dihydro-1H-pyrrolo[3,2-c]quinoline 179 (13 examples) core from substituted 2-iodoanilines 176 and 2,3-dihydro-1H-pyrrole 177 was achieved using 10 mol% Pd(PPh3)4 and K2CO3 in 1,4-dioxane at 170°C for 1 h in an MW oven in 60% yield (). But, the reaction of 176 and 177 in refluxing toluene for 24 h produces 179 in 44% Citation86.
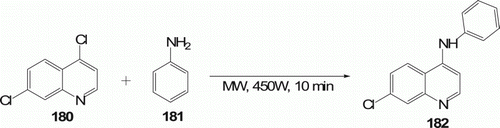
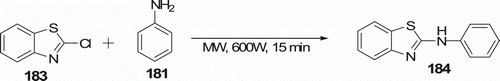
An efficient synthesis of 4-aminoaryl/alkyl-7-chloroquinolines 182 (14 examples) and 2-aminoaryl/alkylbenzothiazoles 184 (seven examples) has been developed by MW-accelerated regioselective aromatic nucleophilic substitution of 4,7-dichloroquinoline 180 (or 2-chlorobenzothiazole 183) with aliphatic and aromatic amines 181 under solvent-free conditions in the absence of added protic or Lewis acid catalyst with 70–95% yield in 10 min ( and 53). Conventional heating at 100°C affords with 20–40% conversion in 1 h Citation87.
2.10. Pyrimidine
Pyrimidine derivatives serve both as biomimetic and reactive pharmacophores due to their diverse medicinal properties. The importance of partially hydrogenated pyrimidine derivatives in medicinal chemistry is widely known Citation88. Most popular method for pyrimidine synthesis is Biginelli reaction Citation89. In order to improve the yields obtained in the described classic conditions for the synthesis of pyrimidines, much effort has been made.
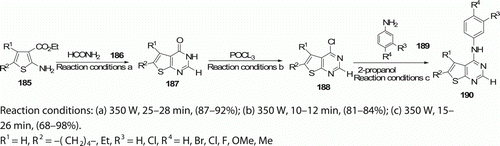
Various 2-unsubstituted 4-(substituted)anilinothieno[2,3-d]pyrimidines 190 (14 examples) were synthesized by Phoujdar et al. Citation90 through the chlorination of the corresponding 2-unsubstituted-thieno[2,3-d]-pyrimidin-4-ones 187, followed by the nucleophilic displacement of the 4-Cl group of 188, with a variety of anilines 189. Compared to conventional method in refluxing iso-propanol that completes in 5–9 h with 59–85% yield, the entire four-step MW-assisted reactions require 2 h in high yield ().
Novel chromeno[2,3-b] pyrimidine derivatives 194 (11 examples) were obtained by Rai et al. Citation91. MW irradiation of intermediate 193 with different amines in acetic acid gave 194 within 5 min in reasonably good yields (64–75%; ). All these compounds were screened for their antimicrobial activity.
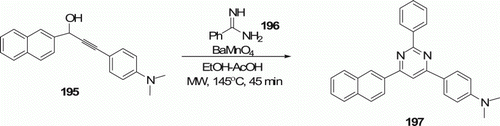
2,4,6-Triarylpyrimidines 197 were synthesized by Bagley et al. Citation92 by MW-assisted tandem oxidation/heterocyclocondensation using BaMnO4 () at 145°C in 45 min. In contrast, traditional method under conductive heating by cyclocondensation of 195 and 196 at reflux in EtOH in the presence of NaOH, moisture, and air for 10 h produces 197 in 0-40% yield.
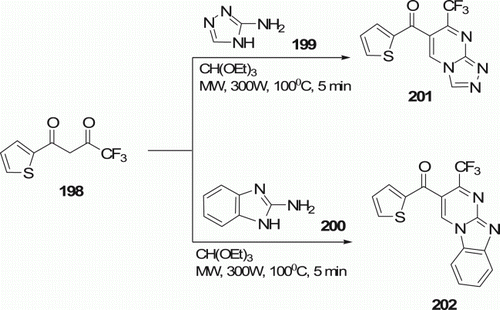
Shaaban Citation93 has synthesized 6-thienoyl-7-(trifluoromethyl)-Citation1 Citation2 Citation4triazolo[4,3-a]pyrimidine 201 in 91% yields (and 6-thienoyl-7-(trifluoromethyl) benzimidazo[1,2-a]pyrimidine 202 in 83% yields) by MW irradiation of 4,4,4-trifluoro-1-(thien-2-yl)butane-1,3-dione 198, 3-amino-1,2,4-triazole 199 (or 2-amino-benzimidazole 200), and triethylorthoformate at 100°C for 5 min (). Most of the thermal syntheses of such compounds consume a lot of time and/or lack of high selectivity Citation93.
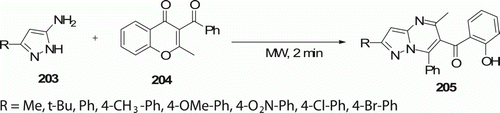
6-(2-Hydroxybenzoyl)-5-methyl-7-phenylpyrazolo[1,5-a]pyrimidines 205 (eight examples) have been synthesized directly by the solvent-free reaction between 5-amino-1H-pyrazoles 203 and 3-benzoyl-2-methyl-4H-chromen-4-one 204 using MW-assisted route in 2 min in 88–93% yield (). This work was reported by Quiroga et al. Citation94. Conventional heating at 180°C for 20 min gives the product in 70–76% yield.
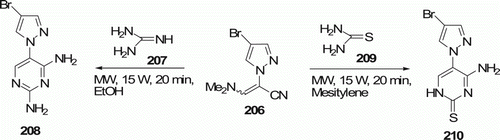
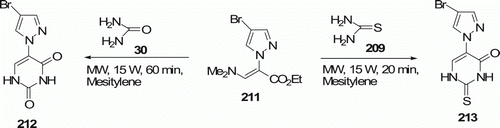
Aminopyrimidines 208 and 210 were made by reaction of 206 with guanidine 207 and thiourea 209, respectively (). Urea 30 and thiourea 209 were also used to obtain a wide variety of pyrazolyl-substituted pyrimidines 212 and 213 (). These reactions were reported by Hoz et al. Citation95. All reactions were performed under MW irradiation, and reaction times were in the range of 20–60 min. These conditions gave good yields of 208 and 210 (38–56%) and 212 and 213 (58–70%).
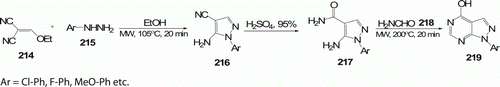
Daniels et al. Citation96 synthesized a series of functionalized pyrazolo[3,4-d]pyrimidines 219 (seven examples) on MW irradiation (). The last reaction step that required >48 h at reflux and provided 40–65% yield under conventional method has been optimized to 20 min with 74–91% yields on MW heating.
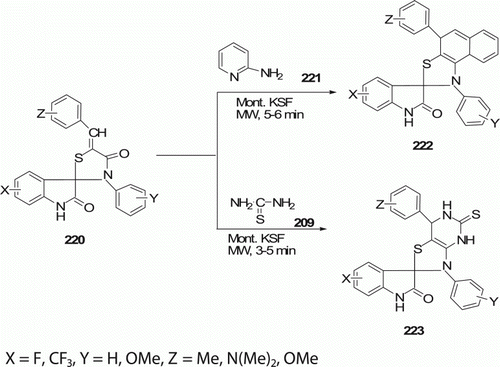
Arya et al. Citation97 synthesized fluorinated spiro [indole-3,2′-pyrido[1,2-a]thiazolo[5,4-e]pyrimidines] 222 (four examples) and spiro [indole-3,2′-thiazolo[4,5-d]pyrimidines] 223 (four examples) under MWs in the presence of montmorillonite KSF (Mont. KSF). Micheal addition of 2-aminopyridine 221 and arylidenes derivatives 220 yielded 222 in the presence of Mont. KSF in 85–91% yield in 5–6 min. Reaction of arylidenes derivatives 220 with thiourea 209 afforded 223 in 85–90% yield in 3–5 min ().
2.11. Pyrimidinones
There is a continuous widespread interest in the synthesis of pyrimidinones because of the diverse biological properties associated with this system Citation98 Citation99. Synthetic procedures are generally based on the modifications of the century-old Biginelli's reaction Citation89, involving an acid catalyzed three-component condensation of a 1,3-dicarbonyl compound, aldehyde, and urea.
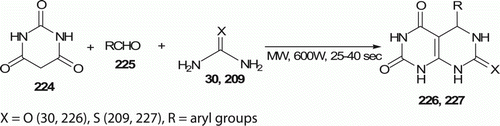
Shingare et al. Citation100 synthesized various pyrimido[4,5-d]pyrimidine-2,4,7-triones 226, 227 (16 examples) by multicomponent reaction of aldehyde 225, urea 30/thiourea 209, barbituric acid 224, and alumina (Al2O3) on MW irradiation (600 W) within a time of 25–40 sec (). Traditional procedures typically involved longer reaction time and less yield Citation100.
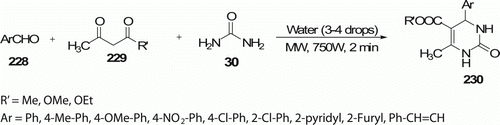
An efficient, simple MW-assisted synthesis of 3,4-dihydropyrimidinones 230 (14 examples) in excellent yields was obtained in the presence of water without additional solvent/acid catalyst in 2 min with 88–98% yield. This reaction has been reported by Singhal et al. Citation101 as shown in . The same reaction was also been executed under conventional heating which was found to be much slower (gets completed in 45–75 min).
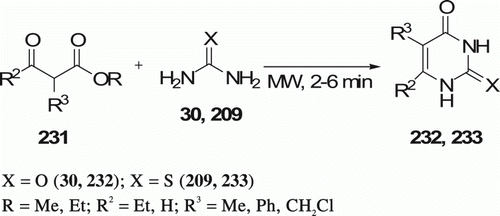
Mojtahedi et al. Citation102 reported the preparation of pyrimidinones 232 (four examples) and thiopyrimidinones 233 (three examples) in solvent-free and MW-assisted conditions () with good yields (53–81%) in 2–6 min. Conventional method, in refluxing ethanol (containing metallic sodium), completes within 6–7 h with 4–78% yields.
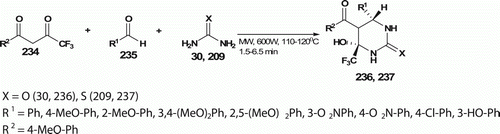
Khunt et al. Citation103 developed a solvent-free MW-assisted process for the preparation of tetrahydropyrimidinones 236 and tetrahydrothiopyrimidinones 237 employing equimolar amounts of neat 1,3-dicarbonyl compounds 234, different aromatic aldehydes 235 and urea 30 (or thiourea 209; ). The reaction mixture was irradiated at 110–120°C with MW for 1.5–6.5 min with 78–85% yield (15 examples). In contrast, conventional thermal heating method involving THF (as solvent) and InCl3 (as catalyst) furnishes the products in hours.
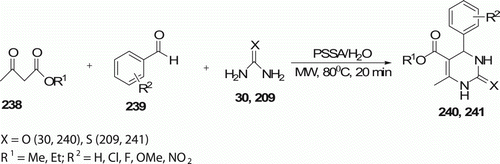
Dihydropyrimidinones 240 and 241 (15 examples) were synthesized by aqueous Biginelli protocol using polystyrenesulfonic acid (PSSA) as a catalyst under MW irradiation Citation104 at 80°C for 20 min with an excellent yield of 86–92% (). This reaction proceeds without using any phase-transfer catalyst (PTC). Conventional heating condition (oil bath) requires 5–6 h for completion.
2.12. Quinazoline
Quinazoline and its derivatives are a class of heteroaromatic compounds that have drawn much attention because of their biological and pharmaceutical activities Citation105.
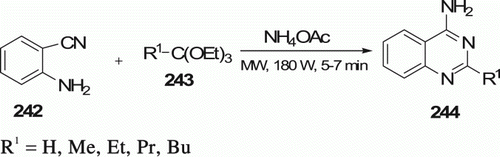
Rad-Moghadam et al. Citation106 synthesized various quinazolines 244 (five examples) under both MW irradiation and conventional heating conditions from a mixture of 2-aminobenzonitrile 242, orthoester 243, and ammonium acetate under solvent-free conditions (). MW method offers products in high yields (82–89%), in 5–7 min, whereas conventional method occurs in oil bath at 120°C, in 30–80 min with 83–92% yield.
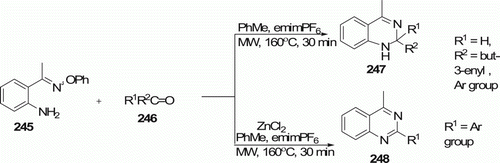
2-(Aminoaryl)alkanone O-phenyl oximes 245 and carbonyl compounds 246 in toluene solution with emimPF6 as ionic liquid were irradiated with MWs () at 160°C for 30 min to afford dihydroquinazolines 247 (five examples) in high yields (72–94%). This reaction was reported by Portela-Cubillo and his coworkers Citation107. When 0.3 equivalents of ZnCl2 is included in the mixture, quinazolines 248 (eight examples) are obtained instead. With the exception of the CuCl2 catalyzed reaction of aldehydes with anthranilamide, methods of forming the quinazoline ring either require multi-step preparations of special reagents/reactants or give moderate yields Citation107.
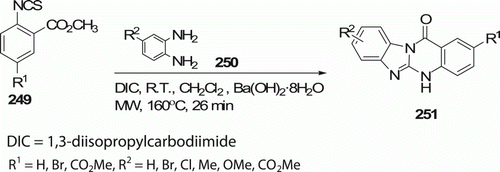
An effective route to the formation of benzimidazo[2,1-b]quinazolin-12(5H)-ones 251 (11 examples) from o-aryl isothiocyanate esters 249 and o-phenylenediamines 250 has been reported by Carpenter et al. Citation108 in 91–98% overall yield via tandem DIC-mediated benzimidazole cyclization and MW-assisted benzimidazoquinazolinone cyclization with barium hydroxide (). Common approaches to 251require ~200°C and give low yields or sometimes together with byproduct.
2.13. Miscellaneous
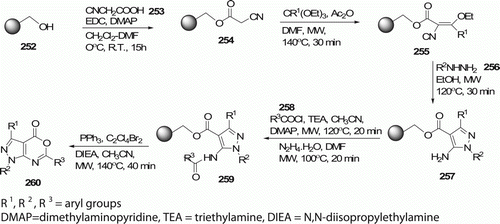
Fused 1,3-oxazin-6-ones constitute an interesting class of pharmacologically active compounds because they have shown a multitude of biological activities Citation109. A MW-assisted solid-phase synthesis of heteroannulated 1,3-oxazin-6-ones 260 has been developed by Che et al. Citation109 (). Overall reaction time has been dramatically shortened when compared to the conventional procedures.
Sondhi et al. Citation48 recently reported the new products 2-(2-aminophenyl)isoquinoline-1,3(2H,4H)-dione 263 (three examples) and 11H-benzimidazo [1,2b]isoquinoline-11-one 264 (three examples) from the reaction between 2-(carboxymethyl)benzoic acid 261 and diamines 71 (or 262) under MW irradiation that gets completed within 5–6 min in 99% yield ().
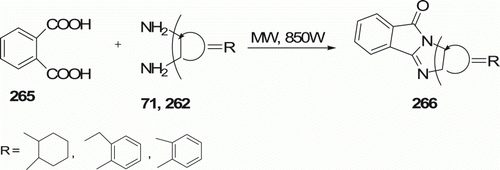
In the similar way, the same group Citation48 has investigated the condensation reaction of phthalic acid 265 and diamines 71 (or 262) under MW irradiation that gives another set of tetracyclic heterocyclic compound 266 (three examples), in good yields (95–98%) in 4–5 min ().
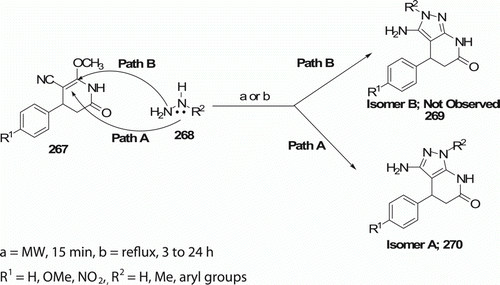
Pyrazolo[3,4-b]pyridin-6-ones have been shown to be inhibitors of cyclin-dependent protein kinase-2 (cdk-2), cyclin-dependent protein kinase-5 (cdk-5), and phosphatidylinositol 3-kinase (PI3-K) Citation110. Thus, these compounds have potential in the treatment of bipolar disorder, diabetes, dementia, Alzheimer's disease, schizophrenia, depression, and cancer Citation110. Rodrigues-Santos et al. Citation111 have reported the synthesis of pyrazolo[3,4-b]pyridin-6-ones 270 (12 examples) both by the conventional method and by MW irradiation (). 270 were obtained in moderate to good yields (50–85%) using the conventional methanol reflux method in 4–24 h. Using this MW method, 270 were produced in moderate yield (50–65%), which is low compared to the conventional methodology, but is 12–96 times faster than conventional method.
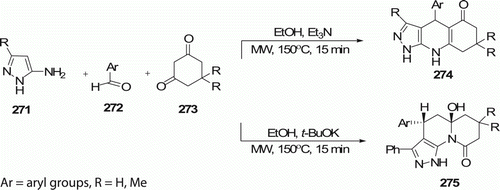
Regio- and chemoselective synthesis of 1,4,6,7,8,9-hexahydro-1H-pyrazolo[3,4-b]quinolin-5-ones 274 (eight examples) and 5a-hydroxy-4,5,5a,6,7,8-hexahydropyrazolo[4,3-c]quinolizin-9-ones 275 (nine examples) starting from 5-amino-3-phenylpyrazole 271, cyclic 1,3-dicarbonyl compounds 273, and aromatic aldehydes 272 under MW irradiation is described by the Chebanov et al. Citation112. Whereas in most cases, the three-component coupling in ethanol under reflux conditions provides mixtures of both possible regioisomers pyrazoloquinolinones and pyrazoloquinazolinones in varying ratios (). 274 were produced in 15 min at 150°C with 70–91% yield using MW-assisted route and 275 were obtained in the presence of t-BuOK instead of Et3N in 15 min with 38-75% yield.
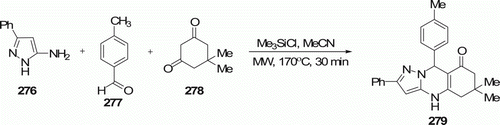
Pyrazoloquinazolinones 279 (eight examples) were also produced (with 54–70% yield) by the same group [112] when the three-component condensation was executed in the presence of trimethylsilylchloride (Me3SiCl) as reaction mediator at higher temperature (170 ° C) for 30 min (). Reaction involving the condensation of these three reactants in refluxing ethanol at ~80°C is rather unpredictable Citation112.
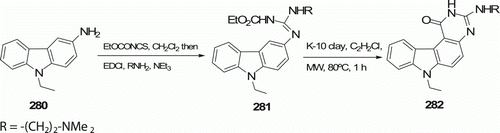
A new heterocycle tetracyclic pyrimido[4,5-c]carbazole 282 was prepared by Debray et al. Citation113 in three steps from 3-aminocarbazole 280 (). A mixture of 281 and montmorillonite K-10 (K-10) clay was irradiated in an MW oven at 80°C for 1 h to furnish 282 in 77% yield. In classical methods, the Friedel–Craft type cyclization of 281 catalyzed by Me3SiCl in dimethylformamide (DMF) works well; however, the efficiency of the work-up is very sensitive to the nature of the R group, and sometimes produce lower yields.
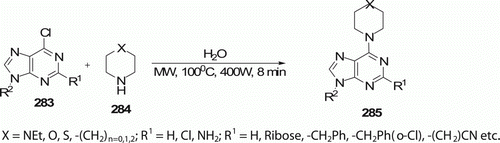
Nucleosides play important roles in various biological processes. Kinase inhibitors, C6-cycloamine-substituted purines and their analogs 285 (42 examples) were synthesized by Qu et al. Citation114 via a mild aqueous MW protocol that afforded the desired compounds at 100°C in 8 min in higher purity and yield (73–94%), making this methodology suitable for rapid drug discovery (). Traditional routes often involve long reaction time (15% yield after 24 h), toxic-solvent, and labor-intensive operation Citation114.
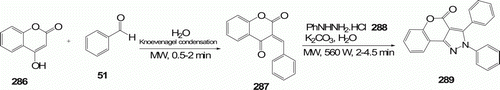
Pyrazole nucleus in fusion with benzopyrans confers pharmacological properties Citation115. Benzopyrano[4,3-c]pyrazoles 289 (seven examples) were synthesized by heterocondensation reaction between in situ-generated 3-arylidene-2,4-chromanediones 287 and N-substituted hydrazine 288 moieties under MW irradiation conditions in 2–4.5 min (). This high yielding (75–85%) protocol was reported by Kidwai et al. Citation115. Under conventional method, second stage requires 5–8 h to obtain 45–78% yield.
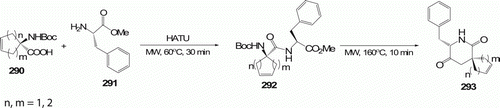
Spiro-amino acids incorporated into bioactive peptides are useful for both restricting the flexibility of the peptide and providing information on the topographical requirements of peptide receptors. An efficient method for the synthesis of spiro-2,5-diketopiperazines (spiro-DKPs) 293 (four examples) was developed by Jam and his co-workers Citation116 by cyclization of Boc-protected dipeptides 292 containing spiro-amino acids using MW heating in water in the presence of O-(7-Azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) at 160°C in 10 min with 80–86% (). Older methods used for peptide bond formation are inefficient with sterically congested amino acid residues like spiro-amino acids, offer low yield and need excess of reagents to complete the reaction Citation116.
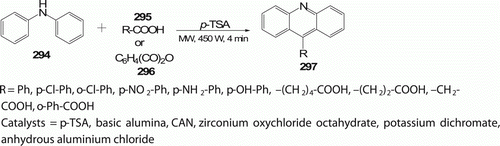
Das and his co-workers Citation117 reported a simple and general solventless reaction for the synthesis of 9-substituted acridines 297 (11 examples), by a modification of classical Bernthsen reaction (), using p-TSA as the catalyst using MW irradiation method. This paves the way for an environmentally benign condition without compromising viability and speed. The reaction time has been dramatically reduced to 4 min with 80% yield at 450 W than the conventional method (75% yields in 20 h).
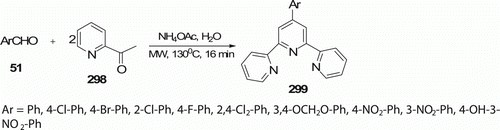
Tu et al. Citation74 have developed a first facile and clean Kröhnke reaction for the synthesis of 4′-aryl-2,2′:6′,2″-terpyridines 299 (11 examples) in water under both MW irradiation or conventional heating conditions at 130°C (). The use of MW irradiation at 200 W significantly reduces the reaction time to 16 min from 240 min required under conventional synthesis and improves the reaction yields 82–93% from 71% to 81%.
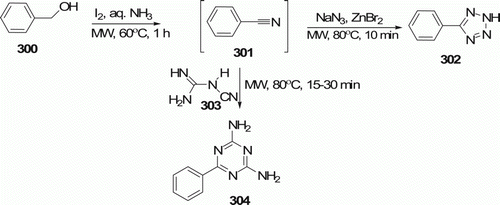
Triazines and tetrazoles are important class of nitrogen heterocycles that form an integral part of therapeutically interesting compounds which display diverse biological activities. Shie et al. Citation118 reported a method for the direct conversion of aldehydes to triazines 304 (five examples, 69–83% yields) and tetrazoles 302 (five examples, 70–83% yields) with high optical purity via cycloadditions of intermediate nitriles with dicyandiamide and sodium azide under MW irradiation (). Formation of triazines and tetrazoles requires refluxing (e.g., ~100°C) for a prolonged period (12–48 h) in traditional methods.
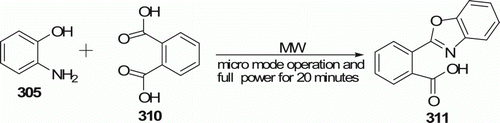
The benzoxazole moiety is an important pharmacophore due to the biological activity (melatonin receptor agonist and cyclooxygenase inhibitory, anticancer antimycobacterial, elastase inhibitory, etc.) displayed by 2-substituted benzoxazoles Citation119. Chakraborti et al. Citation119 Citation120 described an efficient new methodology for the synthesis of 2-substituted benzoxazoles 308 (16 examples)/benzothiazoles 309 (18 examples) from carboxylic acids 307 by direct condensation with 2-aminothiophenol 306/2-aminophenol 305 following the MW procedure in absence of any metal catalyst and under solvent-free conditions (). Reaction of 2-aminophenol 305 with various carboxylic acids 307 under MW irradiation in a domestic MW oven using micro-mode operation and full power for 20 min provided good yields (up to 90%). In contrast, the conventional methodologies suffer from harsh reaction condition (refluxing at higher temperature ~200°C for days/hours).
Investigation Citation119 by this group reveals that in the case of dicarboxylic acids 310, the reaction proceeds via the formation of the corresponding anhydride and affords predominant formation of the mono-benzoxazole 311 ().
4. Conclusion
The development of more environment-friendly methodologies is certainly a very current topic. Synthetic organic reactions performed under MW irradiation are gaining popularity, because this technique offers simple, clean, fast, efficient and economic for the synthesis of a large number of organic compounds including N-containing heterocycles. This technique enables a number of reactions to be completed under mild reaction condition, in shorter reaction times, and with higher yields that either do not occur under conventional heating or occur at much higher temperatures. We herein describe the current developments in the synthesis of N-containing heterocyclic compounds using MW heating. We believe that in the coming future many more MW-assisted reactions could be done on industrial scales thereby increasing the overall efficiency of the processes. The future for the application of MW technology looks bright because of its efficiency and its potential to contribute to clean products.
Microwave-assisted synthesis of Nitrogen containing heterocycles
Download ()Acknowledgements
We thank DST, New Delhi, India (Ref: SR/WOS-A/CS-29/2010) for financial support. Prof. Gautam Mandal, Department of Physics, TIFR, India, is also gratefully acknowledged for his assistance.
References
- Bacolini G. Topics Heterocycl. Syst. Synth. React. Prop . 1996 ; 1 : 103 .
- Alcázar J , Oehlrich D. Recent applications of microwave irradiation to medicinal chemistry . Future Med. Chem . 2010 ; 2 : 169 – 176 .
- Katritzky AR , Rees CW. Comprehensive heterocyclic chemistry . New York : Pergamon Press ; 1984 1–8 .
- Balaban AT , Oniciu DC , Katritzky AR. Aromaticity as a Cornerstone of Heterocyclic Chemistry . Chem. Rev . 2004 ; 104 : 2777 – 2812 .
- Brichacek M , Njardarson JT. Creative approaches towards the synthesis of 2,5-dihydro- furans, thiophenes, and pyrroles. One method does not fit all . Org. Biomol. Chem . 2009 ; 7 : 1761 – 1770 .
- Sheldon RA. Catalysis: the key to waste minimization . J. Chem. Technol. Biotechnol . 1997 ; 68 : 381 – 388 .
- Dabholkar VV , Ansari FY. Novel pyrimidine derivatives by sonication and traditional thermal methods . Green Chem. Lett. Rev . 2010 ; 3 : 245 – 248 .
- Ballini R Eco-friendly synthesis of fine chemicals . UK : Royal Society of Chemistry ; 2009 . p. 275 – 292 .
- Santagada V , Perissutti E , Caliendo G. The application of microwave irradiation as new convenient synthetic procedure in drug discovery . Curr. Med. Chem . 2002 ; 9 : 1251 – 1283 .
- Santagada V , Frecentese F , Perissutti E , Fiorino F , Severino B , Caliendo G. The application of microwave irradiation as new convenient synthetic procedure in drug discovery . Mini Rev. Med. Chem . 2009 ; 9 : 340 – 358 .
- Phukan M , Borah KJ , Borah R. Henry reaction in environmentally benign methods using imidazole as catalyst . Green Chem. Lett. Rev . 2009 ; 2 : 249 – 253 .
- Anastasa PT , Beacha ES. Green chemistry: the emergence of a transformative framework . Green Chem. Lett. Rev . 2007 ; 1 : 9 – 24 .
- Villar H , Frings M , Bolm C. Ring closing enyne metathesis: a powerful tool for the synthesis of heterocycles . Chem. Soc. Rev . 2007 ; 36 : 55 – 66 .
- Polshettiwar V , Varma RS. Microwave-assisted organic synthesis and transformations using Benign Reaction Media . Acc. Chem. Res . 2008 ; 41 : 629 – 639 .
- Tucker JL. Green chemistry, a pharmaceutical perspective . Org. Process Res. Dev . 2006 ; 10 : 315 – 319 .
- Daştan A , Kulkarnia A , Török B. Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches . Green Chem . 2012 ; 14 : 17 – 37 .
- de la Hoz A , Díaz-Ortiz A , Moreno A , Sanchéz-Migallón A , Prieto P , Carrillo JR , Vázquez E , Gómez MV , Herrero MA. Microwave-assisted reactions in heterocyclic compounds with applications in medicinal and supramolecular chemistry . Comb. Chem. High Throughput Screen . 2007 ; 10 : 877 – 902 .
- Varma RS , Polshettiwar V. Aqueous microwave chemistry: a clean and green synthetic tool for rapid drug discovery . Chem. Soc. Rev . 2008 ; 37 : 1546 – 1557 .
- Aydogan F , Basarir M , Yolacan C , Demir AS. New and clean synthesis of N-substituted pyrroles under microwave irradiation . Tetrahedron . 2007 ; 63 : 9746 – 9750 .
- Wilson MA , Filzen G , Welmaker GS. A microwave-assisted, green procedure for the synthesis of N-aryl sulfonyl and N-aryl pyrroles . Tetrahedron Lett . 2009 ; 50 : 4807 – 4809 .
- Knorr L. Synthese von pyrrolderivaten . Ber. Dtsch. Chem. Ges . 1884 ; 17 : 1635 – 1642 .
- Paal C. Synthese von thiophen-und pyrrolderivaten (synthesis of thiophene and pyrrole) . Ber. Dtsch. Chem. Ges . 1885 ; 18 : 367 – 371 .
- Hantzsch A. Neue Bildungsweise von Pyrrolderivaten . Ber. Dtsch. Chem. Ges . 1890 ; 23 : 1474 – 1476 .
- Jayagobi M , Raghunathan R. An efficient synthesis of pyrano [4,5-c] pyrrole derivatives through microwave–accelerated intramolecular Knoevenagal hetero Diels–Alder reaction . Tetrahedron Lett . 2009 ; 50 : 6886 – 6890 .
- Deb I , Seidel D. Retro-Claisen condensation versus pyrrole formation in reactions of amines and 1,3-diketones . Tetrahedron Lett . 2010 ; 51 : 2945 – 2947 .
- Wyr(bek P , Sniady A , Bewick N , Li Y , Mikus A , Wheeler KA , Dembinski R. Microwave-assisted zinc chloride-catalyzed synthesis of substituted pyrroles from homopropargyl azides . Tetrahedron . 2009 ; 65 : 1268 – 1275 .
- Portela-Cubillo F , Scottb JS , Walton JC. Microwave-assisted preparations of dihydropyrroles from alkenone O-phenyl oximes . Chem. Commun . 2007 ; 4041 – 4043 .
- Oskooie HA , Heravi MM , Behbahani FK. A facile, mild and efficient one-pot synthesis of 2-substituted indole derivatives catalyzed by Pd(PPh3)2Cl2 . Molecules . 2007 ; 12 : 1438 – 1446 .
- Karthikeyan SV , Perumal S , Shetty KA , Yogeeswari P , Sriram D. A microwave-assisted facile regioselective Fischer indole synthesis and antitubercular evaluation of novel 2-aryl-3,4-dihydro-2H-thieno[3,2-b]indoles . Bioorg. Med. Chem. Lett . 2009 ; 19 : 3006 – 3009 .
- Lehmann F , Holm M , Laufer S. Rapid and easy access to indoles via microwave-assisted Hemetsberger–Knittel synthesis . Tetrahedron Lett . 2009 ; 50 : 1708 – 1709 .
- Borthakur M , Gogoi Y , Gogoi J , Boruah RC. Lewis acid catalyzed rapid synthesis of 5-hydroxy-benzo[g]indole scaffolds by a modified Nenitzescu reaction . Tetrahedron Lett . 2010 ; 51 : 5160 – 5163 .
- Bhella SS , Pannu APS , Elango M , Kapoor A , Hundal MS , Ishar MPS. Investigations on synthesis of indole based constrained mimetic scaffolds through 1,3-dipolar cycloadditions of the C-(3-indolyl)-N-phenylnitrone with a variety of olefinic and allenic dipolarophiles under microwave irradiation . Tetrahedron . 2009 ; 65 : 5928 – 5935 .
- Carpita A , Ribecai A. Microwave-assisted synthesis of indole-derivatives via cycloisomerization of 2-alkynylanilines in water without added catalysts, acids, or bases . Tetrahedron Lett . 2009 ; 50 : 6877 – 6881 .
- Carpita A , Ribecai A , Stabile P. Microwave-assisted synthesis of indole- and azaindole-derivatives in water via cycloisomerization of 2-alkynylanilines and alkynylpyridinamines promoted by amines or catalytic amounts of neutral or basic salts . Tetrahedron . 2010 ; 66 : 7169 – 7178 .
- Chen Y , Markina NA , Larock RC. An efficient, microwave-assisted, one-pot synthesis of indoles under Sonogashira conditions . Tetrahedron . 2009 ; 65 : 8908 – 8915 .
- Waldmann H , Eberhardt L , Wittsteinab K , Kumar K. Silver catalyzed cascade synthesis of alkaloid ring systems: concise total synthesis of fascaplysin, homofascaplysin C and analogues . Chem. Commun . 2010 ; 46 : 4622 – 4624 .
- Zang H , Su Q , Mo Y , Cheng BW , Jun S. Ionic liquid [EMIM]OAc under ultrasonic irradiation towards the first synthesis of trisubstituted imidazoles . Ultrason. Sonochem . 2010 ; 17 : 749 – 751 .
- Debus H. Über die einwirkung des ammoniaks auf glyoxal . Liebigs Ann. Chem . 1858 ; 107 : 199 – 208 .
- Wallach O , Schulze E. Ueber Basen der Oxalsäurereihe . Ber. Dtsch. Chim. Ges . 1881 ; 14 : 420 .
- Lantos I , Zhang W-Y , Shui X , Eggleston DS . Synthesis of imidazoles via Hetero-Cope Rearrangements . J. Org. Chem . 1993 ; 58 : 7092 – 7095 .
- Tymoshenko DO. On the development of organic chemistry in Ukraine . Arkivoc . 2005 ; viii : 1 – 3 .
- Crouch RD , Howard JL , Zile JL , Barker KH. Microwave-mediated synthesis of lophine: developing a mechanism to explain a product . J. Chem. Edu . 2006 ; 83 : 1658 – 1660 .
- Xia M , Lu Y-D. A novel neutral ionic liquid-catalyzed solvent-free synthesis of 2,4,5-trisubstituted imidazoles under microwave irradiation . J. Mol. Catal. A: Chem . 2007 ; 265 : 205 – 208 .
- Wolkenberg SE , Wisnoski DD , Leister WH , Wang Y , Zhao Z , Lindsley CW. Efficient synthesis of imidazoles from aldehydes and 1,2-diketones using microwave irradiation . Org. Lett . 2004 ; 6 : 1453 – 1456 .
- Sparks RB , Combs PA. Microwave-assisted synthesis of 2,4,5-triaryl-imidazole; A novel thermally induced N-hydroxyimidazole N–O bond cleavage . Org. Lett . 2004 ; 6 : 2473 – 2475 .
- Shih M-H , Tsai C-H , Wang Y-C , Shieh MY , Lin , G-L , Wei C-Y. Microwave-assisted synthesis of sydnonyl-substituted imidazoles . Tetrahedron . 2007 ; 63 : 2990 – 2999 .
- Hoz A de la , Díaz-Ortiz Á , Mateo M del C , Moral M , Moreno A , Elguero J , Foces-Foces C , Rodríguez ML , Sánchez-Migallón A. Microwave assisted synthesis and crystal structures of 2-imidazolines and imidazoles . Tetrahedron . 2006 ; 62 : 5868 – 5874 .
- Sondhi SM , Rani R. Microwave-mediated one step synthesis of tri- and tetracyclic heterocyclic molecules . Green. Chem. Lett. Rev . 2010 ; 3 : 115 – 120 .
- Meng L , Fettinger JC , Kurth MJ. Intramolecular cycloaddition of azomethine ylides in the preparation of pyrrolidino[2¢,3¢:3,4]pyrrolidino[1,2-a]benzimidazoles . Org. Lett . 2007 ; 9 : 5055 – 5058 .
- Li J-T , Yin Y , Li Li , Sun M-X. A convenient and efficient protocol for the synthesis of 5-aryl-1,3-diphenylpyrazole catalyzed by hydrochloric acid under ultrasound irradiation . Ultrason. Sonochem . 2010 ; 17 : 11 – 13 .
- Sahu SK , Banerjee M , Samantray A , Behera C , Azam MA. Synthesis, analgesic, anti-inflammatory and antimicrobial activities of some novel pyrazoline derivatives . Trop. J. Pharm. Res . 2008 ; 7 : 961 – 968 .
- Polshettiwar V , Varma RS. Nano-organocatalyst: magnetically retrievable ferrite-anchored glutathione for microwave-assisted Paal–Knorr reaction, aza-Michael addition, and pyrazole synthesis . Tetrahedron . 2010 ; 66 : 1091 – 1097 .
- Hatem AA-A , Heba SAE-Z , Kamal MD. Microwave-assisted synthesis and in-vitro anti-tumor activity of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides . Eur. J. Med. Chem . 2010 ; 45 : 2427 – 2432 .
- Paul SH , Jennifer MF. Microwave-assisted synthesis utilizing supported reagents: a rapid and versatile synthesis of 1,5-diarylpyrazoles . Tetrahedron Lett . 2006 ; 47 : 2443 – 2446 .
- Sauzem PD , Machado P , Rubin MA , Sant'Anna G da S , Faber HB , De Souza AH , Mello CF , Beck P , Burrow RA , Bonacorso HG , Zanatta N , Martins MAP. Design and microwave-assisted synthesis of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles: Novel agents with analgesic and anti-inflammatory properties . Eur. J. Med. Chem . 2008 ; 43 : 1237 – 1247 .
- Radi M , Bernardo V , Bechi B , Castagnolo D , Pagano M , Botta M. Microwave-assisted organocatalytic multicomponent Knoevenagel/hetero Diels–Alder reaction for the synthesis of 2,3-dihydropyran[2,3-c]pyrazoles . Tetrahedron Lett . 2009 ; 50 : 6572 – 6575 .
- Ju Y , Varma RS . Aqueous N-heterocyclization of primary amines and hydrazines with dihalides: microwave-assisted syntheses of N-Azacycloalkanes, isoindole, pyrazole, pyrazolidine, and phthalazine derivatives . J. Org. Chem . 2006 ; 71 : 135 .
- Candeias NR , Branco LC , Gois PMP , Afonso CAM , Trindade AF. More sustainable approaches for the synthesis of N-based heterocycles . Chem. Rev . 2009 ; 109 : 2703 – 2802 .
- Manna K , Agrawal YK. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity . Bioorg. Med. Chem. Lett . 2009 ; 19 : 2688 – 2692 .
- Martins MAP , Beck P , Machado P , Brondani S , Moura S , Zanatta N , Bonacorso HG , Flores AFC. Microwave-assisted synthesis of novel 5-trichloromethyl-4,5-dihydro-1H-1-pyrazole methyl esters under solvent free conditions . J. Braz. Chem. Soc . 2006 ; 17 : 408 – 411 .
- Patel VM , Desai KR. Eco-friendly synthesis of pyrazoline derivatives over potassium carbonate . Arkivoc . 2004 ; i : 123 – 129 .
- Al-Mutairi AA , El-Baih FEM , Al-Hazimi HM. Microwave versus ultrasound assisted synthesis of some new heterocycles based on pyrazolone moiety . J. Saudi Chem. Soc . 2010 ; 14 : 287 – 299 .
- Pal S , Mareddy J , Devi NS. High speed synthesis of pyrazolones using microwave-assisted neat reaction technology . J. Braz. Chem. Soc . 2008 ; 19 : 1207 – 1214 .
- Deshmukh MB , Jagtap SS , Desmukh SA. Solvent free accelerated synthesis of 2-hydrazinobenzothiazoles derivatives using microwave . J. Indian Chem. Soc . 2006 ; 83 : 1055 – 1057 .
- Hänni KD , Leigh DA. The application of CuAAC ‘click’ chemistry to catenane and rotaxane synthesis . Chem. Soc. Rev . 2010 ; 39 : 1240 – 1251 .
- Huisgen R. 1,3-Dipolar cycloadditions, past and future . Angew. Chem. Int. Ed. Engl . 1963 ; 2 : 565 – 598 .
- Tsai C-W , Yang S-C , Liu Y-M , Wu M-J. Microwave-assisted cycloadditions of 2-alkynylbenzonitriles with sodium azide: selective synthesis of tetrazolo[5,1-a]pyridines and 4,5-disubstituted-2H-1,2,3-triazoles . Tetrahedron . 2009 ; 65 : 8367 – 8372 .
- Yang D , Kwon M , Jang Y , Jeon HB. A convenient and efficient synthesis of C-carbamoyl-1,2,3-triazoles from alkyl bromide by a one-pot sequential addition: conversion of ester to amide using Zr(Ot-Bu)4 . Tetrahedron Lett . 2010 ; 51 : 3691 – 3695 .
- Meng J , Kung P-P. Rapid, microwave-assisted synthesis of N1-substituted 3-amino-1,2,4-triazoles . Tetrahedron Lett . 2009 ; 50 : 1667 – 1670 .
- Ball C , Dean DK , Lorthioir O , Page LW , Smith CL , Watson SP. [1,3]Oxazolo[3,2-b][1,2,4]triazoles: a versatile synthesis of a novel heterocycle . Tetrahedron Lett . 2010 ; 51 : 3907 – 3909 .
- Lucas R , Neto V , Bouazza AH , Zerrouki R , Granet R , Krausz P , Champavier Y. Microwave-assisted synthesis of a triazole-linked 3′–5′ dithymidine using click chemistry . Tetrahedron Lett . 2008 ; 49 : 1004 – 1007 .
- Gupta R , Jain A , Jain M , Joshi R. ‘One Pot’ synthesis of 2-Amino-3-cyano-4,6-diarylpyridines under ultrasonic irradiation and grindstone technology . Bull. Kor. Chem. Soc . 2010 ; 30 : 3180 – 3182 .
- Khadijah M Al-Zaydia , Laila M Nharia , Rita M Borika , Mohamed H Elnagdib . Green technologies in organic synthesis: self-condensation of enamines, enaminones and enaminoesters under microwave irradiation in ionic liquid . Green Chem. Lett. Rev . 2010 ; 3 : 93 – 99 .
- Tu S , Jia R , Jiang B , Zhang J , Zhang Y , Yao C , Ji , S. Krohnke reaction in aqueous media: one-pot clean synthesis of 4′-aryl-2,2′:6′,2″-terpyridines . Tetrahedron . 2007 ; 63 : 381 – 388 .
- Yin G , Liu Q , Maa J , She N. Solvent- and catalyst-free synthesis of new hydroxylated trisubstituted pyridines under microwave irradiation . Green Chem . 2012 ; 14 : 1796 – 1798 .
- Yan C-G , Cai X-M , Wang Q-F , Wang T-Y , Zheng M. Microwave-assisted four-component, one-pot condensation reaction: an efficient synthesis of annulated pyridines . Org. Biomol. Chem . 2007 ; 5 : 945 – 951 .
- Shi F , Li C , Xia M , Miao K , Zhao Y , Tu S , Zheng W , Zhang G , Ma N. Green chemoselective synthesis of thiazolo[3,2-a]pyridine derivatives and evaluation of their antioxidant and cytotoxic activities . Bioorg. Med. Chem. Lett . 2009 ; 19 : 5565 – 5568 .
- Kantevari S , Chary MV , Vuppalapati SVN , Lingaiah N. Microwave-assisted regioselective one-pot synthesis of trisubstituted pyridine scaffolds using K5CoW12O40.3H2O under solvent free conditions . J. Heterocyclic Chem . 2008 ; 45 : 1099 – 1102 .
- Duvelleroy D , Perrio C , Parisel O , Lasne M-C. Rapid synthesis of quinoline-4-carboxylic acis derivatives from arylimines and 2-substituted acrylates or acrylamides inder indium (III) chloride and microwave activations. Scope and limitations of the reaction . Org. Biomol. Chem . 2005 ; 3 : 3794 – 3804 .
- Kouznetsov VV , Mendez LYV , Gomez CMM. Recent progress in the synthesis of quinolines . Curr. Org. Chem . 2005 ; 9 : 141 – 161 and references cited therein .
- Muscia GC , Bollini M , Carnevale JP , Bruno AM , Asís SE. Microwave-assisted Friedländer synthesis of quinolines derivatives as potential antiparasitic agents . Tetrahedron Lett . 2006 ; 47 : 8811 – 8815 .
- Balamurugan K , Jeyachandran V , Perumal S , Manjashetty TH , Yogeeswari P , Sriram D. A microwave-assisted, facile, regioselective Friedländer synthesis and antitubercular evaluation of 2,9-diaryl-2,3-dihydrothieno-[3,2-b]quinolines . Eur. J. Med. Chem . 2010 ; 45 : 682 – 688 .
- Dandia A , Gautam S , Jain AK. An efficient synthesis of fluorine-containing substituted spiro[piperidine-4,4′-pyrano[3,2-c]quinoline]-3′-carbonitrile by nonconventional methods . J. Fluorine Chem . 2007 ; 128 : 1454 – 1460 .
- Mali JR , Pratap UR , Jawale DV , Mane RA. Water-mediated one-pot synthetic route for pyrazolo[3,4-b]quinolines . Tetrahedron Lett . 2010 ; 51 : 3980 – 3982 .
- Tu S , Zhang Y , Jia R , Jiang B , Zhang J , Ji S. A multi-component reaction for the synthesis of N-substituted furo[3,4-b]quinoline derivatives under microwave irradiation . Tetrahedron Lett . 2006 ; 47 : 6521 – 6525 .
- Tomaszewski MJ , Whalley A , Hu Y-J. A one-pot synthesis of 2,3-dihydro-1H-pyrrolo[3,2-c]quinolines . Tetrahedron Lett . 2008 ; 49 : 3172 – 3175 .
- Motiwala HF , Kumar R , Chakraborti AK. Microwave-accelerated solvent- and catalyst-free synthesis of 4-aminoaryl/alkyl-7-chloroquinolines and 2-aminoaryl/alkylbenzothiazoles . Aust. J. Chem . 2007 ; 60 : 369 – 374 .
- Adib M , Mahmoodi N , Mahdavi M , Bijanzadeh HR. Microwave-assisted simple, one-pot, four-component synthesis of 2,4,6-triarylpyrimidines under solvent-free conditions . Tetrahedron Lett . 2006 ; 47 : 9365 – 9368 .
- Biginelli P. Aldehyde-urea derivatives of aceto- and oxaloacetic acids . Gazz. Chim. Ital . 1893 ; 23 : 360 – 413 .
- Phoujdar MS , Kathiravan MK , Bariwal JB , Shah AK , Jain KS. Microwave-based synthesis of novel thienopyrimidine bioisosteres of gefitinib . Tetrahedron Lett . 2008 ; 49 : 1269 – 1273 .
- Rai US , Isloor AM , Shetty P , Vijesh AM , Prabhu N , Isloor S , Thiageeswaran M , Fun H-K. Novel chromeno [2,3-b]-pyrimidine derivatives as potential anti-microbial agents . Eur. J. Med. Chem . 2010 ; 45 : 2695 – 2699 .
- Bagley MC , Lin Z , Pope SJA. Barium manganate in microwave-assisted oxidation reactions: synthesis of solvatochromic 2,4,6-triarylpyrimidines . Tetrahedron Lett . 2009 ; 50 : 6818 – 6822 .
- Shaaban MR. J. Microwave-assisted synthesis of fused heterocycles incorporating trifluoromethyl moiety . Fluorine Chem . 2008 ; 129 : 1156 – 1161 .
- Quiroga J , Portilla J , Abonía R , Insuasty B , Nogueras M , Cobo J. Regioselective synthesis of novel substituted pyrazolo[1,5-a]pyrimidines under solvent-free conditions . Tetrahedron Lett . 2008 ; 49 : 6254 – 6256 .
- Hoz A , Díaz A , Elguero J , Jiménez A , Moreno A , Ruiz A , Sánchez-Migallón A. Microwave-assisted synthesis of bipyrazolyls and pyrazolylsubstituted pyrimidines . Tetrahedron . 2007 ; 63 : 748 – 753 .
- Daniels RN , Kim K , Lebois EP , Muchalski H , Hughes M , Lindsley CW. Microwave-assisted protocols for the expedited synthesis of pyrazolo[1,5-a] and [3,4-d]pyrimidines . Tetrahedron Lett . 2008 ; 49 : 305 – 310 .
- Arya K , Dandia A. Synthesis of biologically important novel fluorinated spiro heterocycles under microwaves catalyzed by montmorillonite KSF . J. Fluorine Chem . 2007 ; 128 : 224 – 231 .
- Pasunooti KK , Chai H , Jensen CN , Gorityala BK , Wang S , Liu X-W. A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions . Tetrahedron Lett . 2011 ; 52 : 80 – 84 .
- Kidwai M , Saxena S , Mohan R , Venkataramanan R. A novel one pot synthesis of nitrogen containing heterocycles: an alternate methodology to the Biginelli and Hantzsch reactions . J. Chem. Soc. Perkin Trans . 2002 ; 1 : 1845 – 1846 .
- Kategaonkar AH , Sadaphal SA , Shelke KF , Shingate BB , Shingare MS. Microwave assisted synthesis of pyrimido[4,5-d]pyrimidine derivatives in dry media . Ukr. Bioorg. Acta 2009 ; 1 : 3 – 7 .
- Singhal S , Joseph JK , Jain SL , Sain B. Synthesis of 3,4-dihydropyrimidinones in the presence of water under solvent free conditions using conventional heating, microwave irradiation/ultrasound . Green Chem. Lett. Rev . 2010 ; 3 : 23 – 26 .
- Mojtahedi MM , Saidi MR , Shirzi JS , Bolourtchian M. Microwave promoted efficient synthesis of substituted uracils and thiouracils under solvent-free conditions . Synth. Commun . 2002 ; 32 : 851 – 855 .
- Khunt RC , Akbari JD , Manvar AT , Tala SD , Dhaduk MF , Joshi HS , Shah A. Green chemistry approach to synthesis of some new trifluoromethyl containing tetrahydropyrimidines under solvent free conditions . Arkivoc . 2008 ; 11 : 277 – 284 .
- Polshettiwar V , Varma RS. Biginelli reaction in aqueous medium: a greener and sustainable approach to substituted 3,4-dihydropyrimidin-2(1H)-ones . Tetrahedron Lett . 2007 ; 48 : 7343 – 7346 .
- Rivero IA , Espinoza K , Somanathan R. Syntheses of quinazoline-2,4-dione alkaloids and analogues from Mexican Zanthoxylum species . Molecules . 2004 ; 9 : 609 – 616 .
- Rad-Moghadam K , Samavi L. One-pot three-component synthesis of 2-substituted 4-aminoquinazolines . J. Heterocycl. Chem . 2006 ; 43 : 913 – 916 .
- Portela-Cubillo F , Scott JS , Walton JC. 2-(Aminoaryl)alkanone O-phenyl oximes: versatile reagents for syntheses of quinazolines . Chem. Commun . 2008 ; 2935 – 2937 .
- Carpenter RD , Lam KS , Kurth MJ. Microwave-mediated heterocyclization to benzimidazo[2,1-b]quinazolin-12(5H)-ones . J. Org. Chem . 2007 ; 72 : 284 – 287 .
- Che J , Raghavendra MS , Lam Y. Traceless solid-phase synthesis of heteroannulated 1,3-Oxazin-6-ones . J. Comb. Chem . 2009 ; 11 : 378 – 384 .
- Kung DWS , Fuller G. US Patent 266 , 815 , 2004 .
- Rodrigues-Santos CE , Echevarria A. Convenient syntheses of pyrazolo[3,4-b]pyridin-6-ones using either microwave or ultrasound irradiation . Tetrahedron Lett . 2011 ; 52 : 336 – 340 .
- Chebanov VA , Saraev VE , Desenko SM , Chernenko VN , Knyazeva IV , Groth U , Glasnov TN , Kappe CO. Tuning of chemo- and regioselectivities in multicomponent condensations of 5-aminopyrazoles, dimedone, and aldehydes . J. Org. Chem . 2008 ; 73 : 5110 – 5118 .
- Debray J , Zeghida W , Baldeyrou B , Mahieu C , Lansiaux A , Demeunynck M. Montmorillonite K-10 catalyzed cyclization of N-ethoxycarbonyl-N0-arylguanidines: Access to pyrimido[4,5-c]carbazole and pyrimido[5,4-b]indole derivatives . Bioorg. Med. Chem. Lett . 2010 ; 20 : 4244 – 4247 .
- Qu G-R , Zhao L , Wang D-C , Wu J , Guo H-M. Microwave-promoted efficient synthesis of C6-cyclo secondary amine substituted purine analogues in neat water . Green Chem . 2008 ; 10 : 287 – 289 .
- Kidwai M , Singhal PK , Rastogi S. A convenient K2CO3 catalysed regioselective synthesis for benzopyrano[4,3-c]pyrazoles in aqueous medium . Heterocycles . 2007 ; 71 : 569 – 576 .
- Jam F , Tullberg M , Luthman K , Grøtli M. Microwave assisted synthesis of spiro-2,5-diketopiperazines . Tetrahedron . 2007 ; 63 : 9881 – 9889 .
- Das S , Thakur AJ. A green development of Bernthsen 9-substituted acridine synthesis in the absence of solvent catalyzed by p-toluenesulphonic acid (p-TSA) . Green Chem. Lett. Rev . 2011 ; 4 : 131 – 135 .
- Shie J-J , Fang J.-M. Microwave-assisted one-pot tandem reactions for direct conversion of primary alcohols and aldehydes to triazines and tetrazoles in aqueous media . J. Org. Chem . 2007 ; 72 : 3141 – 3144 .
- Kumar R , Selvam C , Kaur G , Chakraborti AK. Microwave-assisted direct synthesis of 2-substituted benzoxazoles from carboxylic acids under catalyst and solvent-free conditions . Synlett . 2005 ; 9 : 1401 – 1404 .
- Chakraborti AK , Selvam C , Kaur G , Bhagat S. An efficient synthesis of benzothiazoles by direct condensation of carboxylic acids with 2-aminothiophenol under microwave irradiation . Synlett . 2004 ; 5 : 0851 – 0855 .