Abstract
A greener, efficient, and chemoselective protocol for O-Boc protection/deprotection of a wide range of phenol derivatives is reported under catalyst-free conditions in water-related systems. Unlike previous reports, no additional reagents or catalysts were used, and workup fulfils green chemistry requirements, making the present method even more interesting.
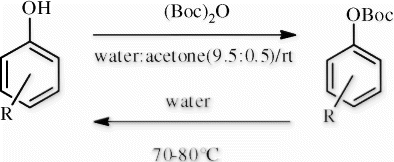
Introduction
Efforts have been made to identify mild and efficient chemoselective methods for the protection/deprotection of functional groups, crucial interest to organic multi-step synthesis Citation1 Citation2. Tert-butyl carbamates are widely used as amine-protecting groups in various fields of organic synthesis due to its stability toward nucleophilic conditions and due to its easy removal in various environments. The insertion of Boc group is usually achieved using di-tert-butyl dicarbonate (Boc)2O, being the best available reagent used for protecting substrates containing a labile-hydrogen moiety such as phenol-type Citation3 Citation4.
Acylation is a common approach in protecting hydroxyl groups Citation5 Citation6, but its regeneration requires harsh conditions incompatible with polyfunctional molecules. Furthermore, O-tert-butoxycarbonylation is a suitable and preferred alternative process to protect hydroxyl group Citation1 Citation2 due to both sustainable compatibility toward reaction conditions applied in organic synthesis and regeneration practices conducted under soft conditions.
In the last decade, various methods and reagents have been developed to achieve the protection/deprotection of Boc group on phenol functionality. The introduction of Boc moiety into phenols is generally achieved by the reaction of (Boc)2O in the presence of a phase transfer catalyst Citation7, 4-dimethylaminopyridine (DMAP) as catalyst Citation8 and using Lewis acids such as BiCl3 Citation9, Zn(OAc)2 Citation10, 1-tert-butoxy-2-tert-butoxycarbonyl-1,2-dihydroisoquinoline (BBDI) Citation3 Citation4 and NaTiO4 Citation11, or using 6,7-dimethoxyisoquinoline Citation12 as an organocatalyst.
On the other hand, the O-Boc deprotection is carried out under mild acidic conditions such as trifluoroacetic acid (TFA) Citation13 and the use of a large excess of base Citation14. In spite of their importance, there are a few methods available for the protection/deprotection of O-Boc groups under eco-sustainable conditions. Recently, Chankeshwara et al. Citation15 reported the O-tert-butoxycarbonylation of functionalized phenols using carbon tetrabromide (CBr4) as catalyst and their regeneration from the O-tert-Boc derivatives using the complex system CBr4–PPh3. More recently, Procopio et al. Citation16 described a new method for the protection/deprotection of the O-tert-butoxy carbonates of alcohols and phenols using mesoporous silica-supported (ErIII-MCM-41).
However, these conditions are not chemoselective, require harsh conditions, long reaction time, and nucleophilic organocatalysts used sometimes involve the generation of side products in significant quantities such as symmetrical carbonates, cyclic carbonates, and carbonic–carbonic anhydrides Citation8.
In recent years, organic reactions in water have received considerable attention Citation17–20. The use of water as a solvent offers several advantages such as improving reactivity and selectivity, mild reaction conditions, and minimization of energy requirements Citation21.
Thus, in the continuation of our previous work on the use of catalyst-free water-related system for the N-Boc protection/deprotection Citation22–24, we herein report an efficient protocol for O-tert-butoxycarbonyl protection/deprotection of phenol derivatives under catalyst-free conditions and in aqueous media, meeting all requirements for a green chemical process.
Results and discussion
In order to determine the best reaction system model, we chose phenol (, Entry 1) as model substrate and treated it with (Boc)2O (1 mmol) under catalyst-free conditions in aqueous media at room temperature, and the expected product was obtained in excellent yield. To find the role of water in O-Boc protection, the reaction was performed in several polar solvents at room temperature (). The best result was obtained with water, affording tert-butyl phenyl carbonate in 95% yield after 45 min. The reaction was carried out under catalyst-free conditions in polar protic solvents (MeOH and EtOH), affording, respectively, expected products in 10% and 4% yields after 72 h. The O-tert-butoxycarbonylation in polar aprotic solvents (THF, MeCN, CH3Cl, and CCl4) did not give any significant results. The readings of these results show well the efficacy of water in the O-Boc protection of phenol. Whereas, when the reaction carried using polar and protic solvents under catalyst-free conditions, the corresponding carbonate is in failure. Only the properties of water molecule cause “electrophilic activation” making the carbonyl group more susceptible to the nucleophilic attack of phenol. What may be explained the role of water compared to other solvents.
Table 1. Evaluation of different solvents for O-Boc protection of phenol under catalyst-free conditions.
The amount of aqueous media has a significant effect on the reaction rate and product yield. The minimal required amount for O-Boc protection is 5 mL/mmol (water:acetone 9.5:0.5). Increasing the amount of water to 7–10 mL/mmol affects the reaction rate by prolonging reaction time by 1.5–4 h in tert-butyl phenyl carbonate conversion, which could be explained by the outsized dispersion of reagents in the solution.
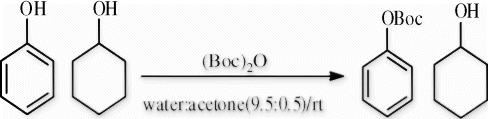
The use of water eliminates the drawbacks found in reported protocols as well as replaces the use of toxic solvents undesired by most pharmaceutical companies Citation25. Various phenols were converted to the O-tert-Boc derivatives in excellent yield after 30–120 min, proving the advantage of this procedure. No side product was formed as previously cited Citation8. The compatibility of the reaction conditions in the presence of other functional groups such as NH-Boc, NH-Ac, CHO, NO2, and OCH3 can be observed in , showing the absence of undesired products such as N-Boc at NH-Ac or N-di-Boc and without damaging substituted functional groups. This study encourages the development of the general strategy used in this method. We tried to test the reaction conditions on aliphatic hydroxyls, but no O-Boc formation was observed at room temperature over time. This may confirm the advantage of this method over reported procedures. The chemoselectivity of O-Boc formation on phenol derivatives rather than on aliphatic hydroxyl groups was also verified by studying the reactivity when applying our protocol conditions on mixtures containing both compounds. This showed that only phenol substrates were affected and that aliphatic hydroxyl groups were left intact. When phenol and cyclohexanol were tested using previous conditions, no tert-butyl cyclohexyl carbonate was formed ().
Table 2. O-Boc protection/deprotection of hydroxy compounds.
The reaction was affected by the nature of the substituent in the aromatic ring; the electron-donating effect of the methoxy (, entry 2) increased the rate of the reaction compared to entry 1. On the contrary, electron-withdrawing substituents impede the process.
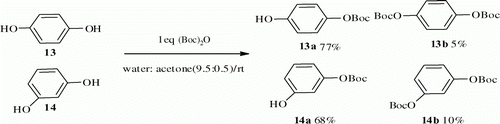
Encouraged by these experimental results, the protocol conditions were also applicable to the resorcinol and hydroquinone 13 and 14 containing two hydroxyl groups. The O-tert-butoxycarbonylation of 13 and 14 using 1 equiv. of (Boc)2O in the same conditions affords a mixture of mono-O-t-Boc and di-O-t-Boc within 30 min. A significant amount of the mono-O-t-Boc was formed (13a in 77% and 14b in 68%) ().
We further investigated the O-Boc deprotection, finding that heating water (80°C) could efficiently catalyze the deprotection of the O-Boc group on phenol derivatives in good to excellent yields within 10 min (). Moreover, it seems obvious that the electronic effect of substituents does not have any significant influence on the deprotection reaction's speed in comparison to the O-Boc protection.
By enhancing the solubility of substrates in water with hydrogen bond-forming groups such as OMe, NH-Ac, NH-Boc, NO2, reaction yields are increased which is in perfect accord with Prof. Qu's research group concerning the N-Boc deprotection in boiling water Citation26. The chemoselective O-Boc deprotection in the presence of other moieties such as NH-Boc and NH-Ac can find interesting applications in protecting group chemistry.
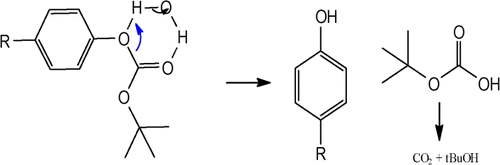
To demonstrate the general act of water as catalyst for the reaction, we used a depressurized system under argon atmosphere to avoid dissolving CO2 in water, released during the removal of the Boc group. Therefore, we propose the following mechanism, where water can provide hydrogen bonding with the carbonyl and tert-butyl oxygen atoms, leading to an electrophilic activation, intermolecular rearrangement, and regeneration of the parent phenol and tert-butyl hydrogen carbonate, followed by the elimination of CO2 and t-BuOH.
Mechanistic proposal: O-Boc deprotection
Experimental
All commercial chemicals and solvents were used without further purification. All reactions were carried out under an inert argon atmosphere. Melting points were determined in open capillary tubes on a Büchi apparatus and are uncorrected. 1H and 13C NMR spectra were recorded in a 250 MHz Brücker spectrometer. Chemical shifts are reported in δ units (ppm) with Trimethylsilane (TMS) as reference. All coupling constants (J) are reported in Hertz. Multiplicity is indicated as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and combinations of these signals. All reactions were monitored by Thin layer chromatography (TLC) on silica Merck 60 F254 percolated aluminum plates. Column chromatography was performed on Merck silica gel (230–400 mesh).
General procedure of O-Boc protection on phenols derivatives
To 1 mmol of substrate dissolved in 3.5 mL of water:acetone (9.5:0.5) was added dropwise 1 mmol of (Boc)2O in 1.5 mL of the same solvent. The mixture was stirred at room temperature. The reaction was monitored by TLC. After the appropriate time, the reaction mixture was extracted with ethyl acetate (3×5mL), the organic layer was separated and dried with anhydrous Na2SO4, and the solvent was eliminated in vacuo. The products was purified in a silica gel column (hexane:diethylether 3:1) to give O-Boc phenols in oil ond solid form.
Tert-butyl 4-acetamidophenyl carbonate Citation11
Yield 97%; R f (CHCl3–MeOH 95:5) 0.7; RMN1H δ (ppm) (250 MHz, CDCl3): 1.55 (s, 9H, O-t-Bu), 2.07 (s, 3H, CH3CONH), 7.04 (d, 2H, HA, HD, J = 2.1 Hz), 7.43 (d, 2H, HB, HC, J = 2.1 Hz), 8.43 (s, 1H, NH). RMN13C δ (ppm) (62.89 MHz, CDCl3): 24.12 (CH3), 27.65 (3CH3), 83.67 (C), 121.03 (2CHB,C), 121.47 (2CHA,D), 135.85 (CCNHAc), 147,00 (CCOBoc), 169.04 (CCOMe).
Tert-butyl (4-((tert-butoxycarbonyl)oxy)phenyl)carbamate Citation12
Yield 95%; R f (CHCl3–MeOH 95:5) 0.63; RMN1H δ (ppm) (250 MHz, CDCl3): 1.51 (s, 9H, NHCOOt-Bu), 1.56 (s, 9H, OCOOt-But), 6.68 (s, 1H, NH), 7.08 (d, 2H, HA, HD, J = 2.2 Hz), 7.43 (d, 2H, HB, HC, J = 2.0Hz). RMN13C δ (ppm) (62.89 MHz, CDCl3): 27.67 (3CH3), 28.30 (3CH3), 80.53 (C), 83.40 (C), 119.35 (2CHB,C), 121.59 (CHA,D), 136.00 (CCNHBoc), 146.34 (CCOBoc), 152.03 (CNCOOtBu), 152.76 (COCOOtBu).
General procedure of O-Boc deprotection on phenols derivatives
One millimoles of O-Boc-phenol and 10 mL of freshly double distilled water were loaded into 50 mL round-bottomed flask related with depressurized system. The reaction mixture was heated at 80°C for particular time and conducted under argon atmosphere. After completion, the mixture was extracted with ethyl acetate (3×5mL) and concentrated in vacuum, all products were obtained pure.
Conclusions
In summary, we report an efficient method for chemoselective O-Boc protection/deprotection of various phenolic structures under catalyst-free conditions, in aqueous media, and in high yields. The present protocol avoids the use of organocatalysts and prevents the generation of side products, has a discrete advantage by having a short reaction time, is easy of manipulation, is chemoselective to phenol hydroxyls rather than aliphatic alcohols, enabling greener reactions in multi-step synthesis.
A simple and eco-sustainable method for the O-Boc formation/cleavage of various phenolic structures under water-mediated/catalyst-free conditions
Download MS Word (1.5 MB)Acknowledgements
This work was generously supported by the Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRS-DT) and Algerian Ministry of Scientific Research (FNR, PNR). Fruitful discussions with Dr. Jamel Zoubir are greatly appreciated.
References
- Greene TW , Wuts PGM . Protecting groups in organic synthesis. , 4th ed . New York (NY) : Wiley ; 2007 .
- Kocienski PJ . Protecting groups. , 3rd ed . New York (NY) : George Thieme Verlag ; 2004 .
- Ouchi H , Saito Y , Yamamoto Y , Takahata . H . 1-tert-Butoxy-2-tert-butoxycarbonyl-1,2-dihydroisoquinoline: A Novel and Chemoselective tert-Butoxycarbonylation reagent . Org. Lett . 2002 ; 4 : 585 – 587 . doi: 10.1021/ol017183u
- Saito Y , Ouchi H , Takahata H . A novel tert-butoxycarbonylation reagent: 1-tert-butoxy-2-tert-butoxycarbonyl-1,2-dihydroisoquinoline (BBDI) . Tetrahedron . 2006 ; 62 : 11599 – 11607 .
- Shivari R , Chakraborti AK . Zinc perchlorate hexahydrate [Zn(ClO4)2.6H2O] as acylation catalyst for poor nucleophilic phenols, alcohols and amines: Scope and limitations . J. Mol. Catal. A: Chem . 2007 ; 264 : 208 – 213 . doi: 10.1016/j.molcata.2006.09.015
- Chakraborti , AK , Shivari R. J . Org. Chem . 2006 ; 71 : 5785 – 5788 . doi: 10.1021/jo0605142
- Houlihan F , Bouchard F , Fichet JM , Wilson CG . Phase transfer catalysis in the tert-butyloxycarbonylation of alcohols, phenols, enols, and thiols with di-tert-butyl dicarbonate . Can. J. Chem . 1985 ; 63 : 153 – 156 . doi: 10.1139/v85-025
- Basel Y , Hassner A . Di-tert-butyl Dicarbonate and 4-(Dimethylamino)pyridine revisited. Their reactions with amines and alcohols . J. Org. Chem . 2000 ; 65 : 6368 – 6380 . doi: 10.1021/jo000257f
- Suryakiran N , Prabhakar P , Venkateswarlu Y . ChemInform Abstract: Facile tert-Butoxycarbonylation of Alcohols, Phenols, and Amines Using BiCl3 as a Mild and Efficient Catalyst . Synth. Commun . 2008 ; 38 : 177 – 185 . doi: 10.1080/00397910701749617
- Bartoli G , Bosco M , Carlone A , Dalpozzo R , Locatelli M , Melchiorre P , Palazzi P , Sambri L . Synlett 2006 ; 2104 – 2108 . doi: 10.1055/s-2006-949609
- Singh SJ , Jayaram RV . Tetrahedron Lett . 2008 ; 49 : 4249 – 4256 . doi: 10.1016/j.tetlet.2008.04.149
- Saito Y , Yoshimura Y , Takahata H . Chemoselective O-tert-butoxycarbonylation of phenols using 6,7-dimethoxyisoquinoline as a novel organocatalyst . Tetrahedron Lett . 2010 : 51 ; 6915 – 6917 . doi: 10.1016/j.tetlet.2010.10.114
- Hansen MM , Riggs JR . A Novel Protecting Group for Hindered Phenols . Tetrahedron Lett . 1998 ; 39 : 2705 – 2706 . doi: 10.1016/S0040-4039(98)00411-0
- Nakamura K , Nakajima T , Kayahara H , Nomura E , Taniguchi H . Base-labile tert-butoxycarbonyl (Boc) group on phenols . Tetrahedron Lett . 2004 ; 45 : 495 – 499 . doi: 10.1016/j.tetlet.2003.11.008
- Chankeshwara SV , Chebolu R , Chakraborti AK . Organocatalytic Methods for Chemoselective O-tert-Butoxycarbonylation of Phenols and Their Regeneration from the O-t-Boc Derivatives . J. Org. Chem . 2008 ; 73 : 8615 – 8618 . doi: 10.1021/jo8013325
- Procopio A , Cravotto G , Oliverio M , Costanzo P , Nardic M , Paonessa R . An eco-sustainable erbium(III)-catalyzed method for formation/cleavage of O-tert-butoxy carbonates . Green Chem . 2011 ; 13 : 436 – 443 . doi: 10.1039/c0gc00728e
- Matlack AS . Introduction to green chemistry . New York (NY) : Marcel Dekker Inc .: 2001 .
- Lancester M . Green chemistry: an introductory text . Cambridge : Royal Society of Chemistry ; 2002 .
- Clark JH , Macquarrie D . Handbook of green chemistry & technology . Oxford : Blackwell Publishers ; 2002 .
- Griero PA . Organic synthesis in water . London : Blakie ; 1998 .
- Tundo P , Annastas P , Black DS , Breen J , Collins T , Menoli S , Miyamoto J , Polyakoff M , Tumas W . Synthetic pathways and processes in green chemistry. Introductory overview . Pure Appl. Chem . 2000 ; 72 : 1207 – 1211 . doi: 10.1351/pac200072071207
- Klai N , Berredjem M , Khettache N , Belghit M-Y , Regainia Z , Aouf N . Simple and efficient cleavage reaction of the boc group in heterocyclic compounds . J. Heterocycl. Chem . 2004 ; 41 : 57 – 60 . doi: 10.1002/jhet.5570410109
- Cheraiet Z , Ouarna S , Jamel Z , Berredjem M , Aouf N . A Simple and Efficient Green Method for the Deprotection of N-Boc in Various Structurally Diverse Amines under Water-mediated Catalyst-free Conditions . Int. J. of Chem . 2012 ; 4 : 73 – 79
- Cheraiet Z , Ouarna S , Hessainia S , Berredjem M , Aouf N . N-tert-Butoxycarbonylation of structurally diverse aminesand sulfamides under water-mediated catalyst-free conditions . ISRN org. chem . 2012 ; ID 404235 : 8 doi: 10.5402/2012/404235
- Alfonsi K , Colberg J , Dunn PJ , Fevig T , Jenings S , Johnson TA , Kleine HP , Knight C , Nagy MA , Perry DA , Stefaniak M . Green chemistry oriented organic synthesis in water . Green Chem . 2008 ; 10 : 31 – 36 . doi: 10.1039/b711717e
- Wang J , Liang Y-L , Qu J . Boiling water-catalyzed neutral and selective N-Boc deprotection . Chem. Commun . 2009 ; 5144 – 5146 . doi: 10.1039/b910239f