Abstract
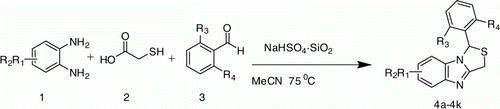
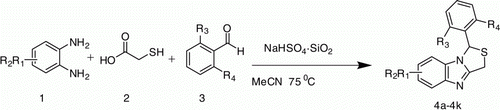
Silica-supported sodium hydrogen sulfate (NaHSO4·SiO2) efficiently catalyzed the three-component reactions of o-phenylenediamine, 2-mercaptoacetic acid, and aromatic aldehydes, and it formed the corresponding 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole. The catalyst is inexpensive and eco-friendly and works under heterogeneous conditions.
Introduction
In natural products and drugs, one of the most widely observed active pharmacophores is benzimidazole. In modern days, it is attracting the interest of researchers for its various significant biological activities Citation1–5; besides this, its derivatives have also found to be potential HIV-1 RT inhibitors. Furthermore, benzimidazoles have found and will continue to find extensive use in a myriad of synthetic contexts. So far, the reported synthetic methodologies for synthesis of benzimidazole and its derivatives involve condensation of various substrates under harsh reaction conditions Citation6–10 which suffer from demerits like poor yield, long reaction time, formation of side products, etc. Citation11. The disadvantages faced during the synthesis of benzimidazole and its derivatives have made it an urgent necessity to develop new methods/protocols, which would eliminate many of the drawbacks of existing synthetic protocols and may meet the requirements of green chemistry to protect human health and also the environment. In this context, microwave-assisted and ionic liquid-mediated synthesis of benzimidazoles is reported by Pietro Monforte and coworkers and Ashok Yadav et al., respectively Citation12 Citation13.
Indeed, multicomponent reactions have recently attracted a considerable attention in organic synthesis owing to their ability to produce the target products in a single operation without isolating the intermediates. They not only reduce the reaction time and energy but also reduce the waste product generation Citation14–16. In the quest of selecting a promising, sustainable catalyst for multicomponent reactions, heterogeneous catalysts provide an efficient and a promising avenue toward realization of high yields of product pertinent to their inherent environmental and eco-friendly nature Citation17 Citation18.
Thus, considering the above reports, advantages and applications of silica-supported sodium hydrogen sulfate as a heterogeneous catalyst, and as part of our ongoing project to explore green methodologies for the synthesis of bioactive heterocyclic compounds Citation19–21, herein we report the effective and practical one-pot synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazoles via the simple three-component condensation of 1,2-phenylenediamine, 2-mercaptoacetic acid, and aromatic aldehydes using catalytic amount of silica-supported sodium hydrogen sulfate, which served as heterogeneous catalyst (). The catalyst can easily be prepared Citation22 from readily available inexpensive ingredients like NaHSO4.and properly activated silica gel (finer than 200 mesh). The mild reaction conditions and simple experimental procedure offer a better alternative to the existing methods.
Results and discussion
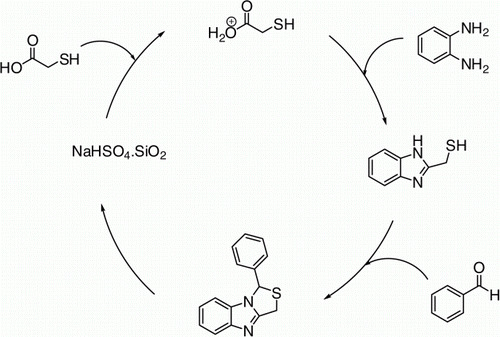
With a viewpoint to accomplish the best reaction condition, the initial efforts were devoted toward the investigation of an appropriate and efficient catalyst for the three-component synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole. In order to observe the need of catalyst, we had run two sets of reactions, namely, control and test. In control set of reaction, o-phenylenediamine, 2-mercaptoacetic acid, and benzaldehyde in acetonitrile were reacted without addition of any catalyst. While in the test sets of reaction, all of the same substrates as mentioned in control set were allowed to react in the presence of various catalysts, namely, Amberlyst-15, PMA-SiO2, mantmorillonite clay, KF-Al2O3, NaHSO4·SiO2, bismuth (III) salts, and Indion 190 resin. In the control set of reaction only trace yield was observed after 20 h at 75 °C, while in the test sets of reaction the yields obtained were in the range of 43–93%. During screening for efficiency considered in terms of product yield, NaHSO4·SiO2 was found to be the most efficient catalyst with 93% of product yield. This high yield of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole is presumably due to the ability of NaHSO4·SiO2 to elevate the formation of imidazole through protonation, which apparently enhances the rate of reaction ( and ).
Table 1. Synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole using various catalysts.a
Further to elucidate the effect of solvents, reactions were carried out in various solvents in the presence of NaHSO4·SiO2 as the catalyst. The control reaction was set in a neat condition. After completion of reactions, the organic solvents were removed under reduced pressure. The dichloromethane was added in the reaction mass and filtered off. The residue was further washed twice with the dichloromethane. The combined dichloromethane filtrate on evaporation afforded the product in the range of 40–93%. To our delight, best result was observed with acetonitrile ().
Table 2. Effect of solvents on preparation of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole.a
We observed that catalyst concentration also plays a pivotal role in the synthesis of 1H,3H-thiazolo[3,4-a]benzimidazoles. After experimenting with various concentrations of NaHSO4·SiO2, we got optimum yield of product with 200 mg of catalyst (). On further increasing the amount of catalyst, the yield of corresponding product decreased, ascribable to the increased acidity.
Table 3. Optimization of the amount of NaHSO4·SiO2 for the synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole.a
Furthermore, temperature also played a crucial role in the synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole. At 30 °C, only 40% conversion was observed. On subsequent rises in temperature the percentage yields of product were as follows: at 50 °C, 65%; at 75 °C, 93%, respectively ().
Table 4. Optimization of temperature for the synthesis of 1-aryl-1H, 3H-thiazolo[3,4-a]benzimidazole.a
All the aforementioned results revealed that NaHSO4·SiO2 in acetonitrile is the best system for the synthesis of 1-aryl-1H, 3H-thiazolo[3,4-a]benzimidazoles. To investigate the generality and feasibility of the protocol, we treated diverse substituted aldehydes and o-phenylenediamines with 2-mercaptoacetic acid. Good to high yields of the products were obtained. The optimized results are summarized in . Electron-releasing group on aldehyde and electron-withdrawing group on amine reduced the rate as well as yield of reaction. Products were characterized by 1H-NMR, 13C NMR, mass, and physical constant. Physical and spectral data of known compounds are in agreement with those reported in the literature Citation12 Citation13.
Table 5. Synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole.a
We emphasized on studying the recyclability and reusability of the catalyst so that our protocol can become more environment-friendly method and thus could belong to the domain of green chemistry methods. Upon the completion of the reaction, the catalyst was separated by filtration, further washed twice with dichloromethane and dried first under vacuum and then in oven. The activated catalyst was used for two more subsequent cycles. Interestingly, consistent performance of the catalyst was observed in all the cycles (Table 6).
Table 6. Reusability of the catalyst.a
Experimental
All commercial reagents were used as received without further purification, and all solvents were of reagent grade. The reaction was monitored by TLC using 0.25-mm E-Merck silica gel 60 F254 precoated plates, which were visualized using UV light. Melting points were measured in open capillaries. The IR spectra were recorded on a PerkinElmer 257 spectrometer using KBr disks. 1H NMR and 13C NMR spectra were recorded on a VXR-300 MHz instrument using TMS as an internal standard.
General experimental procedure
To a mixture of an o-phenylenediamine (1 mmol), 2-mercaptoacetic acid (1 mmol) and aromatic aldehydes (1 mmol) in MeCN (5 mL) NaHSO4·SiO2 (200 mg) was added and then stirred at 75 °C for 1 h. After cooling to room temperature, the reaction mixture was filtered and the residue was washed thoroughly with dichloromethane. The catalyst was recovered from the residue. The filtrate was concentrated under reduced pressure to isolate the crude product which was purified by column chromatography over silica gel using hexane-DCM (7:3) as eluent and eventually recrystalized using ethanol.
Representative spectral data
1–(2/,6/-Dichlorophenyl)-1H 3H-thiazolo[3,4-a]benzimidazole(4a): 1H NMR (DMSO-d 6): 4.21 (d, J=14.1 Hz, 1H, H3), 4.54 (dd, J=1.75 and 14.2 Hz, 1H, H3), 6.63 (s, 1H, H1), 6.6–7.71 (m, 7H, Ar-H) ppm; 13C NMR (DMSO-d 6): 162, 159.8, 159, 158.6, 156.2, 152, 149, 137.4, 135.1, 133, 126.5, 125, 123.3, 76, 70 ppm; IR (KBr):1617, 772, 707 cm−1; MS: (M + 2): 323.2.
Conclusion
In this study, we have investigated a novel, simple, and efficient protocol for the synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole derivatives via cyclocondensation reaction of o-phenylenediamine, 2-mercaptoacetic acid, and aromatic aldehydes using NaHSO4·SiO2 as a catalyst. The method is associated with the benefits derived from multicomponent reaction and the application of a heterogeneous catalyst. We feel this eco-friendly and economically viable catalyst will find practical utility for the one-pot synthesis of various 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazole derivatives.
Acknowledgements
The authors are grateful to the University of Mumbai for financial support. The authors thank Dr. S.T. Gadade, Principal, Changu Kana Thakur College, for providing laboratory and other facilities.
References
- Yildiz-Oren I , Yalcin I , Aki-Sener E , Carturk N . Eur. J. Med. Chem. Synthesis and structure-activity relationships of new antimicrobial active multisubstituted benzazole derivatives . 2004 ; 39 : 291 – 298 .
- Yamato M . J. Pharm. Soc. Jpn. Study on the Development of Biological-Active Compounds after the Model of Natural Products. 1992 ; 112 : 81 – 99 .
- Benazzouz A , Boraud T , Dubedat P , Boireu A , Stutzmann JM , Gross C . Eur. J. Pharmacol. Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: a pilot study. 1995 ; 284 : 299 – 307 .
- Kumar D , Jacob MR , Reynolds MB , Kerwin SM . Bioorg. Med. Chem . 2002 ; 10 : 3997 – 4004 . doi: 10.1016/S0968-0896(02)00327-9
- Evans DA , Sacks CE , Kleschick WA , Taber TR . J. Am. Chem. Soc. Polyether Antibiotics Synthesis. The Total Synthesis and Absolute Configuration of the Ionophore A-23187 . 1979 ; 101 : 6789 – 6791 .
- So YH , Heeschem JP . J. Org. Chem. Mechanism of Polyphosphoric Acid and Phosphorus Pentoxide–Methanesulfonic Acid as Synthetic Reagents for Benzoxazole Formation . 1997 ; 62 : 3552 – 3561 .
- Villemin D , Hammadi M , Martin B . Synth. Commun. Clay Catalysis: Condensation of Orthoesters with O-Substituted Aminoaromatics into Heterocycles . 1996 ; 26 : 2895 – 2899 .
- Terashima M , Ishii M . Synthesis. A facile synthesis of 2-substituted benzoxazoles 1982 ; 484 .
- Hein DW , Alheim RJ , Leavitt J . J. Am. Chem. Soc. The Use of Polyphosphoric Acid in the Synthesis of 2-Aryl- and 2-Alkyl-substituted Benzimidazoles, Benzoxazoles and Benzothiazoles . 1957 ; 79 : 427 – 429 .
- Salehi P , Dabiri M , Zolfigol MA , Otokesh S , Baghbanzadeh M . Tetrahedron Lett. Selective synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles in water at ambient temperature . 2006 ; 47 : 2557 – 2560 .
- Chimirri A , Monforte P , Musumeci L , Rao A , Zappala M , Monforte A . Arch. Pharm. Synthesis and antitumour activity of 1H,3H-thiazolo[3,4-a]benzimidazole derivatives . 2001 ; 334 : 203 – 208 .
- Rao A , Chimirri A , Ferro S , Monforte A , Monforte P , Zappala M . Arkivoc. Microwave-assisted synthesis of benzimidazole and thiazolidinone derivatives as HIV-1 RT inhibitors . 2004 ; V : 147 – 155 .
- Yadav AK , Kumar M , Yadav T , Jain R . Tetrahedron Lett. An ionic liquid mediated one-pot synthesis of substituted thiazolidinones and benzimidazoles . 2009 ; 50 : 5031 – 5034 .
- Bärfacker L , Buss C , Hollmann C , Kitsos-Rzychon BE , Kranemann CL , Rische T , Roggenbuck R , Eilbracht P , Schimdt A . Chem. Rev. Tandem Reaction Sequences under Hydroformylation Conditions: New Synthetic Applications of Transition Metal Catalysis . 1999 ; 99 : 3329 – 3366 .
- Montgomery J . Acc. Chem. Res . 2000 ; 33 : 467 – 473 . doi: 10.1021/ar990095d
- Domling A , Ugi I . Angew. Chem. Int. Ed. Engl. Multicomponent Reactions with Isocyanides . 2000 ; 39 : 3168 – 3210 .
- Ramesh C , Ravindranath N , Das B . J. Org. Chem. Simple, Efficient, and Selective Deprotection of Phenolic Methoxymethyl Ethers Using Silica-Supported Sodium Hydrogen Sulfate as a Heterogeneous Catalyst . 2003 ; 68 : 7101 – 7103 .
- Das B , Venkateswarlu K , Mahender G , Mahender I . Tetrahedron Lett. A simple and efficient method for α-bromination of carbonyl compounds using N-bromosuccinimide in the presence of silica-supported sodium hydrogen sulfate as a heterogeneous catalyst . 2005 ; 46 : 3041 – 3044 .
- Chaskar A , Padalkar V , Phatangare K , Patil K , Bodkhe A , Langi B . Appl. Catal. A: Gen. Heteropoly Acids as a Useful Recyclable Heterogeneous Catalyst for the Facile and Highly Efficient Aza-Cope rearrangement of N-allylanilines . 2009 ; 359 : 84 – 87 .
- Phatangare K , Padalkar V , Mhatre D , Patil K , Chaskar A . Synth. Commun . 2009 ; 39 : 4117 – 4121 . doi: 10.1080/00397910902885533
- Gawand P , Deokar H , Langi B , Yadav A , Chaskar A . Synth. Commun . 2009 ; 39 : 4171 – 4179 . doi: 10.1080/00397910902885541
- Breton GW . J. Org. Chem. Selective Monoacetylation of Unsymmetrical Diols Catalyzed by Silica Gel-Supported Sodium Hydrogen Sulfate . 1997 ; 62 : 8952 .