Abstract
The results of the studies carried out in our laboratories during the last 15 years, aimed at developing green methodologies for the synthesis of polyfunctionalized heteroaromatic substances, are surveyed. The results of the investigations demonstrate that green methodologies are not only less hazard than classical preparative methods but they also are more efficient and economical. For example, short reaction times and higher yields are observed for reactions in which conventional heating is replaced by microwave or ultrasound irradiation. The implementation of multicomponent reactions in green preparative routes also reduces the cost of carrying out the reactions because multiple separation and crystallization steps are avoided. In general, by employing the new green methodologies we have been able to produce a large number of polyfunctional aromatic substances in a highly efficient manner.
Keywords:
Introduction
As a result of pollution prevention legislation in the USA in 1990 Citation1, a green chemistry campaign was initiated so that organic substances could be produced in an environmentally clean manner. The basic principles guiding this new synthetic strategy are simply summarized in the following goals:
| 1. | Reducing potential waste production by utilizing atom economical processes, which minimize the use of solvents. | ||||
| 2. | Reducing the use of hazardous reagents or the production of hazardous by-products. | ||||
| 3. | Utilizing energy economically and looking at energy sources that do not rely on fossil fuel combustion. | ||||
| 4. | Minimizing time so that energy consumption is reduced. | ||||
| 5. | Maximizing yields by utilizing catalysts when appropriate. | ||||
Owing to the fact that the 12 principles of green chemistry have been reviewed several times recently Citation2–5, they will not be elaborated in detail here. Instead, the review below focuses only studies carried out in our group that were targeted at developing green methodologies for the preparation of heterocyclic compounds.
Synthetic approaches
Solvent-free reactions
Long before the recognition and development of green chemistry concepts, we utilized methodologies employing solvent-free reactions for the production of 1,2,4-triazole carboxamides Citation6–8, recently patented as cannabinoid receptor blockers Citation9.
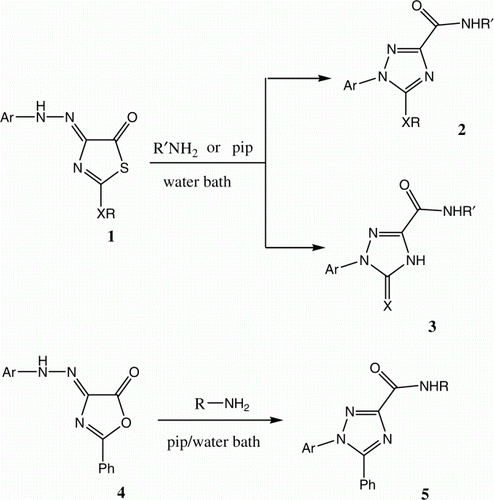
The goal of our more recent efforts was to design economical reactions. We observed that mixtures of 1 and a variety of amines when stirred in refluxing water yield either 2 or 3 depend on the nature of the amine utilized Citation6–8. Recently we have also observed that 4 undergoes rearrangement to form 5 under these reaction conditions ( and ) Citation10.
Table 1. Yields of compounds 2a–c and 3a–c.
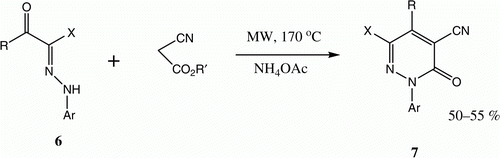
In the past decade, we have also described the conversion of 6 to 7 via reaction with ethyl cyanoacetate in the presence of a catalytic amount of both AcOH and ammonium acetate. This finding opens a new route in which 7 is employed as a precursor in generation of condensed pyridazines () Citation11.
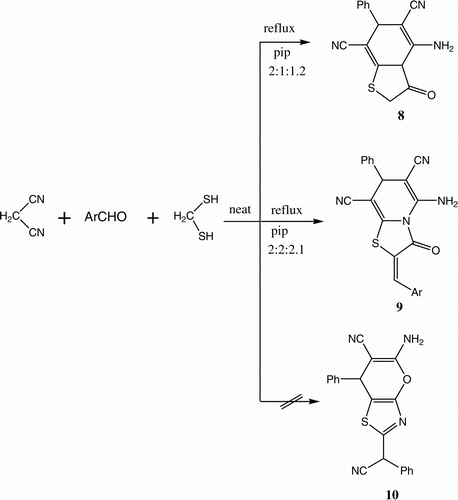
Recently, a green, highly efficient, three-component process for the synthesis of thiazolo[3,2-a]pyridine derivatives 8 and 9 was described. Accordingly, reactions were observed to occur between malononitrile, aromatic aldehydes, and 2-mercaptoacetic acid in respective molar ratios of 2:2:1.2 and 2:2:2.1 and in the presence of catalytic amounts of piperidine in the absence of solvent to produce 8 and 9 ( and ) Citation12. The structures of the products of these reactions were established using HMBC and heteronuclear multi-quantum correlation (HMQC) techniques. The findings contrast to the reported formation of pyronothiazoles 10 in similar reactions Citation13.
Table 2. Yields of compounds 8 and 9a–c.
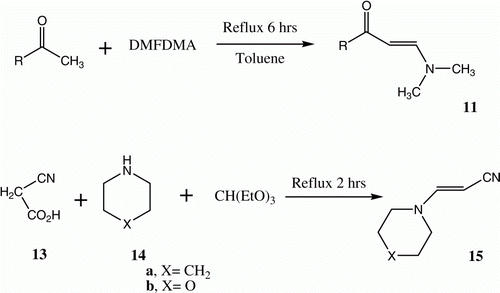
Enaminones 11 are produced via reactions of methyl ketones with dimethylformamide dimethylacetal (DMFDMA) Citation14 in refluxing toluene Citation15, but the yields of these processes are rather poor. Heating methyl ketones with DMFDMA in absence of solvent for 4–8 h, however, leads to the formation of 11 in much improved yields () Citation14. A similar methodology has been developed for the synthesis of 15 from 10, triethylorthoformate and morpholine or piperidine 14a,b () Citation16.
Utilization of microwaves and ultrasound as energy sources
Since Gidey and Gidey described Citation17 the utility of microwave irradiation produced in domestic ovens as an energy source for conducting organic reactions in short time periods, the utility of this technique in organic synthesis has gained great attention Citation17 Citation18. This is especially true when microwave ovens that are specially designed for chemical reactions were constructed.
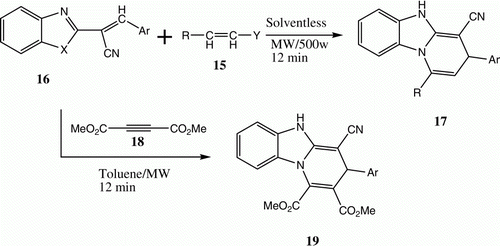
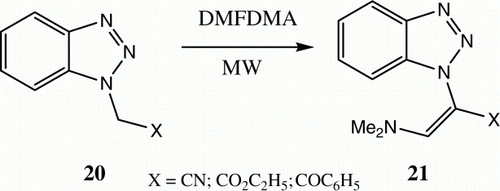
Our interest in adopting microwaves as energy sources started in 1997, when we observed that benzo[g]imidazo[1,2-a]pyridines could be synthesized via reactions of arylidene-1H-benzimidazole-2-acetonitriles 16 with different electron poor olefins and diethyl acetylenedicarboxylate under microwave heating ( and ) Citation19. In 2003, we reported the synthesis of 21 from the reaction of 20 and DMFDMA utilizing this technique Citation20 ().
Table 3. Yields of compounds 17a–d and 19a–b.
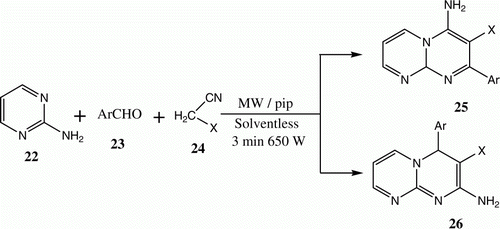
In connection with our interest in applying green methodologies to the synthesis of condensed azines, we developed a novel and efficient route for the preparation of pyrimido[1,2-a]pyrimidines using microwave irradiation. Thus, subjecting equimolecular mixtures of 2-aminopyrimidine 22, aromatic aldehydes 23, and active nitriles 24 in the presence of piperidine as a catalyst to microwave irradiation leads to the formation of either 4-amino isomer 25 or 2-amino isomer 26 ( and ) Citation21, depending on the active methylene compound employed. In this study, we employed the microwave organic reaction enhancement technology (MORE technology) Citation22 in a domestic oven. Moreover, we noted several difficulties with reproducing results and also hazards caused by solvent evaporation. Consequently, we instead began to utilize the controlled microwave heating technique.
Table 4. Yields of compounds 25a-d and 26a-b.
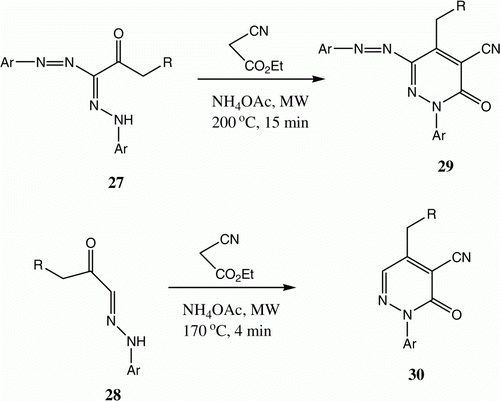
We have described the syntheses of 7 from 6 in shorter times and improved yields using a microwave lab station. Also, we observed that 27 and 28 can be converted to 29 and 30, respectively, by heating under controlled microwave conditions ( and ) Citation20 Citation23–25.
Table 5. Yields of compounds 29a–b and 30.
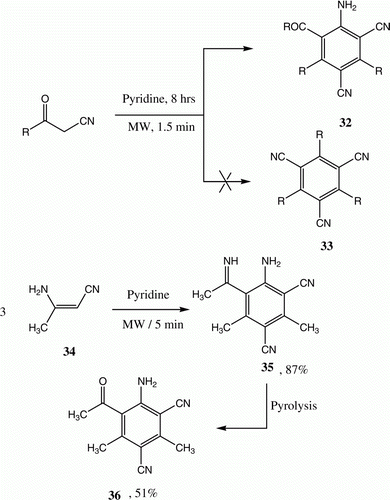
Benzoylacetonitrile 31 has long Citation26 been known to yield the self-tricondensation product 32 upon refluxing in pyridine. Abdelrazek and Michael Citation27 have subsequently reported that 33 is actually the reaction product.
Similarly, heating 3-aminocrotononitrile 34 in pyridine leads to the formation of the aniline derivative 35 that undergoes hydrolysis to afford 36 Citation26. We reinvestigated this process and found that indeed 32 is the product formed. Moreover, instead of requiring the reaction to be carried out in refluxing pyridine for at least 5 h Citation26, we found that heating neat 19 in a microwave oven for 5 min afforded 32 Citation26 ( and ).
Table 6. Yields of compounds 32a–c.
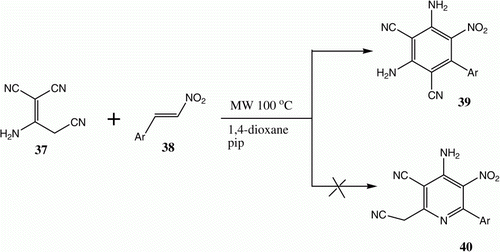
Recently, a novel, simple, and efficient method for the synthesis of polysubstituted diaminobenzonitriles has been developed in our group. The process involves the reaction of 1,1,3-tricyano-2-aminopropionitrile with nitro olefins under controlled microwave irradiation conditions. Thus, when equimolar amounts of 37 and (E)-2-nitrophenylarenes 38 in dioxane are irradiated using a microwave lab station at 100 °C in the presence of piperidine (2 drops), the corresponding polysubstituted diaminobenzonitriles 39 are generated rather than the corresponding 2-cyanomethylpyridine derivatives 40. The structures of 39 were established using 1H NMR and 13C NMR spectroscopic analysis ( and ) Citation27.
Table 7. Yields of compounds 39a–f.
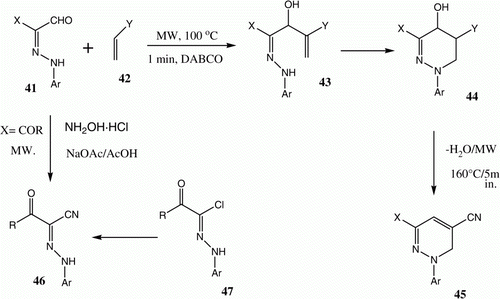
Microwave promoted heating mixtures of 2-arylhydrazonals 41 with acrylonitrile 42a or methyl vinylketone 42b in the presence of 1,4-diazabicyclo[2,2,2]octane (DABCO) for 5 min in the absence of solvent results in the formation of 45 via intermediates 43 and 44 that can be isolated in some cases () Citation28 Citation29. Reactions 41 (X=COR) with hydroxylamine hydrochloride in acetic acid in presence of sodium acetate under microwave irradiation conditions afforded 46 Citation30, which is an ecofriendly route to this substance that serves as an alternative to Shawali et al.'s synthesis Citation31, which utilizes the reaction of 47 with cyanide ion, or the use of Elnagdi's coupling method of aroylacetonitrile with aryldiazonium salts Citation32 (). Notably, this reaction can be conducted on a 0.01 mol scale giving a reported yield of 93% for 45.
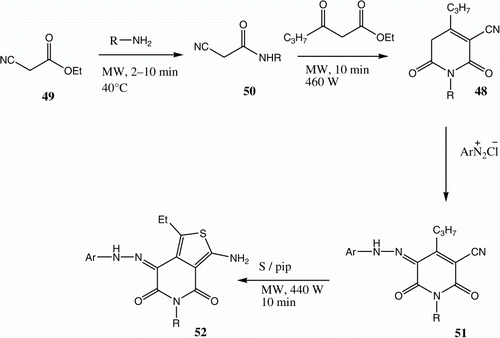
Recently Al-Zaydi et al. Citation33 described a procedure for the formation of pyridines 48 that involves microwave or ultrasound irradiation of a mixture of 49 and an amine followed by treatment of the intermediate 50 with ethyl cyanoacetate also under either microwave or ultrasound irradiation (). Generally, microwave irradiation proved to be more efficient in producing the desired pyridines in higher yields. Pyridones 48 couple with aromatic diazonium salts to yield arylhydrazones 51, which react with elemental sulfur in the presence of piperidine, either under conventional heating or microwave or ultrasound irradiation conditions, to yield thienopyridines 52. Again, the use of microwave and ultrasound irradiation requires less time to bring reactions to completion. Microwave reactions were observed to proceed faster than those promoted by ultrasound ( and ) Citation33. The structure of compound 52a was assigned by using X-ray crystallography.
Table 8. Yields of compounds 52a–d.
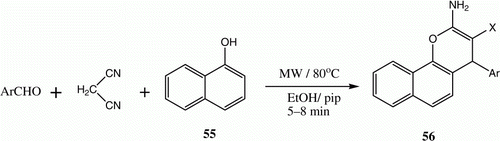
Substituted 2-amino-2-chromenes 56 have received considerable attention owing to their importance as pigments, agrochemicals, and cosmetics Citation34 Citation35. The synthesis of 2-amino-2-chromenes using conventional heating techniques requires prolonged times, and products are formed in only moderate yields. In contrast, an efficient, high-yielding three-component synthesis of these target molecules involves the utilization of controlled microwave heating of aldehydes with active methylene nitriles and α-naphthol 55 in ethanol containing a catalytic amount of piperidine at 80 °C for 5–8 min ( and ) Citation36.
Table 9. Yields of compounds 56a–d.
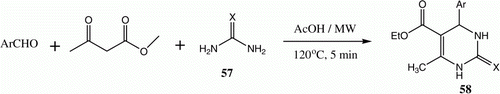
We also developed a simple and efficient method for carrying out a one-pot three-component synthesis of the Biginelli 4-aryl-3,4-dihydropyrimidine-2(1H)-ones 58 through reaction of aldehydes, ethyl acetoacetate, and urea or thiourea under controlled microwave heating conditions ( and ) Citation37.
Table 10. Yields of compounds 58a–f.
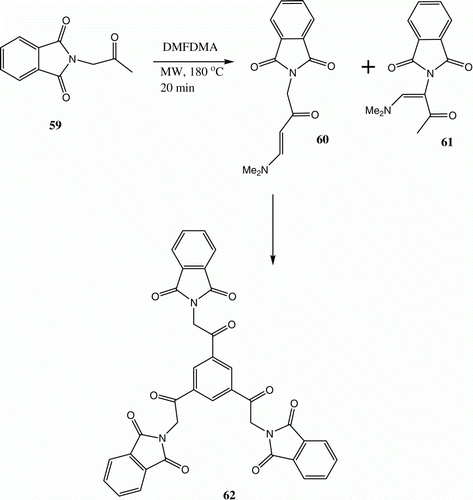
Under microwave irradiation conditions, 59 reacts to afford a mixture of 60 and 61, of which the former undergoes self-condensation to yield 62 upon microwave irradiation in an acetic acid solution in the presence of acidic zeolite () Citation38.
Solar thermal energy as an energy source
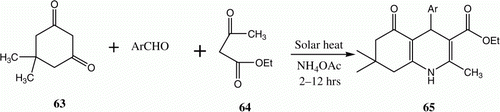
A great demand now exists for alternative and freely available clean energy sources. An important indicator of the greenness of a chemical reaction is found in its energy efficiency (the cost and environmental friendliness of the energy, and its applicability on a large scale). Solar energy is essentially free and, because its use does not require raw materials, no pollutants are produced. Thus, solar energy can be regarded as a clean reagent Citation39. A description of our first utilization of solar thermochemical energy in organic synthesis was published in 2008. The process involved a four-component synthesis of polyhydroquinoline derivative 65 via the reaction of dimedone 63, aromatic aldehydes, ethyl acetoacetate 64, and ammonium acetate employing solar heating ( and ) Citation40.
Table 11. Yields of compounds 65a–g.
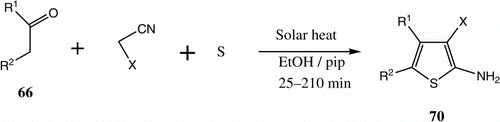
Green conditions have been developed for the synthesis of substituted 2-aminothiophenes 70, employing multicomponent reactions of a ketone 66 with active methylene nitrile and elemental sulfur induced by solar thermal energy. The procedure proved to be efficient and simple in terms of conducting the reaction and isolating products ( and ) Citation41.
Table 12. Yields of compounds 70a–f.
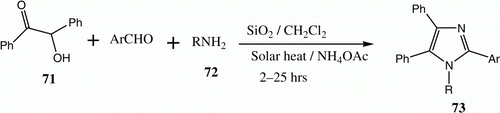
The tetra-substituted imidazole scaffold is a core component of many biological systems as well as several natural products Citation42. A number of synthetic approaches for the construction of this scaffold have been published, but all involve the use of high temperatures, expensive metal precursors, and catalysts that may be harmful to the environment. We observed that exposing a solution of benzoin 71, aromatic aldehydes, aromatic amines 72, and ammonium acetate in dichloromethane in the presence of catalytic amount of high surface area SiO2 to direct sunlight for 2–25 h leads to the formation of the corresponding tetra-substituted imidazoles 73 in excellent yields ( and ) Citation42.
Table 13. Yields of compounds 73a–f.
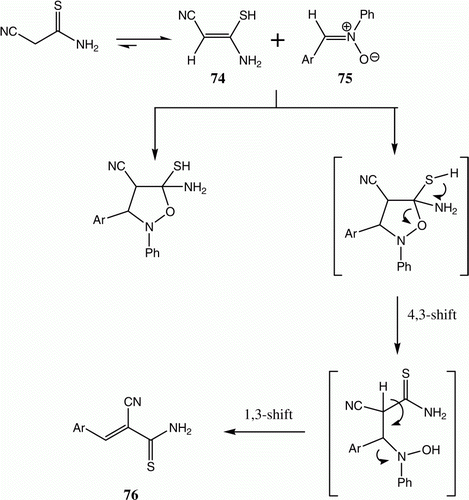
A novel synthesis of 2-cyano-3-aryl-2-enthioamides 76 has been recently developed in our laboratories. We observed that reactions of cyanothioacetamide 74 with nitrones 75 in EtOH under solar thermal energy conditions afford 76 in good yields. The reaction of 74 with diphenylnitrone furnished the corresponding 2-cyano-3-phenylprop-2-ene 76a. It is worth mentioning that 76a has not previously isolated as a solid product. A mechanism that accounts for the formation of 76 is presented in () Citation43.
Table 14. Yields of compounds 76a–f.
Utility of heterogeneous acid and base catalysis to replace homogenous alternatives
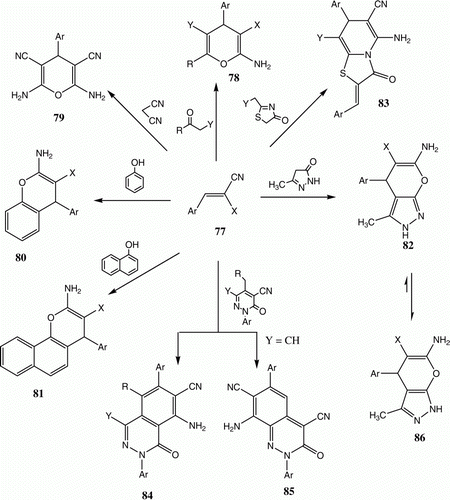
In the 1970s and 1980s, we extensively investigated the reactivity of functionally substituted cinnamonitriles 77 toward active methylene compounds, electron rich aromatics, azolones, and alkyl heteroaromatic carbonitriles in the presence of homogeneous base catalysts, usually piperidine or pyridine Citation44–56. This effort led to the preparation of a variety of pyrans 78, thiopyrans 79, benzopyrans 80, naphthopyrans 81, pyranoazoles 82, thiazolopyridines 83, phthaloazinones 84, and cinnolines 85. Several of the compounds synthesized in this effort showed interesting biological activities, which have been reported in the literature Citation57 Citation58 and described in patents Citation59 Citation60. As a consequence, synthetic approaches to these types of compounds have attracted much attention recently () Citation61–64.
In 2008, we reported on utility of chitosan, a naturally occurring biopolymer obtained by treating chitin with strong alkali, as a heterogeneous catalyst for these reactions Citation65. It occurred to us that it might be valuable to probe in detail the actual structures of some of these products, which have been questioned Citation67 Citation68. Chitosan is hydrophilic basic catalyst and, as such, it has been used successfully as a catalyst for Michael addition reactions () Citation69. It was observed that reactions of 77 (X=CN) with acetoacetic ester and malononitrile in the presence of chitosan afford 78 and 79, respectively, in almost the same yields described for reactions in the presence of piperidine.
Similarly, reactions of this substance with phenols, naphthols, and pyrazolone afforded the respective products 80, 81, and 82. The latter product was incorrectly assigned as 86 earlier. The reaction of 77 with thiazolylacetonitriles and ethyl thiazolylacetate afforded 83 as expected despite claims to the contrary Citation67 (). Pyridazinylcarbonitriles (Y=CO2Et) afforded 84 whereas when Y=CN 85 was formed (). All of the observed yields are similar to those given in the original reports Citation69.
Table 15. Yields of compounds 80, 81 and 83.
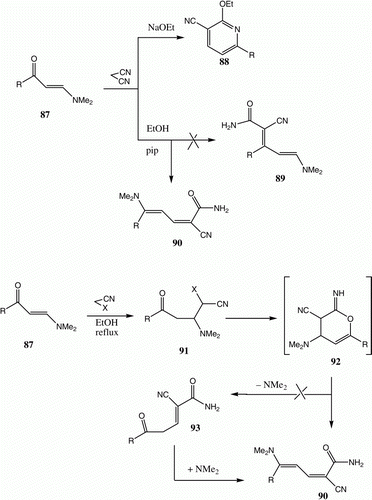
We have also utilized chitosan as base catalyst to promote addition reactions of malononitrile and ethyl cyanoacetate to form the enaminone 87. Initially, Al-Omran et al. reported that 88 is the product of the reaction of these substances promoted by ethanolic sodium ethoxide, but suggested that 89 is produced when the reaction occurred in ethanolic piperidine ( and ) Citation70. Utilizing both 15N NMR, HMBC NMR methods Citation71 Citation72, and a subsequent X-ray crystal structural analysis, we demonstrated that the reaction product is really 90. It is quite strange that the results of our work were available in open-access journals at least a year before the report of similar results by Abdelrazek et al. Citation68.
Table 16. Yields of compounds 90a–c.
We have recently found that 87 reacts not only with malononitrile but also with ethyl cyanoacetate and benzoylacetonitrile to yield dienamides, which are believed to be formed via intermediates 91, 92, and 93. The possibility that formation of 92 takes place, which then reacts further by elimination of dimethylamine, was excluded based on experimental observations () Citation73.
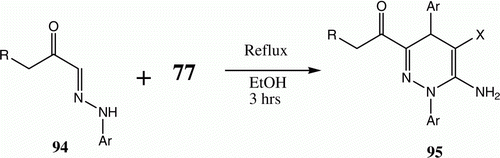
Chitosan has also been used as catalyst for the addition of 94 to 77 yielding 95, thus replacing piperidine reported in the initial paper as the catalyst of choice ( and ) Citation74 Citation75.
Table 17. Yields of compounds 95a–d.
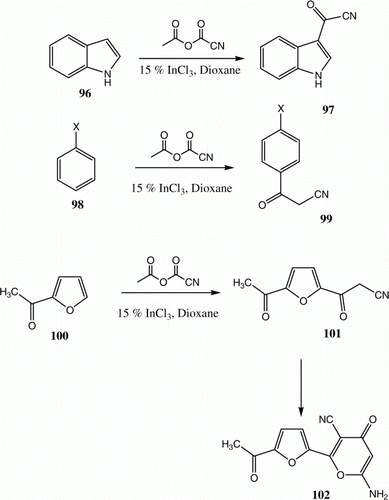
Eco-friendly, solid Lewis acid catalysts as well as ionic liquids have also been employed as replacement for their noneco-friendly counterparts. In 2007 we reported Citation76 the utilization of 97, prepared by the reaction of indole 96 with cyanoacetic acid/acetic anhydride mixture following the procedure reported by Slatt et al. Citation77 as a building block for the synthesis of substituted indoles. This approach appeared to us to be an interesting route to 3-oxoalkanomitriles. However, less electron-rich aromatics failed to undergo the reaction employing the conditions developed. We envisioned that InCl3 would act as an efficient catalyst for this condensation and, indeed, this catalyst did promote the reaction of 98 to form oxoalanonitriles 99. Also, 101 and 102 have been produced from 100 in 70% yields () Citation78.
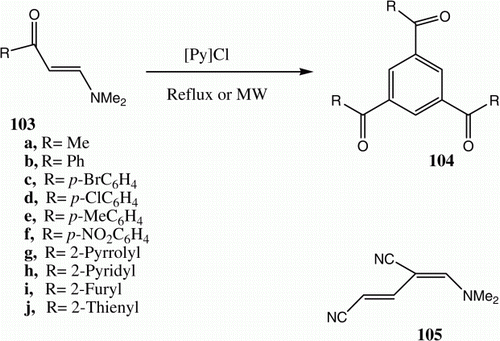
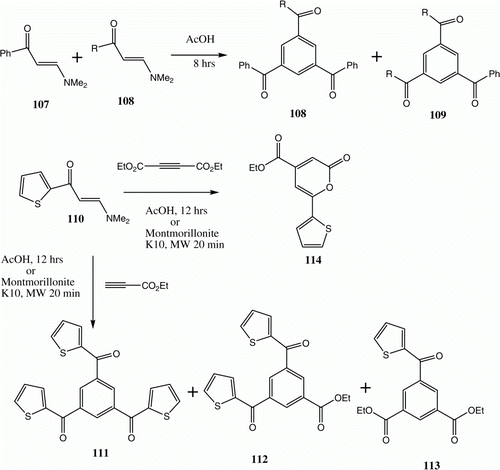
Abdel-Khalik and Elnagdi Citation79 reported that enaminones 103a are converted into 1,2,5-triaroylbenzenes 104a in refluxing in acetic acid (). Subsequently Makhseed et al. Citation80 extended this approach to the synthesis of benzene-1,3,5-trials 104 (R=H) and triethylbenzene-1,3,5-tricarboxylates 104 (R=OH). Recently Al-Zaydi et al. were able to affect the conversion of 103d to 104d more efficiently using pyridine hydrochloride as an ionic liquid and MW as the energy source () Citation81. Although 103e in refluxing acetic acid reacts to generate 105 only, when the process is carried out in the presence of pyridinium hydrochloride 104e is formed Citation81. Very recently, Al-Mousawi et al. showed that these conversions can be affected by fusing enaminones in the presence of montmorillonite K10. Moreover, these workers provided evidence that the reaction takes place in a stepwise manner. Specifically, the results of crossover experiments showed that mixtures of 106 and 107 afford both 108 and 109. In addition, the reaction of 110 with ethyl propiolate was shown to afford a mixture of 111–113 () Citation14. On heating with dimethyl acetylenedicarboxylate in the presence of montmorillonite K10, 110 is converted to 114 ().
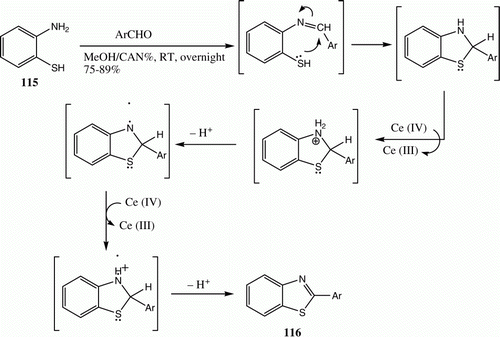
Ceric (IV) ammonium nitrate (CAN) is a convenient and widely used reagent for affecting a broad range of reactions owing to advantages that include solubility in water and various organic solvents, inexpensiveness, eco-friendly nature, uncomplicated handling, fast conversions, and convenient work up procedure. We have initiated a program aimed at exploring the potential of CAN in organic transformation. This effort led to the development of a one-pot CAN-catalyzed synthesis of 2-arylthiazoloes 116 from the reaction of 2-aminothiophenol 115 and aromatic aldehydes ( and ) Citation82.
Table 18. Yields of compounds 116a–h.
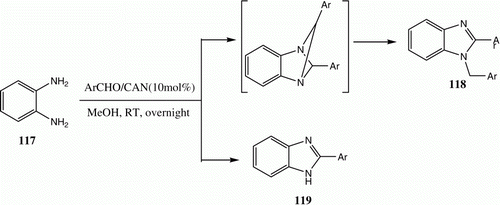
The reactions of o-phenylenediamine 117 with aromatic aldehydes in MeOH at room temperature, catalyzed by Cerium (IV) ammonium nitrate (CAN), afford either 118 and/or 119. The results clearly show that this process proceeds mainly via two different routes. The first involves 1:1 condensation followed by oxidation to afford 2-aryl-1-substituted-1H-benzimidazoles. The second process is a 1:2 condensation that generates 2-aryl-1-arylmethyl-1H-benzimidazoles. Thus, in these reactions both of the expected products are obtained in ratios that depend on nature of the solvent. For example, in methanol both 118 and 119 are obtained ( and ) Citation83.
Table 19. Yields of compounds 118a–c and 119a–b.
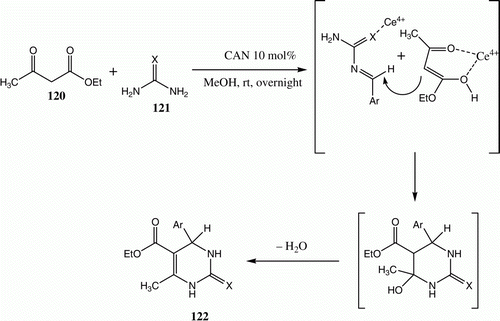
Although extensively utilized as a one-electron oxidant Citation84, CAN's use as a Lewis acid catalyst in C–N bond forming reactions leading to heterocyclic compounds is somehow limited Citation85. We recently observed that a simple and highly efficient procedure for conducting the Biginelli condensation reaction of aldehydes, β-ketoesters 120, urea, or thiourea 121a,b at ambient temperature involves the use of CAN as a Lewis-acid catalyst. A mechanism to account for the formation of the products is shown in () Citation86.
Table 20. Yields of compounds 122a–g.
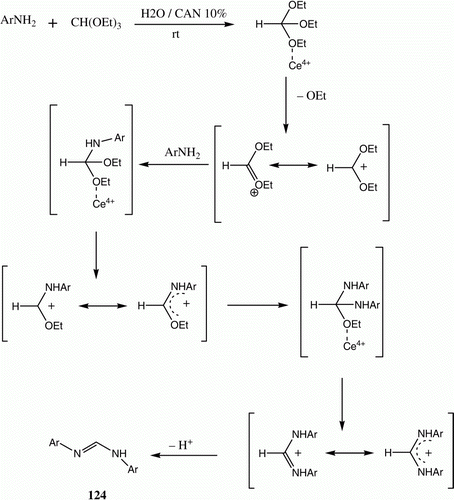
N,N-diaryl-formamidines are of considerable interest in fields related to organic and medicinal chemistry. Exchange reactions of N,N -dimethylformamidines or acetamidines by a variety of amines have been reported to be the most popular method for the synthesis of symmetrical and unsymmetrical formamidines. We have developed a simple and green route for the synthesis of N,N′-diaryl-formamidines 124 via the reaction of aromatic amines with triethylorthoformate 123 in water at room temperature catalyzed by CAN acting as a Lewis acid catalyst ( and ) Citation87.
Table 21. Yields of compounds 124a–h.
Greener synthetic approaches
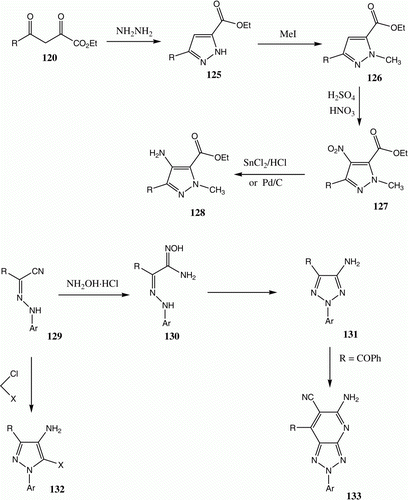
4-Aminopyrazoles-5-carboxylic acid derivatives are interesting intermediates in the synthesis of pharmaceuticals. Moreover, reported synthetic approaches to these substances are rather inefficient hazardous and/or nonconcise Citation88. For example, 128, a precursor of Viagra, is prepared using a multistep sequence that includes production of potentially explosive nitropyrazoles by employing a hazardous nitration reaction mixture followed by a heavy metal reduction. Recently we described a direct synthesis of 132 through the reaction of 129 with functionally substituted alkyl halides (). Similarly 129 was converted to a Zaperinsate analog via reaction with NH4OH to yield intermediate 130 that then reacts with malononitrile to yield 133.
Conclusion
In investigations carried out over a 48-year period, our group has made contributions to green methodologies by providing greener approaches to several biologically interesting polyfunctional heteroaromatic compounds.
References
- Pavia DL , Lampman GM , Kriz GS , Engel RG . Introduction to organic laboratory techniques: a small scale approach. , 2nd ed .; 2005 . 254 .
- Anastas , PT and Warner , JC . 1998 . Green chemistry: theory and practice , New York (NY) : Oxford University Press .
- Mikami K. Green reaction media in organic synthesis . Blackwell ; 2005 .
- Matlack , AS . 2001 . Introduction to green chemistry , New York (NY) : Marcel Dekker .
- Roesky , HW and Kennepohl , DK . 2009 . Experiments in green and sustainable chemistry , 283 Weinheim : Wiley-VCH Verlag GmbH & Co. KGaA .
- Mustafa A , Harhash AH , Elnagdi MH , Abd El-All F. J Liebigs Ann der Chemie . 1971 ; 748 : 70 – 78 . doi: 10.1002/jlac.19717480108
- Mustafa A , Harhash AH , Elnagdi MH , Abd El-Al F. J Liebigs Ann der Chemie . 1971 ; 748 : 79 – 87 . doi: 10.1002/jlac.19717480109
- Asker W , Elnagdi MH , Fahmy SMJ. Prakt. Chemie 1971 ; 313 4 : 715 – 721 . doi: 10.1002/prac.19713130419
- Elnagdi MH. US patent , 0106614 . 2004 .
- Abdel-Megid M , Elnagdi MH , Negm AMJ. Heterocycl Chem . 2002 ; 39 1 : 105 – 108 . doi: 10.1002/jhet.5570390115
- Al-Saleh B , Hilmy NM , El-Apasery MA , Elnagdi MH. J Heterocycl. Chem . 2006 ; 43 6 : 1575 – 1581 . doi: 10.1002/jhet.5570430622
- Mekheimer RA , Hilmy NM , Abdel-Hameed A , Dacrory S , Sadek KU. Synth Commun . 2011 ; 41 : 2511 – 2516 . doi: 10.1080/00397911.2010.505700
- Abdelrazek FM , Michael FA , Mohamed AE. Arch Pharm . 2006 ; 339 : 305 – 312 . doi: 10.1002/ardp.200500259
- Al-Mousawi SM , Moustafa MS , Elnagdi MH. Green Chem Lett Rev . 2011 ; 4 2 : 185 – 193 . doi: 10.1080/17518253.2010.528049
- Tseng S , Esptein JW , Brbaander HJ , Francisco G. J Heterocycl Chem . 1987 ; 24 : 837 – 843 . doi: 10.1002/jhet.5570240357
- Al-Shiekh MA , El-Din AM , Salah H , Ebtisam A , Elnagdi MH. J Chem Res . (S) 2004 ; 3 : 174 – 179 . doi: 10.3184/0308234041640807
- Kappe CO , Dallinger D , Murphree SS. Practical microwave synthesis for organic chemistry strategies, instruments and protocols. , 1st ed .; 2009 . 296 .
- Kappe CO , Stadler A. Microwaves Org Med Chem 2005 ; 25 : 410 .
- Mekheimer R , Shaker RA , Sadek KU , Otto HH. Heterocycl Commun . 1997 ; 13 : 217 – 219 .
- Al-Saleh B , Abdelkhalik MM , El-Apasery MA , Elnagdi MH. J Heterocycl Chem 2003 40 1 : 171 – 175
- Abd El-Latif FM , Barsy AM , Aref AM , Sadek KU. Green Chem . 2002 ; 4 : 196 – 198 . doi: 10.1039/b110723m
- Loupy , A . 2006 . Microwaves in organic synthesis , 2nd ed 1 – 523 .
- Al-Mousawi SM , El-Apasery MA , Al-Kandery N , Elnagdi MH. J. Heterocycl. Chem . 2008 ; 45 2 : 359 – 364 . doi: 10.1002/jhet.5570450210
- El-Apasery , MA. J Appl Polym Sci . 2008 ; 109 : 695 – 699 . doi: 10.1002/app.28129
- Al-Mousawi SM , EL-Apasery MA , Elnagdi MH. Heterocycles 2008 ; 75 5 : 1151 – 1161 . doi: 10.3987/COM-07-11301
- Al-Awadi NA , Abdelkhalik MM , Abdelhamid IA , Elnagdi MH. Synlett 2007 ; 19 : 2979 – 2982 . doi: 10.1055/s-2007-992355
- Abdelrazek FM , Michael FA. J Heterocycl Chem . 2006 ; 43 1 : 7 – 10 . doi: 10.1002/jhet.5570430102
- Singh V , Batra S. Tetrahetdron 2008 ; 64 : 4511 – 4574 . doi: 10.1016/j.tet.2008.02.087
- Al-Awadi NA , Ibrahim MR , Al-Etaibi AM , Elnagdi MH. ARKIVOC 2011 ; ii : 310 – 321 .
- El-Dusouqui OME , Abdelkhalik MM , Al-Awadi NA , Dib HH , George BJ , Elnagdi MH. J Chem Res . (S) 2006 ; 5 : 295 – 302 . doi: 10.3184/030823406777410945
- Shawali AS , Abdelkader MH , Eltalbawy FMA. Tetrahedron 2002 ; 58 : 2875 – 2880 . doi: 10.1016/S0040-4020(02)00157-6
- Elnagdi MH , Elmoghayar MRH , Elgemeie GEH. Synthesis 1984 ; 1 : 1 – 26 . doi: 10.1055/s-1984-30717
- Al-Zaydi KM , Borik RM , Mekheimer RA , Elnagdi MH. Ultraso Sonochem . 2010 ; 17 5 : 909 – 915 . doi: 10.1016/j.ultsonch.2009.12.008
- Elis GP . In : Weisherger A , Taylor EC Chromenes, chromanes and chromanes . New York (NY) : John Wiley ; 1977 . Chapter 2 , 13 .
- Hafez EA , Elnagdi MH , Elagamy AA , El-Taweal FAR. Heterocycles 1987 ; 46 : 903 – 907 . doi: 10.3987/R-1987-04-0903
- Mekheimer RA , Sadek KU. J Heterocycl Chem . 2009 ; 46 : 149 – 152 . doi: 10.1002/jhet.13
- Mekheimer RA , Sadek KU. Arabian J Chem . 2008 ; 19 : 79 – 82 .
- Al-Mousawi SM , El-Apasery MA , Elnagdi MH. Molecules 2010 ; 15 : 58 – 67 . doi: 10.3390/molecules15010058
- Funken KH. Sol Energy Mater 1991 ; 24 : 370 – 375 . doi: 10.1016/0165-1633(91)90076-W
- Mekheimer RA , Abdel Hamed A , Sadek KU. Green Chem . 2008 ; 10 : 592 – 593 . doi: 10.1039/b715126h
- Mekheimer RA , Ameen RA , Sadek KU. Chin Chem Lett . 2008 ; 19 : 788 – 790 . doi: 10.1016/j.cclet.2008.04.041
- Mekheimer RA , Abdel Hamed A , Mansour SAA. Chin Chem Lett . 2009 ; 20 : 812 – 814 .
- Mekhamer RA , Al-Zaydi KM , Al-Shamary A , Sadek KU. Green Sustain Chem . 2011 ; 1 : 176 – 181 . doi: 10.4236/gsc.2011.14027
- El-Saka IA , El-Kousy SM , Kandil ZE. J Fur Prakt Chem . 1991 ; 333 : 345 – 350 . doi: 10.1002/prac.19913330222
- Abed NM , Hafez EAA , Elsakka I , Elnagdi MH. J Heterocycl Chem . 1984 ; 21 5 : 1261 – 1264 . doi: 10.1002/jhet.5570210503
- Harb AA , ElMaghraby AM , Metwally SA. Collect Czech Chem Commun . 1992 ; 57 : 1570 – 1574 . doi: 10.1135/cccc19921570
- Elmoghayer MRH , Khalifa MAE , Ibraheim MKA , Elnagdi MH. Monatsh Chem . 1982 ; 113 1 : 53 – 57 . doi: 10.1007/BF00814337
- Abed NM , Ibrahim NS , Elnagdi MHZ. Naturforsch . 1986 ; 41B 7 : 925 – 928 .
- Elnagdi MH , Ghozlan SAS , Abdelrazek FM , Ali SM. J Chem Res . 1991 ; 5 : 116 – 118 .
- Galil FM , Sallam MM , Sherif SM , Elnagdi MH. Liebigs Ann der Chem . 1986 ; 9 : 1639 – 1644 . doi: 10.1002/jlac.198619860914
- Galil FM , El-Taweel FMAA , Khodeir MNM , Elnagdi MH. Bull Chem Soc Jpn . 1993 ; 66 2 : 464 – 486 . doi: 10.1246/bcsj.66.464
- Elmoghayar MRH , Ibraheim MKA , Elghandour AHH , Elnagdi MH. Synthesis 1981 ; 8 : 635 – 637 . doi: 10.1055/s-1981-29554
- Sadek KU , Hafez EAA , Mourad AEE , Elnagdi MH. Z Naturforsch . 1984 ; 39B 6 : 824 – 828 .
- Abdou SF , Sherif M , Sadek KU , Elnagdi MH. Heterocycles 1981 ; 16 12 : 2177 – 2180 . doi: 10.3987/R-1981-12-2177
- Elnagdi MH , Ibrahim NS , Sadek KU , Mohamed MH. Liebigs Ann der Chem . 1988 ; 10 : 1005 – 1008 . doi: 10.1002/jlac.198819881014
- Elnagdi MH , Negm AM , Erian AW. Liebigs Ann der Chem . 1989 ; 12 : 1255 – 1256 . doi: 10.1002/jlac.198919890298
- Thumar NJ , Patel MP. ARKIVOC 2009 ; xiii : 363 – 380 .
- Abyshev AZ , Gindin VA , Semenov EV , Agaev EM , Abdulla-Zade AA , Guseinov AB. Pharm Chem J . 2006 ; 40 : 607 – 610 . doi: 10.1007/s11094-006-0203-7
- Konkoy CS , Fick DB , Cai SX , Lan NC , Keana JFWU. S. patent 6800657 . 2003 .
- Zhang X , Li X , Sui Z. US patent , 7812183 . 2010 .
- Montero JM , Brown DR , Gai PL , Lee AF , Wilson K. Chem Eng J . 2010 ; 161 : 332 – 339 . doi: 10.1016/j.cej.2009.12.035
- Hobson ST , Braue J , Ernest H , Lehnert EK , Klabunde KJ , Koper OP , Decker S. US Patent , 6403653 . 2002 .
- Montero JM , Wilson K , Lee AF. Top Catal . 2010 ; 53 : 737 – 745 . doi: 10.1007/s11244-010-9461-4
- Juarez R , Corma A , Garcia H. Top Catal . 2009 ; 52 : 1688 – 1695 . doi: 10.1007/s11244-009-9303-4
- Al-Matar HM , Khalil KD , Meier H , Kolshorn H , Elnagdi MH. ARKIVOC 2008 ; 16 : 288 – 301 .
- Gomha SM , Riyadh SM. ARKIVOC 2009 ; xi : 58 – 68 .
- Abdelrazek FM , Michael FA , Mohamed AE. Arch die Pharm . 2006 ; 339 : 305 – 312 . doi: 10.1002/ardp.200500259
- Abdelrazek FM , Elsayed AN. J Heterocycl Chem . 2009 ; 46 : 949 – 953 . doi: 10.1002/jhet.181
- Ghozlan SAS , Mohamed MH , Abdelmoniem AM , Abdelhamid IA. ARKIVOC 2009 ; x : 302 – 311 .
- Behbehani H , Abdel-Khalik MM , Elnagdi MH. Org Prep Proced Int . 1999 ; 31 5 : 551 – 557 . doi: 10.1080/00304949909355339
- Alnajjar A , Abdelkhalik MM , Al-Enezi A , Elnagdi MH. Molecules 2009 ; 14 1 : 68 – 77 . doi: 10.3390/molecules14010068
- Al-Mousawi SM , Moustafa MS , Abdelkhalik MM , Elnagdi MH. ARKIVOC 2009 ; ii : 1 – 10 .
- Khalil K , Al-Matar H , Elnagdi M. Eur J Chem . 2010 ; 1 4 : 252 – 285 . doi: 10.5155/eurjchem.1.4.252-258.211
- Ghozlan SAS , Abdelhamid IA , Hassaneen HM , Elnagdi MH. J Heteroatom Chem . 2007 ; 44 1 : 105 – 108 . doi: 10.1002/jhet.5570440118
- Al-Mousawi SM , Moustafa MS , Elnagdi MH. Tetrahedron Lett . 2009 ; 50 46 : 6411 – 6413 . doi: 10.1016/j.tetlet.2009.08.037
- Mohamed NR , El-Saidi MMT , Ali YM , Elnagdi MH. J Heterocyclic Chem . 2007 ; 44 6 : 1333 – 1337 . doi: 10.1002/jhet.5570440615
- Slatt J , Romero I , Bergman J. Synthesis 2004 : 2760 – 2765 .
- Khalil KD , Al-Matar HM , Al-Dorri DM , Elnagdi MH. Tetrahedron 2009 ; 65 45 : 9421 – 9427 . doi: 10.1016/j.tet.2009.08.084
- Abdel-Khalik MM , Elnagdi MH. Synth Commun . 2002 ; 32 2 : 159 – 164 . doi: 10.1081/SCC-120001996
- Makhseed S , Hassaneen HME , Elnagdi MH. Z Naturforsch . 2007 ; 62 4 : 529 – 536 .
- Al-Zaydi KM , Nhari LM , Borik RM , Elnagdi MH Green Chem Lett Rev . 2010 ; 3 2 : 93 – 99 . doi: 10.1080/17518250903567261
- Al-Qalaf F , Mekheimer RA , Sadek KU. Molecules 2008 ; 13 : 2908 – 2914 . doi: 10.3390/molecules13112908
- Sadek KU , Al-Qalaf F , Mekheimer RA , Abdelkhalik MM , Elnagdi MH. Arabian J Chem . 2012 ; 1 : 63 – 66 . doi: 10.1016/j.arabjc.2010.07.024
- Yadav JS , Reddy BVS , Reddy KB , Raj KS , Prasad ARJ. J Chem Soc Perkin I 2001 ; 1939 – 1942 . doi: 10.1039/b102565c
- Nair V , Panicker SB , Nair LG , George TG , Augustine A. Synlett 2003 ; 2 : 156 – 165 . doi: 10.1055/s-2003-36775
- Sadek KU , Al-Qalaf FA , Mervat M , Elnagdi MH. J Heterocycl Chem . 2010 ; 47 2 : 284 – 286 .
- Sadek KU , Alnajjar A , Mekheimer RA , Mohamed NK , Mohamed HA. Green Sust Chem . 2011 ; 1 : 92 – 97 . doi: 10.4236/gsc.2011.13015
- Anwar HF , Elnagdi MH. ARKIVOC 2009 ; 1 : 198 – 250 .