Abstract
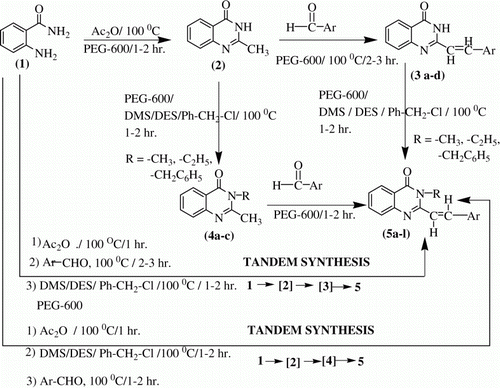
Polyethylene glycol (PEG)-mediated, green and efficient, tandem syntheses of N-subtituted-2-styrylquinazolinones are being reported. Condensation of anthranilamide (1) with acetic anhydride in PEG-600, at 100°C for 1 hr gave 2-methyl-3H-quinazoline-4-one (2). Treatment of 2 with benzaldehydes in PEG-600, at 100°C for 2–3 hr, gave 2-styrylquinazolinone-4-ones (3) in excellent yields. Treatment of 3 (a–d) either with dimethyl sulfate (DMS), diethyl sulfate (DES), or with Ph-CH2-Cl, individually in PEG-600, at 100°C for 1–2 hr without using any base, followed by simple processing resulted in N-substituted-2-styrylquinazolin-4-ones 5 (a–l). In an alternative approach, reaction of 4 (a–c) with benzaldehydes in PEG-600, at 100°C for 1–2 hr, without using any base, followed by simple processing resulted in 5 (a–l). 4 (a–c) were prepared by treatment of 2 with either DMS, DES, or with Ph-CH2-Cl, individually, in the presence of PEG-600 as reaction medium, at 100°C for 1–2 hr without using any base. Both the sequence of reactions, 1 ![]() 2
2 ![]() 3
3 ![]() 5 or 1
5 or 1 ![]() 2
2 ![]() 4
4 ![]() 5 could be carried out in tandem in an efficient manner in PEG-600 without the isolation of any intermediates. The structures of all the new compounds synthesized in this work have been established on the basis of their spectroscopic data and analytical data.
5 could be carried out in tandem in an efficient manner in PEG-600 without the isolation of any intermediates. The structures of all the new compounds synthesized in this work have been established on the basis of their spectroscopic data and analytical data.
Introduction
The quinazolinone ring system forms an important class of N-heterocyclic compounds as it is present in a large number of compounds with useful biological properties such as anti-cancer Citation1, anti-inflammatory Citation2 Citation3, anti-convulsant Citation3, hypotensive Citation4, and anti-malarial Citation5 types. 2-Styrylquinazolinones are associated with inhibitory effects on tubulin polymerization and the growth of L1210 murine leukemia cells Citation6. Some derivatives are also known to possess anti-convulsant activities Citation7.
Although there are several publications Citation8 Citation9 describing the synthesis of functionalized 4(3H)-quinazolinones, there are only a few reports Citation10 on the preparation of substituted-2-styrylquinazolin-4(3H) ones. The general method for the synthesis of these heterocycles is the Knoevenagel condensation of substituted-2-methylquinazolinones with aromatic aldehydes under basic Citation11 Citation12 or acidic Citation13 conditions. However, many of the protocols for the initial generation of the quinazolines core/or preparation of N-substituted-2-styrylquinazolinones suffer from drawbacks such as requiring multistep procedure, harsh reaction conditions, long reaction times, or low yields Citation14 Citation15. Also there is a need to develop an environmentally-friendly method for the synthesis of quinazolinones. Polyethylene glycol (PEG) has attracted increasing interest in the context of green synthesis during recent years Citation16. Indeed, PEG is recognized as an attractive green solvent for various organic reactions Citation16. Our interest in PEG as a green reaction medium was provoked by several factors, such as its thermal stability, commercial availability, non-volatility, immiscibility with a number of organic solvents and recyclability Citation17. Furthermore, PEG-600 is an inexpensive, completely non-halogenated and easily degradable solvent that possesses low toxicity Citation18. PEG has been used as a complexing solvent with unique properties of cationic coordination ability Citation19. On the basis of the complexing properties of PEG, we chose it for the synthesis of quinazolinones. Tandem reactions are commonly referred to under the multistep one-pot reactions.
Tandem reactions are combinations of two or more reactions whose occurrence is in a specific order, and if they involve sequential addition of reagents the secondary reagents must be integrated into the products. Tandem reactions have several advantages over a series of individual reactions. First, they allow constructions of complex structures in as few steps as possible. Finally, employing reactions in tandem will save on cost and amounts of reagents, solvents and reduce the amount of waste that is generated.
Results and discussion
In the first step, condensation of anthranilamide (1) with acetic anhydride in PEG-600, at 100°C for 1hr gave the previously reported Citation20 2-methyl-3H-quinazoline-4-one (2). Its IR spectrum in KBr showed the diagnostic absorption at ≈3300 cm−1 (broad, medium) due to –NH– stretching vibration and another at 1667 cm−1 (strong, sharp) due to –CO- group and the absence of an unequal doublet at 3450, 3480 cm−1 due to asymmetrical and symmetrical stretching vibrations of –NH2 group that was there in 1. Its 1H NMR (DMSO-d6/TMS) spectrum showed signals at δ 3.00 (s, 3H, CH3), 7.40–8.00 (m, 4H, phenyl ring protons), 12.33 (s, 1H, br, –NH, D2O exchangeable). Its APCI mass spectrum showed M+• − 1 ion peak at 159 corresponding to a molecular mass of 160.
In the second step, treatment of 2 with benzaldehydes in PEG-600, at 100°C for 2–3 hr, gave 2-styrylquinazolinone-4-ones (3) in excellent yields. The products showed in their IR spectra (KBr), characteristic peaks at ≈3480 cm−1 (broad, medium) due to –NH group and at ≈1660 cm−1 (strong, sharp) due to –CO- group. In their 1H NMR (DMSO-d6/TMS) spectra, the products showed signals at δ 6.98–7.02 (d, J ≈ 14 Hz, 1H), 7.92–7.96 (d, J ≈ 14 Hz, 1H), due to trans disposed vinylic protons) and at ≈δ 12.33 (s, 1H, br, D2O exchangeable), due to the –NH- protons in addition to the signals due to aryl protons. For details, please see Experimental Section.
Treatment of 2 with either dimethyl sulfate (DMS), diethyl sulfate (DES), or with Ph-CH2-Cl, individually, in the presence of PEG-600 as reaction medium, at 100°C for 1–2 hr without using any base, resulted in 4(a–c) in yields of ≈80–85% on processing the reaction mixture. The products showed in their IR spectra (in KBr) absorptions at 1660 cm−1 (strong, sharp) due to the –N–CO– group and absence of any absorption in the 3400–3000 cm−1 due to any –NH- grouping, which was seen in the spectra of starting compounds 2. All the products 4 (a–c) showed in their 1H NMR spectra signals due to alkyl protons in the aliphatic region in addition to aryl protons in the 7.0–8.0 region and absence of the –NH- proton signal (D2O exchangeable) which was observed prominently in the spectra of starting compounds 2.
Treatment of 3 (a–d) either with DMS, DES, or with Ph-CH2-Cl individually in PEG-600, at 100°C for 1–2 hr without using any base, followed by simple processing resulted in 5(a–l) in yields of ≈80–85%. The products showed in their IR spectra (in KBr) characteristic peaks at 1660 cm−1 (strong, sharp) due to –CO- group and absence of any absorption due to –NH grouping. In their 1H NMR (DMSO-d6/TMS) spectra, the products showed signals at δ 6.98–7.02 (d, J ≈ 14 Hz, 1H), 7.92–7.96 (d, J ≈ 14 Hz, 1H), due to trans disposed vinylic protons) and at ≈δ 12.33 (s, 1H, br, D2O exchangeable), due to the –NH- protons in addition to signals due to aryl protons. For details, please see Experimental Section.
In an alternative approach, reaction of 4 (a–c) with benzaldehydes in PEG-600, at 100°C for 1–2 hr, without using any base, followed by simple processing resulted in 5(a–l) in yields of ≈85–90%, identical in m.p., m.m.p., Thin Layer Chromatography (TLC) and IR with that of the same products obtained in the route described above (i.e., 3
![]() 5). For details, please see the Experimental Section.
5). For details, please see the Experimental Section.
It is obvious from the above results that PEG-600 is a very efficient solvent for the alkylation of 2 and 3 (a–d) resulting in the formation of 4 (a–c) and 5 (a–l), respectively. Mechanistic explanation Citation21 of these results is that, probably PEG-600 dissolves the substrates 2 (or 3 as the case may be) and the reagent (i.e., the alkylating agent, DMS, DES etc.), bringing them together thereby providing an effective means for chemical reaction to occur. Furthermore, PEG-600 is able to extract the hydrogen from the –NH of quinazolinone and is able to coordinate it through several lone pairs of electrons in its oxygen containing chain. This role of PEG-600 is very similar to that of the crown ethers or that of the proton sponge (i.e., 1, 8-dimethylaminonaphthalene). The latter acts as a very strong base due to its ability to extract hydrogen from an acidic substrate and then retain it by chelation through lone pair of electrons on the two nitrogen atoms of the two amino groups Citation21.
Both the sequences of reactions mentioned above (1
![]() 2
2 ![]() 3
3 ![]() 5 or 1
5 or 1
![]() 2
2 ![]() 4
4 ![]() 5) could be carried out in tandem in an efficient manner in PEG-600 without the isolation of any intermediates. Thus, treatment of 1 with AC2O in PEG-600 at 100°C for 1hr gives 2 in situ and subsequent alkylation of 2 (without its isolation) with DMS/DES/ Ph-CH2-Cl yields alkyl derivatives of 4 (a–c) again in situ. Subsequent treatment of the resulting reaction mixture with 1 equivalent of an aromatic aldehyde followed by heating at 100°C for 1–2 hr leads to the formation of N-substituted-2-styrylquinazolinones (5a–l), again in situ, as shown by TLC examination of reaction mixtures with authentic intermediates/products. Processing the reaction mixture leads to isolation of 5 (a–l) in good yields. Similarly, the sequence 1
5) could be carried out in tandem in an efficient manner in PEG-600 without the isolation of any intermediates. Thus, treatment of 1 with AC2O in PEG-600 at 100°C for 1hr gives 2 in situ and subsequent alkylation of 2 (without its isolation) with DMS/DES/ Ph-CH2-Cl yields alkyl derivatives of 4 (a–c) again in situ. Subsequent treatment of the resulting reaction mixture with 1 equivalent of an aromatic aldehyde followed by heating at 100°C for 1–2 hr leads to the formation of N-substituted-2-styrylquinazolinones (5a–l), again in situ, as shown by TLC examination of reaction mixtures with authentic intermediates/products. Processing the reaction mixture leads to isolation of 5 (a–l) in good yields. Similarly, the sequence 1
![]() 2
2 ![]() 3
3 ![]() 5 could be carried out in tandem in an efficient manner in PEG-600 without the isolation of any intermediates in the sequence of reactions. Thus, treatment of 1 with AC2O in PEG-600 at 100°C for 1hr gives 2 in situ. Subsequent treatment of the resulting reactions mixture with 1 equivalent of an aromatic aldehyde followed by heating at 100°C for 2–3 hr leads to the formation of 3 (a–d) again, in situ. Subsequent alkylation of 3 (without its isolation) with DMS/DES/ Ph-CH2-Cl gives methyl/ethyl/benzyl derivatives, respectively, of 5 (a–l), as shown by TLC examination of reaction mixtures with authentic intermediates/products.
5 could be carried out in tandem in an efficient manner in PEG-600 without the isolation of any intermediates in the sequence of reactions. Thus, treatment of 1 with AC2O in PEG-600 at 100°C for 1hr gives 2 in situ. Subsequent treatment of the resulting reactions mixture with 1 equivalent of an aromatic aldehyde followed by heating at 100°C for 2–3 hr leads to the formation of 3 (a–d) again, in situ. Subsequent alkylation of 3 (without its isolation) with DMS/DES/ Ph-CH2-Cl gives methyl/ethyl/benzyl derivatives, respectively, of 5 (a–l), as shown by TLC examination of reaction mixtures with authentic intermediates/products.
Conclusion
In summary, we have developed efficient, mild, and convenient tandem syntheses of N-substituted-2-styryl-4(3H)-quinazolinones (5a–l) in PEG-600 in two routes (i.e., 1
![]() 2
2 ![]() 3
3 ![]() 5 or 1
5 or 1
![]() 2
2 ![]() 4
4 ![]() 5). Probably, this is the first case of two variable but identical end-products- giving tandem syntheses, under green conditions, of substituted-2-styryl-4(3H)-quinazolinones. Obviously, as figures pointed out, tandem routes gave good yields compared to the stepwise routes.
5). Probably, this is the first case of two variable but identical end-products- giving tandem syntheses, under green conditions, of substituted-2-styryl-4(3H)-quinazolinones. Obviously, as figures pointed out, tandem routes gave good yields compared to the stepwise routes.
Experimental section
General
Melting points are uncorrected and were determined in open capillary tubes in sulfuric acid bath. TLC was performed on silica gel-G and spotting was done using iodine or UV-light. IR spectra were recorded with Perkin-Elmer 1000 instrument in KBr phase, 1H-NMR on VARIAN 400 MHz instrument and Mass spectra on Agilent-LC-MS instrument giving only M+ values using Q + 1or Q-1 mode.
Preparation of 2 from 1 (general procedure)
A mixture of 1 (2.74 g, 20 mmol), acetic anhydride (2.5 mL, 30 mmol) and PEG-600 (40 mL), was heated at 100°C for 1 hr. After completion of reaction (as indicated by TLC using hexane: ethyl acetate, 8:2 as elluent), water (2×40 mL) was added to the reaction mixture and the separated solid was filtered, washed with water (2×10 mL) and dried. Yield = 2.75 gm (85%). The crude product was recrystallized from ethanol to obtain pure 2. M.P.=228–230°C (Lit.20 M.P. 230–232°C).
Preparation of 3 from 2 (general procedure)
A mixture of 2 (2.74 g, 15 mmol), aromatic aldehyde (15 mmol) and PEG-600 (40 mL) was heated at 100°C for 1–2 hr. After completion of reaction (as indicated by TLC using hexane:ethyl acetate, 8:2 as elluent) water (2×40 mL) was added to the reaction mixture and the separated solid was filtered, washed with water (2×10 mL) and dried. The crude product was recrystallized from ethanol to obtain pure 3.
3a: Yield = 3.40 g (80%); M.P. 251°C (Lit.10 M.P. 252°C); IR (KBr): ≈ 3400 cm−1 (broad, medium –NH-), 1667 cm−1 (strong, sharp, –CO–). 1H-NMR (400 MHz, DMSO/d6/TMS): δ 6.98–7.02 (d, 1 H , J=14 Hz, vinylic proton), 7.40–7.4 (m, 4H, aromatic ring protons), 7.64–8.11 (m, 6H, four quinazolinone ring protons + one aromatic benzene ring proton + one vinylic proton). 12.33 (s, 1H, br, –NH, D2O exchangeable). MS: m/z 249 (M+• + 1).
3b: Yield = 3.90 g. (82%); M.P. 259°C (Lit.10 M.P. 260°C); IR (KBr): ≈ 3400 cm−1 (broad, medium –NH-), 1667 cm−1 (strong, sharp, –CO–). 1H NMR (400 MHz, DMSO/d6/TMS): δ 3.80 (s, 3H, OCH 3 ), 6.98–7.02 (d, 1 H , J=14 Hz, vinylic proton), 7.00–8.21 (m, 9H, four quinazolinone ring protons + four aromatic benzene ring + one vinylic proton), 12.33 (s, 1H, br, –NH, D2O exchangeable). MS m/z 279 (M+• + 1).
3c: Yield = 4.50 g (81%); M.P. 253°C (Lit.10 M.P. 255°C); IR (KBr): ≈ 3400 cm−1 (broad, medium –NH-), 1667 cm−1 (strong, sharp,–CO–). 1H-NMR (400 MHz, DMSO/d6/TMS): δ 3.80 (s, 3H, OCH 3 ), 4.20 (t, 3H, –CH 3 ), 4.50 (m, 2H, –CH 2 –), 6.98–7.02 (d, 1 H, J=14 Hz, vinylic proton), 7.00–8.21 (m, 8H, four quinazolinone ring protons + three aromatic benzene ring proton + one vinylic proton), 12.33 (s, 1H, br, –NH, D2O exchangeable). MS m/z 323 (M+• + 1).
3d: Yield = 3.60 g (80%); M.P. 255°C (Lit.10 M.P. 258°C); IR (KBr): ≈ 3400 cm−1 (broad, medium –NH-), 1667 cm−1 (strong, sharp, –CO–). 1H-NMR (400 MHz, DMSO/d6/TMS): δ 6.98–7.02 (d, 1 H, J=14 Hz, vinylic proton), 6.87–8.21 (m, 9H, four quinazolinone ring protons + four aromatic benzene ring proton + one vinylic proton), 10.22 (s, 1H, br, –OH, D2O exchangeable), 12.33 (s, 1H, br, –NH, D2O exchangeable). MS m/z 265 (M+• + 1).
Preparation of 4 from 2 (general procedure)
A mixture of 2 (2.74 g, 15 mmol), DMS/DES/ Ph-CH2-Cl (15 mmol) and PEG-600 (40 mL), was heated at 100°C for 1–2 hr. After completion of reaction (as indicated by TLC using hexane:ethyl acetate, 8:2 as elluent), water (2×40 mL) was added to the reaction mixture and the separated solid was filtered, washed (2×10 mL) with water and dried. The crude product was recrystallized from ethanol to obtain pure 4.
4a: Yield = 2.50 g (84%); M.P. 63°C (Lit.22 M.P. 65°C); IR (KBr): 1667 cm−1 (strong, sharp,–CO–). 1H-NMR (400 MHz, DMSO/d6/TMS): δ 2.5 (s, 3 H, –CH3), 3.50 (s, 3H, –CH 3 ), 7.5–8 (m, 4H, aromatic benzene ring proton). MS m/z 175 (M+• + 1).
4b: Yield = 2.60 g (81%); M.P. 85°C (Lit.22 M.P. 87°C); IR (KBr): 1667 cm−1 (strong, sharp, –CO–). 1H-NMR: (400 MHz, DMSO/d6/TMS): δ 2.5 (s, 3 H, –CH3), 4.20 (t, 3H, –CH 3 ), 4.50 (m, 2H, –CH 2 -), 7.5–8 (m, 4H, aromatic benzene ring). MS m/z 189 (M+• + 1).
4c: Yield = 3.50 g (81%); M.P. 95°C (Lit.22 M.P. 97°C); IR (KBr): 1667 cm−1 (strong, sharp,–CO–). 1H-NMR: (400 MHz, DMSO/d6/TMS): δ 2.5 (s, 3 H, –CH3), 4.50 (s, 2H, –CH 2 –), 7–7.5 (m, 5H, aromatic benzene ring protons), 7.5–8 (m, 4H, quinazolinone ring protons). MS: m/z 251 (M+• + 1).
Preparation of 5 from 3 (general procedure)
A mixture of 3 (2.48 g, 10 mmol), DMS/DES/ Ph-CH2-Cl (10 mmol) and PEG-600 (40 mL), was heated for 1–2 hr at 100°C. After completion of reaction (as indicated by TLC using hexane: ethyl acetate, 8:2 as elluent), water (2×40 mL) was added to the reaction mixture and the separated solid was filtered, washed with water (2×10 mL) and dried. The crude product was recrystallized from ethanol to obtain pure 5.
5a: Yield = 2.27 g. (87%); M.P. 120–22°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–). 1H-NMR (400 MHz, DMSO/d6/TMS): δ 3.50 (s, 3H, –CH 3 ), 6.98–7.02 (d, 1 H, J=14 Hz, vinylic proton), 7.40–7.4 (m, 4H, aromatic benzene ring), 7.64–8.11 (m, 6H, four quinazolinone ring protons + one aromatic benzene ring + one vinylic proton). MS: m/z 263 (M+• + 1). [Found: C: 77.83, H: 5.36, N: 10.68, C17H14N2O requires C: 77.84, H: 5.38, N: 10.68%].
5b: Yield = 2.62 g. (90%); M.P. 125–26°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–). 1H-NMR (400 MHz, DMSO/d6/TMS): δ 3.70 (s, 3H, –CH 3 ), 3.80 (s, 3H, OCH 3 ), 6.98–7.02 (d, 2H, J=16 Hz, vinylic protons), 7.28–8.11 (m, 8H, four quinazolinone ring protons + four aryl protons). MS m/z 293 (M+• + 1). [Found: C: 73.85, H: 5.50, N: 9.55, C18H16N2O2 requires C: 73.95, H: 5.52, N: 9.58%].
5c: Yield = 2.95 g. (88%); M.P. 120–22°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 3.70 (s, 3H, –CH 3 ), 3.80 (s, 3H, OCH 3 ), 4.20 (t, 3H, –CH 3 ), 4.50 (m, 2H, –CH 2 –), 6.98.–7.02 (d, 1 H, J=14 Hz, vinylic proton), 7.00–8.21 (m, 8H, four quinazolinone ring protons + three aromatic benzene ring + one vinylic proton). MS m/z 337 (M+• + 1). [Found: C: 71.39, H: 5.95, N: 8.30, C20H20N2O3 requires C: 71.41, H: 5.99, N: 8.33%].
5d: Yield = 2.50 g. (90%); M.P. 121–23°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–); 1H-NMR: (400 MHz, DMSO/d6/TMS) δ 3.70 (s, 3H, –CH 3 ), 6.98–7.02 (d, 1 H, J=14 Hz, vinylic proton), 6.87–8.21 (m, 9H, four quinazolinone ring protons + four aromatic benzene ring + one vinylic proton), 10.22 (s, 1H, br, OH, D2O exchangeable). MS m/z 279 (M+• + 1). [Found: C: 73.35, H: 5.04, N: 10.04, C17H14N2O2 requires C: 73.37, H: 5.07, N: 10.07%].
5e: Yield = 2.34 g. (85%); M.P. 121–23°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–); 1H NMR (400 MHz, DMSO/d6/TMS,δ): 1.60 (t, 3H, –CH 3 ) 4.50 (s, 2H, –CH 2 –) 6.98–7.02 (d, 1 H , J=14 Hz, vinylic proton), 7.40–7.4 (m, 4H, aromatic benzene ring) 7.64–8.11 (m, 6H, four quinazolinone ring protons + one aromatic benzene ring + one vinylic proton); MS m/z 277 (M+• + 1). [Found: C: 78.20, H: 5.80, N: 10.10, C18H16N2O requires C: 78.24, H: 5.84, N: 10.14%].
5f: Yield = 2.69 g. (88%); M.P. 120–22°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 1.60 (t, 3H, –CH 3 ), 4.50 (s, 2H, –CH 2 –), 3.80 (s, 3H, OCH 3 ), 6.98.–7.02 (d, 2 H, J=16 Hz, vinylic protons), 7.28–8.11 (m, 8H, four quinazolinone ring protons + four aromatic benzene ring). MS m/z 307 (M+• + 1). [Found: C: 74.45, H: 5.92, N: 9.14, C19H18N2O2 requires C: 74.49, H: 5.92, N: 9.14%].
5g: Yield = 2.80 g. (80%); M.P. 119–21°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 1.60 (t, 3H, –CH 3 ), 4.50 (s, 2H, –CH 2 –), 3.80 (s, 3H, OCH 3 ), 4.20 (t, 3H, –CH 3 ), 4.50 (m, 2H, –CH 2 –), 6.98.–7.02 (d, 1 H, J=14 Hz, vinylic proton), 7.00–8.21 (m, 8H, four quinazolinone ring protons + three aromatic benzene ring protons + one vinylic proton). MS m/z 351 (M+• + 1). [Found: C: 71.95, H: 6.30, N: 7.95, C21H22N2O3 requires C: 71.98, H: 6.33, N: 7.99%].
5h: Yield = 2.56 g. (88%); M.P. 121–23°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 1.60 (t, 3H, –CH 3 ), 4.50 (s, 2H, –CH 2 –), 6.98–7.02 (d, 1H, J =14 Hz, vinylic protons), 6.87–8.21 (m, 9H, four quinazolinone ring protons + four aromatic benzene ring + one vinylic proton), 10.22 (s, 1H, br, OH, D2O exchangeable). MS m/z 293 (M+• + 1). [Found: C: 73.90, H: 5.50, N: 9.54, C18H16N2O2 requires C: 73.95, H: 5.52, N: 9.58%].
5i: Yield = 3.04 g. (90%); M.P. 118–20°C; IR (KBr): 1667 cm−1 (strong, sharp, –CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 4.50 (s, 2H, –CH 2 –), 6.98–7.02 (d, 1 H, J=14 Hz, vinylic proton), 7–7.5 (m, 5H, phenyl ring protons), 7.40–7.4 (m, 4H, aromatic benzene ring), 7.64–8.11 (m, 6H, four quinazolinone ring protons + one aromatic benzene ring proton + one vinylic proton). MS m/z 339 (M+• + 1). [Found: C: 81.60, H: 5.30, N: 8.24, C23H18N2O requires C: 81.63, H: 5.36, N: 8.28%].
5j: Yield = 2.85 g. (87%); M.P. 120–22°C; IR (KBr): 1667 cm−1 (strong, sharp, –CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 3.80 (s, 3H, –OCH 3 ), 4.50 (s, 2H, –CH 2 –), 6.98–7.02 (d, 2H, J=16 Hz, vinylic protons), 7–7.5 (m, 5H, phenyl ring protons), 7.28–8.11(m, 8H, four quinazolinone ring protons + four aromatic benzene ring protons). MS m/z 369 (M+• + 1). [Found: C: 76.20, H: 5.40, N: 7.55, C24H20N2O2 requires C: 78.24, H: 5.47, N: 7.60%].
5k: Yield = 3.51 g. (85%); M.P. 120–22°C; IR (KBr): 1667 cm−1 (strong, sharp, –CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 3.80 (s, 3H, –OCH 3 ), 4.20 (t, 3H, –CH 3 ), 4.40 (s, 2H, –CH 2 –), 4.50 (m, 2H, –CH 2 –), 6.98–7.02 (d, 2H, J=16 Hz, vinylic protons), 7–7.5 (m, 5H, phenyl ring protons), 7.00–8.21 (m, 7H, four quinazolinone ring protons + three aromatic benzene ring protons). MS m/z 413 (M+• + 1). [Found: C: 75.65, H: 5.80, N: 6.70, C26H24N2O3 requires C: 75.71, H: 5.86, N: 6.79%].
5l: Yield = 2.93 g. (83%); M.P. 118–20°C; IR (KBr): 1667 cm−1 (strong, sharp,–CO–); 1H-NMR (400 MHz, DMSO/d6/TMS): δ 4.50 (s, 2H, –CH 2 –), 6.98–7.02 (d, 1 H, J=14 Hz, vinylic proton), 7–7.5 (m, 5H, phenyl ring protons), 6.87–8.21 (m, 9H, four quinazolinone ring protons + four aromatic benzene ring + one vinylic proton), 10.22 (s, 1H, br, OH, D2O exchangeable). MS m/z 355 (M+• + 1). [Found: C: 77.90, H: 5.05, N: 7.80, C23H18N2O2 requires C: 77.95, H: 5.12, N: 7.90%].
Preparation of 5 from 4 (general procedure)
A mixture of 4 (1.74 g, 10 mmol), aromatic aldehydes (10 mmol) and PEG-600 (40 mL), heated at 100°C for 1–2 hr. After completion of reaction (as indicated by TLC using hexane: ethyl acetate, 8:2 as elluent), water (2×40 mL) was added to the reaction mixture and the separated solid was filtered, washed (2×10 mL) with water and dried. The crude product was recrystallized from ethanol to obtain pure 5.
5a: Yield = 2.35 g. (90%).
5b: Yield = 2.65 g. (91%).
5c: Yield = 3.05 g. (91%).
5d: Yield = 2.50 gm. (90%).
5e: Yield = 2.40 g. (87%).
5f: Yield = 2.75 g. (90%).
5g: Yield = 3.08 g. (88%).
5h: Yield = 2.62 g. (90%).
5i: Yield = 2.90 g. (86%).
5j: Yield = 3.12 g. (85%).
5k: Yield = 3.62 g. (88%).
5l: Yield = 2.93 g. (83%).
Preparation of 5 from 1: (i.e., 1 2
2 3
3 5). (Tandem synthesis) – (First route)
5). (Tandem synthesis) – (First route)
A mixture of 1 (2.74 g, 20 mmol), acetic anhydride (2.5 mL, 30 mmol) and PEG-600 (40 mL), was heated at 100°C. After 1hr, the reaction mixture was cooled to room temperature, aromatic aldehyde (20 mmol) was added to the mixture, the reaction mixture heated again to 100°C and maintained at this temperature for 1–2 hr. Then, the reaction mixture was cooled to room temperature, DMS/DES/ Ph-CH2-Cl (20 mM) was added to the mixture, the reaction mixture heated once again to 100°C and maintained at this temperature for 1–2 hr. After completion of reaction (as indicated by TLC using hexane: ethyl acetate, 8:2 as elluent), water (2×40 mL) was added to the reaction mixture and the separated solid was filtered, washed with water (2×10 mL) and dried. The crude product was recrystallized from ethanol to obtain pure 5. For yields Please see .
Table 1. Synthesis of N-substituted-2-styrylquinazolinones (5a–l).
Preparation of 5 from 1: (i.e., 1 2
2 4
4 5). (tandem synthesis) (second route)
5). (tandem synthesis) (second route)
A mixture of 1 (2.74 g, 20 mmol), acetic anhydride (2.5 mL, 30 mmol) and PEG-600 (40 mL), was heated at 100°C. After 1 hr the reaction mixture was cooled to room temperature, DMS/DES/Ph-CH2-Cl (20 mmol) was added to the reaction mixture and the mixture was heated again to 100°C and maintained at this temperature for 1–2 hr. Then, the reaction mixture was cooled to room temperature, aromatic aldehyde (20 mmol) was added to the mixture and the mixture heated once again to 100°C and maintained at this temperature for 1–2 hr. After completion of reaction (as indicated by TLC using hexane: ethyl acetate, 8:2 as elluent), water (2×40 mL) was added to the reaction mixture and the separated solid was filtered, washed with water (2×10 mL) and dried. The crude product was recrystallized from ethanol to obtain pure 5. For yields Please see .
PEG-600 MEDIATED, GREEN AND EFFICIENT, TANDEM SYNTHESESOF N-SUBTITUTED-2-STYRYLQUINAZOLIN-4- ONES
Download MS Word (295 KB)Acknowledgements
The authors are indebted to the University Grants Commission, Govt. of India, New Delhi. They are also thankful to the authorities of Jawaharlal Nehru Technological University Hyderabad for providing laboratory facilities.
References
- Sirisoma N , Pervin A , Zhang H , Jiang S , Willardsen J , Anderson B , Mather G , Pleiman M , Kasibhatla S , Tseng B , Drewe J , Xiong Cai S. Discovery of N-(4-Methoxyphenyl)-N,2-dimethylquinazolin-4-amine, a Potent Apoptosis Inducer and Efficacious Anticancer Agent with High Blood Brain Barrier Penetration . J Med Chem . 2009 ; 52 : 2341 . doi: 10.1021/jm801315b
- Burbuliene MM , Jakubkiene V , Mekuskiene G , Udrenaite P , Smicius P. Synthesis and anti-inflammatory activity of derivatives of 5-[(2-disubstitutedamino-6- methyl-pyrimidin-4-yl)-sulfanylmethyl]-3H-1,3,4-oxadiazole-2-thiones . Farmaco . 2004 ; 59 : 767 . doi: 10.1016/j.farmac.2004.05.007
- Alagarasamy M , Meena S , Revathi R , Vijaya kumar S , Ramesh KV. Synthesis of some novel 2-mercapto-3(substituted amino) 5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-ones as analgesic and anti-inflammatory agents . Arkivoc . 2006 ; 58 : 149 .
- Ozaki K , Yamada Y , Onine T , Ishizuka T , Iwasawa Y. Studies on 4(1H) Quinazolinones. 5.' Synthesis and Antiinflammatory Activity of 4(1H)quinazolinone Derivatives . J Med Chem . 1985 ; 28 : 568 . doi: 10.1021/jm50001a006
- Bonola G , Dare P , Mgistretti MJ , Massarani E , Setnikar I. l-Aminoacyl-2,3-dihydro-4(1H)-quinazolin Derivatives with Choleretic and Antifibrillatory Activity . J Med Chem . 1968 ; 11 : 1136 . doi: 10.1021/jm00312a007
- Hess HJ , Cronin TH , Scriabine A. Antihypertensive 2-amino-4(3H)-quinazolinones . J Med Chem . 1968 ; 11 : 130 . doi: 10.1021/jm00307a028
- Bhargava PN , Chaurasia MR. Some 6,8-dibromo-S-substited-2-mercapto-3-aryl(or alkyl)-4-quinazolones . J Med Chem . 1968 ; 11 : 140 . doi: 10.1021/jm00307a031
- Reddy PSN , Venugopal KN , Rao GK , Pai PNS. Indian J Heterocyclic Chem . 2007 ; 16 : 243 .
- Zeng F , Alper H. One-Step Synthesis of Quinazolino[3,2-a]quinazolinones via Palladium-catalyzed Domino Addition/Carboxamidation Reactions . Org Letters . 2010 ; 12 : 3642 . doi: 10.1021/ol101428v
- Minoo D , Mustafa B , Sadat DA. Novel and Efficient One-Pot Tandem Synthesis of 2-Styryl-Substituted 4(3H)-Quinazolinones . J Comb Chem . 2008 ; 10 : 700 . doi: 10.1021/cc800067g
- Wolfe JF , Rathman TL , Sleevi MC , Campbell JA , Greenwood TD. Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones . J Med Chem . 1990 ; 33 : 161 . doi: 10.1021/jm00163a027
- Philipova I , Dobrikov G , Krumova K , Kaneti J. J Heterocyclic Chem . 2006 ; 43 : 1057 . doi: 10.1002/jhet.5570430436
- Rafffa D , Edler MC , Daidone G , Maggio B , Merickech M , Plescia S , Schillaci D , Bai R , Hamel E. Eur J Med Chem . 2004 ; 39 : 299 . doi: 10.1016/j.ejmech.2003.12.009
- Xia Y , Yang ZY , Hour MJ , Kuo SC , Xia P , Bastow KF , Nakanishi Y , Nampoothiri P , Hackl T , Hamel E , Lee KH. Biorg Med Chem Lett . 2001 ; 11 : 1193 . doi: 10.1016/S0960-894X(01)00190-1
- Mostafa B , Maja M , Markus D , Claudia R , Minoo D , Oliver KC. Parallel Microwave Synthesis of 2-Styrylquinazolin-4(3H) in a High-Throughput Platform Using HPLC/GC Vials as Reaction Vessels . J Comb Chem . 2009 ; 11 : 676 . doi: 10.1021/cc900036a
- Nana VS , Kiran FS , Sandip A , Bapurao BS , Muralidhar SS. PEG-400 remarkably and recyclable media for one-pot synthesis of various 2-amino-4H-chromenes . Green Chem Lett Rev . 2010 ; 3 : 83 . doi: 10.1080/17518250903567246
- Kidwai M , Jahan A , Divya B. Polyethylene glycol as an efficient and reusable solvent medium for the synthsis of thiohydations using K2CO3 as catalyst . J Sulfur Chem . 2010 ; 3 : 161 . doi: 10.1080/17415991003777409
- Heldebrant DJ , Jessop PG. Liquid Poly(ethylene glycol) and Supercritical Carbon Dioxide: A Benign Biphasic Solvent System for Use and Recycling of Homogeneous Catalysts . J Am Chem Soc . 2003 ; 125 : 5600 . doi: 10.1021/ja029131l
- Kidwai M , Divya B , Kumar MN. Polyethylene glycol (PEG) mediated green synthesis of 2,5-disubstituted 1,3,4-oxadiazoles catalyzed by ceric ammonicum nitrate . Green Chem Lett Rev . 2010 ; 1 : 55 .
- Jiang JB , Hesson DP , Dusak BA , Dexter DL , Kang GJ , Hamel E. J Med Chem . 1990 ; 33 : 1721 . doi: 10.1021/jm00168a029
- Venkatanarayana M , Dubey PK. PEG-600: a facile and eco-friendly raction medium for the synthesis of N-alkyl derivatives of indole-3-carboxyladehyde . Green Chem Lett Rev . 2010 ; 3 : 257 . doi: 10.1080/17518251003749379
- Motohiro A , Teruyuki K , Yoshihisa W. Transition-metal complex-catalyzed reductive N-heterocyclization: synthesis of 4(3H)quinazolinone derivatives from N-(2- nitrobenzoyl)amides . J Org Chem . 1993 ; 58 : 310 .