Abstract
Palladium-catalyzed transfer hydrogenation of some organic substrates in water containing a nonionic surfactant was examined using sodium hypophosphite as a hydrogen source. Hydrogenation of organohalides such as polychloroarenes, alkenes, alkynes, nitro compounds, aromatic aldehydes, and O-benzyl and N-benzyl derivatives efficiently proceeded to give the corresponding reduction products. The addition of a nonionic surfactant, such as Tween 20, proved to be effective in obtaining satisfactory results in most cases.
Introduction
Catalytic hydrogenation reactions are among the most fundamental and important processes in synthetic and industrial chemistry Citation1. Typically, these reactions are performed in organic solvents under a hydrogen atmosphere. For safe and environmentally benign operations, transfer hydrogenation, which does not need a hydrogen cylinder, in aqueous media is preferable.
Hypophosphorous acid and its salts are well-known powerful reducing agents and are mainly used in electroless plating. In organic synthesis, hypophosphites are often used as hydrogen donors in the catalytic transfer reduction of various functional groups such as alkene Citation2–4, alkyne Citation5, halogen Citation2 Citation6 Citation7, nitro Citation8 Citation9, N-oxide Citation2 Citation10, carboxyl Citation11, cyano Citation12 Citation13, carbonyl Citation2 Citation12, O-benzyl Citation2 Citation4 Citation14 Citation15, and N-benzyl Citation16 groups, although most of the methods require an organic co-solvent. Some examples of organic-solvent-free processes include dehalogenation of water-soluble aryl chlorides Citation17, microwave-assisted dechlorination of pentachlorobenzene Citation18, and mechanochemical solid-phase dechlorination of hexachlorobenzene Citation19; however, these methods were optimized for specific targets, and no systematic studies were conducted to explore the substrate scope. In this paper, we report the catalytic transfer hydrogenation of various organic substrates in water using a hypophosphite–palladium system, in which the reaction efficiency remarkably improved by the use of nonionic surfactants. Regarding the effect of coexisting phase-transfer agents, Tundo and coworkers reported rapid hydrodehalogenation of 1,2,4,5-tetrachlorobenzene by hypophosphite and a palladium catalyst in a multiphase system consisting of an organic solvent, a strong aqueous alkali, and a quaternary onium salt Citation7.
Results and discussion
We began our investigation with 1-bromododecane, a highly hydrophobic organic halide, as a substrate to optimize the reaction conditions, and the results of which are shown in . An aqueous solution of 1-bromododecane containing Tween 20 as a surfactant was treated with sodium hypophosphite in the presence of palladium on carbon (Pd/C) at the reflux temperature. The gas chromatography (GC) analysis of the reaction mixture showed an almost quantitative formation of dodecane (entry 1). A similar treatment at 50°C gave only 6% yield (data not shown). The addition of a base was necessary to neutralize the liberated hydrogen bromide and to prevent catalyst poisoning (entries 1–4). In fact, the yield was reduced to 16% without any bases (entry 5). Among the other surfactants tested, nonionic surfactants, such as Triton X series (entries 6 and 7) and Brij 30 (entry 8) produced satisfactory results, whereas ionic surfactants, such as quaternary ammonium salts (entries 9 and 10) and a linear alkylbenzene sulfonate (entry 11), were less effective. In particular, the reaction in the presence of Aliquat 336 resulted in a poor yield in contrast to Tundo's multiphase system Citation7. However, in the absence of any surfactants, the substrate and catalyst were not well dispersed, and the reaction resulted in only 32% yield (entry 12). It is currently unclear why nonionic surfactants are so effective.
Table 1. Reduction of 1-bromododecane.
Using the reaction conditions employed in entry 1 of , hydrogenolysis of other organic halides was also examined, and representative results are shown in . As shown in entries 2 and 3, the dehalogenation of hydrocinnamyl bromide and β-bromophenetole successfully proceeded at 50°C to afford 3-phenylpropane and phenetole in >99 and 90% yields, respectively. A similar reaction without Tween 20 resulted in somewhat lower yields (data shown in parentheses). Aromatic halides, such as 4-chloroanisole and 4-bromobiphenyl, were smoothly reduced to anisole and biphenyl, respectively, in almost quantitative yields even in the absence of a surfactant (entries 4 and 5). This was probably due to the high adsorbability of aromatic compounds on activated carbons.
Table 2. Reduction of various substrates using an NaH2PO2–Pd/C system.
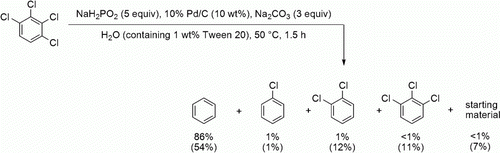
The catalytic hydrodehalogenation of aromatic halides – a promising alternative to traditional high-temperature incineration – is also important as an attractive process for the chemical detoxification of polychlorinated aromatic pollutants, such as polychlorinated biphenyls and dioxins. In this study, we examined the hydrodechlorination of polychlorinated aromatics using the hypophosphite–palladium system using 1,2,3,4-tetrachlorobenzene as a model compound. The reaction was performed in a 1 w/w% aqueous Tween 20 solution containing three equivalents of Na2CO3 at 50°C (), yielding completely dechlorinated benzene in 86% yield and partially reduced products only in trace amounts. However, a similar treatment without Tween 20 produced benzene in 54% yield and considerable amounts of chlorinated intermediates along with a 7% recovery of the starting material (data shown in parentheses), indicating the effectiveness of the surfactant additive.
To explore the scope and limitations of the present protocol, the hydrogenation of other organic substrates, which participate in several reduction processes, was carried out, and the results are also shown in . The reduction of nonactivated alkenes, such as 1-dodecene and trans-stilbene, was achieved by performing the reaction at the reflux temperature, yielding the corresponding saturated hydrocarbons in almost quantitative yields (entries 6 and 7), whereas methyl trans-cinnamate could be reduced even at room temperature to afford the hydrocinnamate in quantitative yield without affecting the ester functionality (entry 8). In these cases, adding Na2CO3 was not necessary. However, for the hydrogenation of diphenylacetylene, the product yield improved from 76 to 97% by adding Na2CO3 despite the fact that the process did not liberate any hydrogen halides (entry 9). We assume that the weakly acidic dihydrogen phosphite generated during the process might act as a weak catalyst poison.
Aliphatic and aromatic nitro compounds, such as nitrocyclohexane, nitrobenzene, and 4-nitrotoluene, were reduced to their corresponding amino compounds in quantitative yields (entries 10–12). In the case of 4-chloronitrobenzene, simultaneous dechlorination occurred during the process to afford aniline in 98% yield (entry 13). Hydrogenation of carbonyl compounds was carried out in a similar manner. Although aliphatic aldehydes and ketones were unreactive, the carbonyl group conjugated with a benzene ring was reduced to its corresponding benzyl alcohol in 68% yield (entry 14). The present method was also effective for the hydrogenolysis of O- and N-benzyl compounds. The reaction of benzyl phenyl ether and N-benzyl-N-ethylaniline gave phenol and N-ethylaniline in 94 and 81% yields, respectively (entries 15 and 16). In these cases, toluene was also produced.
Experimental
General information
All reagents and solvents were commercial products and were used as accepted. GC measurements were performed on a Shimadzu GC-1700 gas chromatograph using a 50 m×0.25 mm methyl silicone capillary column (Quadrex). The GC peaks of the starting materials and reaction products were identified by a comparison of their GC retention times with those of commercially available authentic samples.
General procedure for catalytic transfer hydrogenation
Typically, a mixture of 1-bromodedecane (249 mg, 0.999 mmol), Na2CO3 (107 mg, 1.01 mmol), 10% Pd/C (24.8 mg), and tridecane (107 mg) as an internal standard in 1 w/w% aqueous Tween 20 (6 ml) was placed in a 20-ml recovery flask equipped with a dropping funnel and a reflux condenser and was heated to reflux. To the refluxing mixture, a 1 w/w% aqueous Tween 20 solution (4 ml) of NaH2PO2 H2O (531 mg, 5.01 mmol) was added dropwise over 30 min. After being refluxed for an additional 1 h, the reaction mixture was cooled in an ice bath, and CHCl3 was added as the extraction solvent. Then, the resulting mixture was filtered through Hyflo Super Cel to remove the catalyst. A direct GC analysis of the dried organic phase showed the formation of dodecane in 96% yield, and no starting material was detected.
The hydrogenation of other substrates, which do not liberate any hydrogen halide during the process, was carried out without a base. A mixture of methyl trans-cinnamate (162 mg, 0.999 mmol), 10% Pd/C (16.3 mg), and dodecane (109 mg) as an internal standard in 1 w/w% aqueous Tween 20 (6 ml) was placed in a 20-ml recovery flask equipped with a dropping funnel. To the mixture, a 1 w/w% aqueous Tween 20 solution (4 ml) of NaH2PO2·H2O (532 mg, 5.02 mmol) was added dropwise over 30 min at room temperature. After being stirred at room temperature for an additional 1 h, CHCl3 was added as the extraction solvent. Then, the resulting mixture was filtered through Hyflo Super Cel to remove the catalyst. A direct GC analysis of the dried organic phase showed the formation of methyl 3-phenylpropionate in quantitative yield, and no starting material was detected.
Conclusion
We investigated the palladium-catalyzed transfer hydrogenation of some organic substrates using NaH2PO2 as the hydrogen source in water. The reduction of aliphatic and aromatic halides, alkenes, alkynes, nitro compounds, and aromatic aldehydes, as well as the deprotection of O-benzyl and N-benzyl derivatives, took place smoothly. The hydrodehalogenation of polychlorinated aromatics was also demonstrated using 1,2,3,4-tetrachlorobenzene as a model compound. In most cases, the addition of a nonionic surfactant, such as Tween 20, was necessary to obtain acceptable yields.
Acknowledgements
This work was partly supported by Tokai University and a Tokai University Supporters Association Research and Study Grant. The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- Rylander PN . Catalytic hydrogenation in organic syntheses . New York : Academic Press ; 1979 .
- Boyer SK , Bach J , McKenna J , Jagdmann EJ Jr . Org Chem . 1985 ; 50 : 3408 – 3411 . doi: 10.1021/jo00218a034
- Bakulina GV , Erofeev BV . Russ J Phys Chem . 1972 ; 46 : 125 – 126 .
- Sala R , Doria G , Passarotti C . Tetrahedron Lett . 1984 ; 25 : 4565 – 4568 . doi: 10.1016/S0040-4039(01)81494-5
- Johnstone RAW , Wilby AH . Tetrahedron . 1981 ; 37 : 3667 – 3670 . doi: 10.1016/S0040-4020(01)98896-9
- Boyer SK , McKenna J , Karliner J , Nirsberger M . Tetrahedron Lett . 1985 ; 26 : 3677 – 3680 . doi: 10.1016/S0040-4039(00)89221-7
- Marques CA , Selva M , Tundo P . J Chem Soc Perkin Trans. 1 . 1993 ; 529 – 533 . doi: 10.1039/p19930000529
- Entwistle ID , Jackson AE , Johnstone RAW , Telford RP . J Chem Soc Perkin Trans. 1 . 1977 ; 443 – 444 . doi: 10.1039/p19770000443
- Varma RS , Varma M , Kabalka GW . Synth Commun . 1986 ; 16 : 91 – 96 . doi: 10.1080/00397918608057693
- Balicki R , Kaczmarek Ł . Gazz Chim Ital . 1994 ; 124 : 385 – 386 .
- Gooßen LJ , Ghosh K . Chem Commun . 2002 ; 836 – 837 .
- Khai BT , Arcelli A . J Org Chem . 1989 ; 54 : 949 – 953 . doi: 10.1021/jo00265a040
- Backeberg OG , Staskun B . J Chem Soc . 1962 ; 3961 – 3963 . doi: 10.1039/jr9620003961
- Frija LMT , Cristiano MLS , Guimarães EMO , Martins NC , Loureiro RMS , Bickley JF . J Mol Catal A: Chem . 2005 ; 242 : 241 – 250 . doi: 10.1016/j.molcata.2005.08.022
- Araújo NCP , Brigas AF , Cristiano MLS , Frija LMT , Guimarães EMO , Loureiro RMS . J Mol Catal A: Chem . 2004 ; 215 : 113 – 120 . doi: 10.1016/j.molcata.2003.12.033
- Adger BM , O'Farrell C , Lewis NJ , Mitchell MB . Synthesis . 1987 ; 53 – 55 . doi: 10.1055/s-1987-27841
- Davydov DV , Beletskaya IP . Russ Chem B . 1993 ; 42 : 575 – 577 . doi: 10.1007/BF00698459
- Hidaka H , Saitou A , Honjou H , Hosoda K , Moriya M , Serpone N . J Hazard Mater . 2007 ; 148 : 22 – 28 . doi: 10.1016/j.jhazmat.2007.01.143
- Pri-Bar I , James BR . J Mol Catal A: Chem . 2007 ; 264 : 135 – 139 . doi: 10.1016/j.molcata.2006.07.046