Abstract
A clean and efficient procedure was established to synthesize 1,4-dihydropyridines via one-pot Hantzsch reactions in aqueous medium without the use of a catalyst and/or organic solvent. The reaction with stoichiometric molar ration could be carried out in a sealed vessel with a water steam, air, or nitrogen atmosphere to afford Hantzsch esters in good to excellent yields and purities. After the simple filtration, the 1,4-dihydropyridines were isolated, and the filtrate could be recycled and reused without significantly decreasing the yields and purities. The novel and clean methodology offers the advantages including short reaction time, good yields, operational simplicity, less leaks, and environmentally benign.
Introduction
In recent years, one-pot multicomponent reactions (MCRs) have been extensively studied for their simple procedures, high selectivity, and superior atom economy. Contrary to the classical methods to synthesize complex molecules by sequential procedure, MCRs which are referred to as one-pot synthesis of three or more than three starting materials provide a powerful tool toward the rapid synthesis of diverse and complex heterocyclic compounds.Citation1–4 As one of the known MCRs, Hantzsch reaction has attracted much attention on the synthesis of 1,4-dihydropyridines (1,4-DHPs) due to their significant biological activities and pharmacological properties.Citation5–9 However, the classical Hantzsch method is generally carried out in acetic acid or in refluxing ethanol for long reaction times to afford low yields.Citation10 Additionally, this method has difficulty in the synthesis of different substituted biologically active Hantzsch esters derivatives. Then, some improved synthetic methods for preparing these 1,4-DHPs compounds have been reported by condensation of aldehydes, ammonium salts, and β-keto esters in the presence of Lewis acid,Citation11 Citation12 Bronsted acid,Citation13–15 solid acid,Citation16 base,Citation17 Citation18 biocatalysts,Citation19 Citation20 organo-catalysts Citation21, and ionic liquids Citation22 Citation23 as catalysts. Recently, the progress of modified Hantzsch reactions in aqueous medium, solvent-, and/or catalyst-free synthesis has attracted the attention of chemists because they are environmentally benign processes.Citation24–29 However, the search for new green and facile procedures that are efficient under related mild conditions is still being actively pursued.
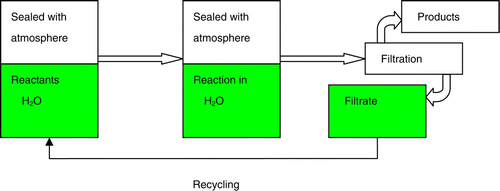
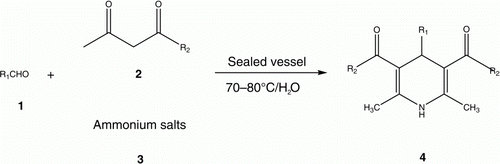
Considering the importance of MCRs and the diverse applications of 1,4-DHPs and the limitation with the previous synthetic routes and our earlier interest in green procedures of MCRs, we now wish to report an efficient and convenient methodology for the Hantzsch reaction by one-pot cyclocondensation of aldehydes, ammonium salts, and β-keto esters under organic solvent-free and catalyst-free conditions ().
Results and discussion
To optimize the one-pot, catalyst-, and organic solvent-free clean procedure conditions, in the initial experiments, benzaldehyde, ethyl acetoacetate, and ammonium carbonate (molar ratio was 1:2:1) were used as model reactants in open and sealed vessels (). As shown in , generally open-vessel reactions (typically, the flask charged with a reflux condenser) offered less efficiency and lower yields (entries 1 and 2), which were different from the reported work.Citation25 Additionally, in the reaction processing, white solid could be found from the bottom to middle in reflux condenser, suggesting that ammonium carbonate decomposed in aqueous media at the reaction temperature to produce ammonia, carbon dioxide, and water, and they combined again in the reflux condenser when cooled. Considering the good solubility of ammonium salts in aqueous medium, in our procedure, the reflux condenser was deleted, and the reactor was sealed by air or other atmospheres (entries 3–6). To our satisfaction, the one-pot Hantzsch reaction with stoichiometric amount of reactants proceeded smoothly with high efficiency in aqueous medium to afford excellent yields in a sealed vessel. Among the several sealed systems, the atmospheric pressure approached to outside when the water steam was used before the reaction, and the principle is in some cases similar to a thermos bottle with a cork rather than an autoclave. It is generally known that the temperature is not more than 75°C, or the volume of water occupies the 85% capacity of bottle, and the cork is stable. In case of the other atmospheres, the pressure increased with the increase of reaction temperatures and the volume remained the same in the vessel. Less leaks of this procedure is not only facilitated for the Hantzsch reaction but also has the potential application for others with easy decomposition and/or ugly smell reactants such as ammonium salts, including NH3·H2O, (NH4)2CO3, NH4HCO3, HCO2NH4 and CH3COONH4, CO(NH2)2. The reaction temperature might range from 0 to 90 °C, below the boiling point of water.
Table 1. Effect of different reaction system on one-pot catalyst-free Hantzsch reaction.
To explore the reaction medium, different solvents were then selected as reaction media with the above model reactants and open-vessel reactors. The results are summarized in . Among the eight reaction conditions, one-pot Hantzsch reaction was accomplished in polar solvents such as ethanol, water, or their mixture with good to excellent yields. Less efficiency and relative lower yields were found in weaker polar solvents, such as toluene and dichloromethane, because of the poor solubility of inorganic ammonium salts in these solvents. Additionally, the reaction proceeded in moderate yield without any solvent. Significant amount of white solid formed in reflux condenser in this process due to the sublimation of ammonium carbonate. From the results of and , it could easily be seen that the designed sealed system has the advantage of being clean, efficient, and high yielding compared with the open system without an organic solvent and a catalyst, although the high specific heat capacity of water makes prolonged heating of the reaction mixture to be undesirable. Water is a polar inorganic solvent in this process of sublimation of ammonium carbonate, or decomposing when heating, they can be composed and/or dissolved in water again in a sealed system, which is of great importance for the reaction.
Table 2. Effect of different solvents on one-pot catalyst-free Hantzsch reaction.a
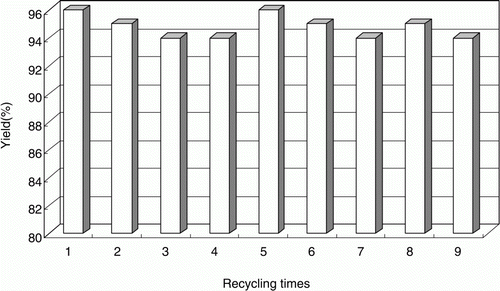
It is of great importance that the recycling performance of the reaction medium and/or the catalyst should be considered while designing or establishing a clean procedure. Hence, the recycling performance of the reaction system was investigated using the same model reaction when optimizing the reaction conditions. After the reaction and cooling to room temperature, the 1,4-DHPs was isolated by simple filtration in good purity. The filtrate containing the excess unreacted reactant (ranged from 2% to 3%) could be recycled and reused in the next cycle without further treatment. The fresh substrates benzaldehyde, ethyl acetoacetate, and ammonium carbonate (stoichiometric molar ratio was 1:2:1) were charged into the filtrate, and the mixture was reacted under the same reaction conditions as model reactants as before, without decreasing the yield and purity. The data listed in showed that the filtrate could be reused at least nine times without decreasing the efficiency and yield. The easy recycling performance is indeed an attractive property of the green procedure for the environmental protection and economic reasons. This recycle and reuse procedure reduced the emission of waste water after the reaction.
From the above experiment, it could be inferred that the reaction temperature might play an important role in the one-pot Hantzsch reaction procedure. Thus, a similar model of MCRs condensation of ethyl acetoacetate, benzaldehyde, and ammonium carbonate was studied in aqueous medium. The increasing of the reaction temperature would on the one hand facilitate this reaction, but on the other hand, result in the decomposition or sublimation of the ammonium carbonate, which was also different from the reported work.Citation25 The results listed in showed that the optimal reaction temperature was 70–75°C, and this sealed system would not only prevent the loss of the ammonium salts and environmental pollution but also proved satisfactory with respect to reaction time and yield of the desired 1,4-DHPs.
Table 3. Effect of reaction temperature on Hantzsch reaction.
Besides the reaction media and other reaction conditions, the reactants might be an important factor and should be considered for the potential application of a green procedure on a large scale. The effect of the nitrogen source (ammonium salts) on the Hantzsch reaction was subsequently explored, a similar model of MCRs condensation of benzaldehyde, ethyl acetoacetate, and an ammonium salt (mole ratio = 1:2:1) under above optimal conditions. It can be seen from that ammonium salts sourced from strong acids such as Cl−, SO4 2 −, and NO3 − showed lower efficiency compared with that from weak acids such as CO3 2 −, HCO3 −, HCOO−, and CH3COO−. This trend suggests that the mild buffered pH of the reaction media played an important role in this procedure. The nucleophilic capability of ammonia decreased with the increase of acidity in aqueous medium because of the hydrogen bonding and protonation of ammonia in reaction medium, which might be responsible for the lower activity. HCO2NH4 and CH3COONH4 have relative lower atom economy and are expensive than inorganic ammonium salts. It is of no doubt that ammonium carbonate should be the optimized nitrogen source in this procedure for its safety, high efficiency, and a bargain price.
Table 4. Effect of different ammonium salts on Hantzsch reaction.
Then, the condensation reactions of various aldehydes with ethyl- or methyl acetoacetate and ammonium carbonate were screened based on the optimized conditions to yield respective 1,4-DHPs, and the results are presented in . It can easily be seen that, in all cases, the reactions gave the products in good yields ranging from 86% to 96%. Most importantly, many of the pharmacologically relevant substitution patterns on the aromatic ring could be introduced without any interruption in efficiency. Aromatic aldehydes carrying either electron-donating or electron-withdrawing substituents afforded good yields of 1,4-DHPs in high purity. Besides the aromatic aldehydes, aliphatic aldehydes (entries 13 and 14) can also be employed without any decrease in yields. Another important feature of this procedure is the survival of a variety of functional groups such as ethers, esters, nitro, hydroxyl, and halides under the present reaction conditions. Additionally, to reduce or eliminate the leaks or emission, after the separation of the product, the filtrate containing the unreacted reactants could be recycled and reused without any treatment, using the same reactants and stoichiometric molar ratio as the previous.
Table 5. Catalyst- and organic solvents-free synthesis of 1,4-dihydropyridines.
In summary, we have designed a novel, clean, and efficient procedure for synthesis of 1,4-dihydropyridines via one-pot Hantzsch reactions with satisfactory yields of 86–96%. The catalyst-free, organic solvent-free, and condenser-free clean procedure was accomplished smoothly in the sealed system. This procedure established has the advantages of least leaks and emission, high efficiency and recyclable performance, which has the potential application for the green synthesis.
Experimental
Melting points were determined on X-6 microscope melting apparatus and reported uncorrected. 1H NMR spectra were recorded on Bruker DRX500 (500 MHz) spectrometer. FTIR spectra were recorded on a Nicolet AVATR-360 spectrometer. All chemicals (AR grade) were commercially available and used without further purification.
General procedure for the one-pot Hantzsch reaction in aqueous medium
To a tube reactor charged with stoichiometric molar ratio of aldehyde (1 mmol) 1, β-keto esters (2 mmol) 2, and ammonium salts (1mmol) 3 was added 2–3 mL water, the remaining space was charged with water steam, air, or nitrogen and then closed quickly. The mixture was then stirred for a desired time at 70–75 °C (). On completion (monitored by TLC), the final mixture was then cooled to room temperature and stayed overnight. The mixture was filtered to isolate the precipitated product, and the resulting solid 1,4-DHPs could be crystallized from 95% ethanol to give the pure crystalline products 4, and identified by IR, 1HNMR, and physical data (m.p.) with those reported in the literature. The filtrate could be recycled and reused in next run without any treatment.
Acknowledgment
This work was financially supported by Key Laboratory of Organic Synthesis of Jiangsu Province (KJS1112).
References
- Dömling A , Ugi I . Multicomponent reactions with isocyanides . Angew Chem Int Ed . 2000 ; 39 18 : 3168 – 3210 . doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
- Zhu , J and Bienayme , H. 2005 . Multi component reactions , Weinheim : Wiley-VCH .
- Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry . Chem Rev . 2006 ; 106 1 : 17 – 89 . doi: 10.1021/cr0505728
- Kalinski C , Lemoine H , Schmidt J , Burdack C , Kolb J , Umkehrer M , Ross G . Multicomponent reactions as a powerful tool for generic drug synthesis Synthesis . 2008 ; 24 : 4007 – 4011 .
- Bocker RH , Guengerich EPJ . Oxidation of 4-aryl- and 4-alkyl-substituted 2,6-dimethyl-3,5-bis(alkoxycarbonyl)-1,4-dihydropyridines by human liver microsomes and immunochemical evidence for the involvement of a form of cytochrome P-450 J Med Chem . 1986 ; 29 : 1596 – 1601 .
- Janis RA , Triggle DA . New developments in calcium ion channel antagonists J Med Chem . 1983 ; 25 : 775 – 779 . doi: 10.1021/jm00360a001
- Sirisha K , Bikshapathi D , Achaiah G , Reddy VM . Synthesis, antibacterial and antimycobacterial activities of some new 4-aryl/heteroaryl-2,6-dimethyl-3,5-bis-N-(aryl)-carbamoyl-1,4-dihydropyridines . Eur J Med Chem . 2011 ; 46 5 : 1564 – 1571 . doi: 10.1016/j.ejmech.2011.02.003
- Lu J , Bai Y-J , Yang B-Q , Ma H-R . Improved synthesis and aromatization of 1,4 - dihydropyridines Chin J Org Chem . 2000 ; 4 : 514 – 517 .
- Shi D-Q , Mou J , Zhuang Q-Y , Wang X-S , Tu S-J . One-pot synthesis of 4-aryl-1,4-dihydropyridines in water Chin J Org Chem . 2004 ; 9 : 1042 – 1044 .
- Hantzsch A . Ueber die synthese pyridinartiger verbindungen aus acetessigäther und aldehydammoniak Justus Liebigs Ann. Chem . 1882 ; 215 : 1 . doi: 10.1002/jlac.18822150102
- Reddy CS , Raghu M . Facile ZrCl4 promoted four-component coupling one-pot synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction Indian J Chem B . 2008 ; 47B : 1578 – 1582 .
- Ko S , Sastry MNV , Lin C , Yao CF . Molecular iodinecatalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction Tetrahedron Lett . 2005 ; 46 : 5771 – 5774 . doi: 10.1016/j.tetlet.2005.05.148
- Zolfigol MA , Safaiee M . Synthesis of 1,4-dihydropyridines under solvent-free conditions Synlett . 2004 ; 5 : 827 – 829 . doi: 10.1055/s-2004-820010
- Nandi GC , Samai S , Singh MS . Biginelli and Hantzsch-type reactions leading to highly functionalized dihydropyrimidinone, thiocoumarin, and pyridopyrimidinone frameworks via ring annulation with β-oxodithioesters . J Org Chem . 2010 ; 75 22 : 7785 – 7795 .
- Liang J-C , Yeh J-L , Wang C-S , Liou S-F , Tsai C-H , Chen I-J . The new generation dihydropyridine type calcium blockers, bearing 4-phenyl oxypropanolamine, display α-/β-Adrenoceptor antagonist and long-Acting antihypertensive activities . Bioorg Med Chem . 2002 ; 10 3 : 719 – 730 . doi: 10.1016/S0968-0896(01)00318-2
- Rafiee E , Eavani S , Rashidzadeh S , Joshaghani M . Silica supported 12-tungstophosphoric acid catalysts for synthesis of 1,4-dihydropyridines under solvent-free conditions . Inorg Chim Acta . 2009 ; 362 : 3555 – 3562 . doi: 10.1016/j.ica.2009.03.049
- Antonyraj CA , Kannan S . Hantzsch pyridine synthesis using hydrotalcites or hydrotalcite-like materials as solid base catalysts Appl Catal A: Gen . 2008 ; 338 : 121 – 129 . doi: 10.1016/j.apcata.2007.12.028
- Shen L , Cao S , Wu J , Li H , Zhang J , Wu M , Qian X . K2CO3-assisted one-pot sequential synthesis of 2-trifluoromethyl-6-difluoromethylpyridine-3,5-dicarboxylates under solvent-free conditions . Tetrahedron Lett . 2010 ; 51 37 : 4866 – 4869 . doi: 10.1016/j.tetlet.2010.07.041
- Lee JH . Synthesis of Hantsch 1,4-dihydropyridines by fermenting bakers' yeast . Tetrahedron Lett . 2005 ; 46 43 : 7329 – 7330 . doi: 10.1016/j.tetlet.2005.08.137
- Wang J-L , Liu B-K , Yin C , Wu Q , Lin X-F . Candida antarctica lipase B-catalyzed the unprecedented three-component Hantzsch-type reaction of aldehyde with acetamide and 1,3-dicarbonyl compounds in non-aqueous solvent Tetrahedron . 2011 ; 67 14 : 2689 – 2692 . doi: 10.1016/j.tet.2011.01.045
- Baghbanian SM , Khaksar S , Vahdat SM , Farhang M , Tajbakhsh M . One-step, synthesis of Hantzsch esters and polyhydroquinoline derivatives using new organocatalyst . Chin Chem Lett . 2010 ; 21 5 : 563 – 567 . doi: 10.1016/j.cclet.2009.12.011
- Pajuste K , Plotniece A , Kore K , Intenberga L , Cekavicus B , Kaldre D . Use of pyridinium ionic liquids as catalysts for the synthesis of 3,5-bis(dodecyloxycarbonyl)-1,4-dihydropyridine derivative Cent Eur J Chem . 2011 ; 9 : 143 – 148 . doi: 10.2478/s11532-010-0132-x
- Reddy BP , Rajesh K , Vijayakumar V . Ionic liquid [tbmim]Cl2/AlCl3 under ultrasonic irradiation towards synthesis of 1,4-DHP's Arab J Chem . 2011 . doi: org/10.1016/j.arabjc.2011.01.027
- Saha M , Roy S , Chaudhuri SK , Bhar S . Microwave-assisted ammonium formate-mediated synthesis of Hanstzch dihydropyridines under solvent-free conditions – a green protocol Green Chem Lett Rev . 2008 ; 1 : 99 – 102 . doi: 10.1080/17518250802095034
- Goswarmi P . One pot synthesis of unsymmetrical dihydropyridines by green, catalyst free and environmentally benign protocol Green Chem Lett Rev . 2009 ; 1 : 173 – 177 .
- Tamaddon F , Razmi Z , Jafari AA . Synthesis of 3,4-dihydropyrimidin-2(1H)-ones and 1,4-dihydropyridines using ammonium carbonate in water . Tetrahedron Lett . 2010 ; 51 8 : 1187 – 1189 . doi: 10.1016/j.tetlet.2009.12.098
- Palmisano G , Tibiletti F , Penoni A , Colombo F , Tollari S , Garella D , Taglipeitra S , Cravotto G . Ultrasound-enhanced one-pot synthesis of 3-(Het)arylmethyl-4-hydroxycoumarins in water . Ultrason Sonochem . 2011 ; 18 2 : 652 – 660 . doi: 10.1016/j.ultsonch.2010.08.009
- Safari J , Banitaba SH , Khalili SD . Cellulose sulfuric acid catalyzed multicomponent reaction for efficient synthesis of 1,4-dihydropyridines via unsymmetrical Hantzsch reaction in aqueous media . J Mol Catal A: Chem . 2011 ; 335 1–2 : 46 – 50 .
- Kumar A , Sharma , S . A grinding-induced catalyst- and solvent-free synthesis of highly functionalized 1,4-dihydropyridines via a domino multicomponent reaction Green Chem . 2011 ; 13 : 2017 – 2020 .