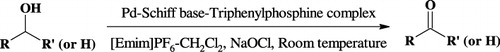Abstract
Palladium (Pd)-catalyzed carbonylation of alcohols proceeds in ionic liquid (IL) media (1-ethyl-3-methylimidazolium hexafluorophosphate). Carbonylation of primary/secondary alcohols to aldehydes/ketones was greatly accelerated by the use of a Pd-based catalyst in the presence of NaOCl as an oxidant. The catalyst was more easier to recycle in the IL [Emim]PF6 with an equal-proportioned CH2Cl2 than in the single CH2Cl2 or IL.
Introduction
Palladium (Pd) is an extraordinarily versatile catalyst for organic synthesis because of its stability and ease of handling. It is however very expensive and it is important knowing that it can be very efficiently recovered from reactions and subsequently recycled. Traditional homogeneous Pd catalysts can be very difficult to separate efficiently. Heterogenization and the use of easily separable solvents are widely cited approaches to ease the problem. Pd-catalyzed carbonylation of alcohols is a highly effective method for the synthesis of various carbonyl compounds such as carboxylic acids, esters, amides, aldehydes, and ketones (Citation1). Pd complexes with Schiff base ligands were found to be efficient homogeneous catalysts under mild reaction conditions (Citation2).
The selective catalytic oxidation of alcohols to their respective aldehydes or ketones is a major challenge for industries. Such reactions are frequently performed at very low conversion rates in order to avoid the formation of carboxylic acids and, if performed in the gaseous phase, decomposition to carbon dioxide (Citation3). These over-oxidations occur due to the large activation energy. Another problem of conventional oxidation is that, if performed in the liquid phase, ethanoic acid is often used as a solvent with a transition metal catalyst. This mixture is corrosive and requires laborious separation from the products; in fact, catalyst recycling is often not feasible (Citation4, Citation5).
One of the alternative approaches for tackling these problems is the usage of ambient temperature ionic liquids (ILs). These solvents have been employed as the promising solvents for the above-mentioned reactions because of their solvating ability, negligible vapor pressure, easy recyclability, and reusability. Over the last several years, many transition metals, such as nickel (Citation6), cobalt (Citation7), ruthenium (Citation8), manganese (Citation9), tungsten (Citation10), rhenium (Citation11), iron (Citation12), and vanadium (Citation13), have been used as catalysts for alcohol oxidation with ILs as green solvents (Citation14).
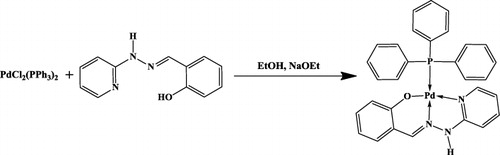
An efficient method for the selective oxidation of alcohols to the corresponding aldehydes and ketones using Pd complex containing triphenylphosphine and a Schiff base [PdL] () as a catalyst in CH2Cl2/NMO system was reported (Citation15). Based on the synthesized catalyst, we describe an extremely effective and reusable oxidation system of alcohols performed in IL [Emim]PF6 with a well-proportioned CH2Cl2. And the catalyst is a simple Pd complex using NaOCl as oxidant at room temperature.
PdL-catalyzed oxidation of alcohols (Citation15) was previously employed as a catalyst for the oxidation of alcohols. It has been studied that the ILs have evident acceleration in the rate on some catalytic reactions, for example, scandium triflate catalyzed Fridel-Crafts alkylation of arenes (Citation16), Mn(salen) catalyzed asymmetric epoxidation of olefins (Citation17), and Mn(Salen) catalyzed oxidation of sec-alcohols (Citation9). Hence, we employed PdL as a catalyst and observed whether there was the same rate acceleration effect on the oxidation of alcohols. Interestingly, the reaction proceeded more rapidly in the IL [Emim]PF6 with an equally proportioned CH2Cl2 than in IL alone; the pure IL did not give a satisfactory rate acceleration in this reaction. A general reaction for the catalytic oxidation of alcohols to corresponding carbonyl compounds is shown in .
Results and discussion
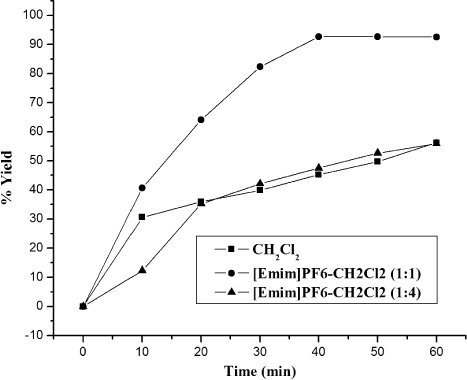
Three different fractions of [Emim]PF6 IL were chosen to make a kinetic study which could clearly illustrate that the acceleration in the rate of the reaction results from the different mixed proportion. As shown in , the reaction was almost complete with 40 minutes in the mixed solvent [Emim (1 ml) + CH2Cl2 (1 ml)]; however, there was no obvious rate acceleration effect in the mixed solvent [Emim (4 ml) + CH2Cl2 (1 ml)].
Benzyl alcohol and PdL were used for the optimization of the reaction conditions in EMIM-NaOCl system (). The initial study was carried out using benzyl alcohol as a substrate and three equivalents of aqueous NaOCl at ambient temperature in the presence of 0.02 mmol of a catalyst. The oxidation proceeded smoothly and 90% isolated yield of benzaldehyde was obtained with 97% conversion and 98% selectivity (2% benzoic acid detected by 1H NMR) after 40 minutes of stirring.
Table 1. Oxidation of benzylalcohol to benzaldehydeFootnotea in EMIM–NaOCl system.
The oxidation occurred only in poor yield by simply bubbling molecular oxygen through reaction mixture under similar reaction conditions (, entry 2). To evaluate the catalytic effect of PdL, the oxidation of benzylalcohol was carried out under similar reaction conditions in the absence of catalyst and no conversion was observed (, entry 3). The reaction was complete at 40 minutes in room temperature (, entry 1). Further, when the oxidation of benzylalcohol was carried out using less amount of PdL, the yield was low (, entry 5). Also the reaction was carried out by varying the oxidant concentration to get the optimum yield (, entries 1 and 6).
The oxidation of other benzylic alcohols was then examined using the optimized reaction conditions (). Benzylic primary and secondary alcohols oxidize smoothly to give aldehydes and ketones, respectively. Lower yields were obtained in the oxidation of aliphatic alcohols and the major products were the corresponding acids (detected by 1H NMR). All the experiments are carried out in air atmosphere since there is no change in conversion if reaction is carried out under argon.
Table 2. Oxidation of alcohols catalyzed by Pd(II) complexes.Footnotea
Inspite of IL being nonvolatile and nonflammable, its improper disposal would still lead to new pollution of environment (because of minimal degradation in the environment). At the same time, ILs are also expensive reagents. Thus, attention needs to be paid to recycling and reuse of ILs.
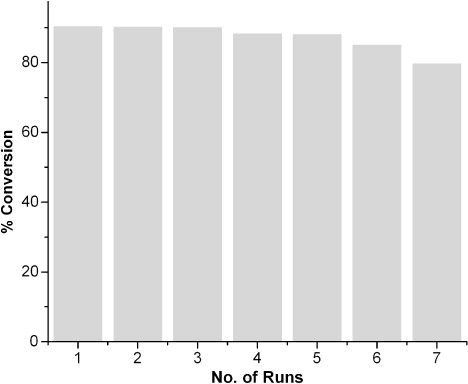
shows the efficiency of the reaction in [EMIM]Cl/catalyst mixture after seven runs with benzylalcohol as substrate. It is clear from the figure that recycling of IL slightly affects the conversion of the product.
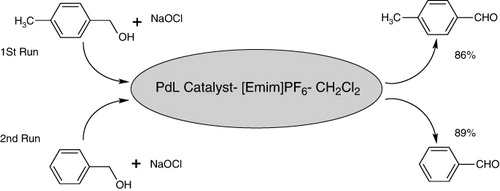
In addition to recycling of the IL CH2Cl2 and catalyst mixture for the same substrate, it is also important to verify the viability of reusing the catalytic system for different substrates, which was confirmed by two consecutive reactions (). After the oxidation of p-methylbenzyl alcohol to the aldehyde, the recovered [Emim]PF6-CH2Cl2 and catalyst were reused for the oxidation of benzyl alcohol to benzaldehyde, and the 1H NMR analysis of the crude mixture of the second reaction indicated no contamination of the earlier product (<1%).
Experimental
General
All chemicals (AR grade) were obtained from commercial resources and used without further purification. Products were all known compounds and were identified by comparing their physical and spectroscopic data with those reported in the literature; 1H NMR spectra were obtained with TMS as internal standard using a Bruker AV 400 (400MHz) spectrometer; IR spectra were recorded on a Shimadzu spectrometer using KBr disks.
Catalytic experiments
A solution of Pd complex (0.02 mmol) in 2 mL of 1:1 mixture of Ethyl-Methyl-Imidazolium (EMIM) IL and dichloromethane was added to the solution of substrate (1 mmol) and NaOCl (1 mmol). The mixture was stirred at room temperature. At the requisite times, aliquots of the reaction mixture were removed and the alcohol and aldehyde/ketone extracted with ether. The ether solution was then analyzed by gas chromatography.
Conclusion
In conclusion, a series of experiments described represents a useful method for the oxidation of alcohols to carbonyl compounds at room temperature. This article substantiates the importance of environmental friendly reaction by using ionic liquids. This will be a step toward green synthesis.
Acknowledgments
The authors thank the Indian Institute of Science, Bangalore, for NMR analysis.
References
- Tsuji, J. Palladium Reagents and Catalysts; John Wiley & Sons: Chichester, 1995; pp 265–275.
- Islam, S.M.; Bose, A.; Palit, B.K.; Saha, C.R. J. Catal. 1998, 173, 268. doi:10.1006/jcat.1997.1836.
- Konietzni, F.; Kolb, U.; Dingerdissen, U.; Maier, W.F. J. Catal. 1998, 176, 527. doi:10.1006/jcat.1998.2076.
- Borgaonkar, H.V.; Raverkar, S.R.; Chandalla, S.B. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 455. doi:10.1021/i300015a026.
- Dugmore, G.M.; Powels, G.J.; Zeelie, B. J. Mol. Catal. A. 1995, 99, 1. doi:10.1016/1381-1169(95)00023-2.
- Ramakrishna, D.; Ramachandra Bhat, B.; Karvembu, R. Catal. Commun. 2010, 11, 498. doi:10.1016/j.catcom.2009.12.011.
- Ramakrishna, D.; Ramachandra Bhat, B. Inorg. Chem. Commun. 2009, 13, 195. doi:10.1016/j.inoche.2009.11.014.
- Wolfson, A.; Wuyts, S.; De Vos, D.E.; Vankelecom, I.F.J.; Jacobs, P.A. Tetrahedron Lett. 2002, 43, 8107. doi:10.1016/S0040-4039(02)01921-4.
- Li, J.W.; Sun, W.; Xu, L.W.; Xia, C.G.; Wang, H.W. Chinese Chem. Lett. 2004, 15, 1437.
- Chhikara, B.S.; Tehlan, S.; Kumar, A. Synlett. 2005, 63.
- Bianchini, G.; Crucianelli, M.; de Angelis, F.; Neri, V.; Saladino, R. Tetrahedron Lett. 2005, 46, 2427. doi:10.1016/j.tetlet.2005.02.056.
- Kumar, A.; Jain, N.; Chauhan, S.M.S. Synlett. 2007, 411. doi:10.1055/s-2007-967951.
- Nan, J.; Arthur, J.R. Tetrahedron Lett. 2007, 48, 273. doi:10.1016/j.tetlet.2006.11.032.
- Muzart, J. Adv. Synth. Catal. 2006, 348, 275. doi:10.1002/adsc.200505273.
- Ramakrishna, D.; Bhat, B.R. Appl. Organometal. Chem. 2010, 24, 663. doi:10.1002/aoc.1683.
- Song, C.E.; Shim, W.H.; Choi, J.H. Chem. Commun. 2000, 1695. doi:10.1039/b005335j.
- Song, C.E.; Roh, E.J. Chem. Commun. 2000, 837. doi:10.1039/b001403f.