Abstract
A simple and efficient one-pot synthetic protocol for the 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s was developed by condensation reaction of aromatic aldehydes, ethyl acetoacetate, and phenylhydrazine/hydrazine hydrate catalyzed by 2-hydroxy ethylammonium propionate under solvent-free conditions. The remarkable features of this new protocol are high yields, short reaction times, easy workup, reusability of inexpensive ionic liquid, and environmentally benignancy.
Introduction
4,4′-(Arylmethylene)bis(1H-pyrazol-5-ol)s have been employed as fungicides (Citation1), pesticides (Citation2), and dyestuffs (Citation3). The condensation of aldehydes with two equivalents of 3-methyl-1-phenyl-5-pyrazolone is a known used route for synthesizing 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s. Xanthan sulfuric acid (Citation4), phosphomolybdic acid (Citation5), silica sulfuric acid (Citation6), 3-aminopropylated silica gel (Citation7), sodium dodecyl sulfate (Citation8), tetramethyl-tetra-3,4-pyridinoporphyrazinato copper(II) methyl sulfate (Citation9), poly(ethylene glycol)-bound sulfonic acid (Citation10), cellulose sulfuric acid (Citation11), lithium hydroxide monohydrate (Citation12), 1,3,5-tris(hydrogensulfato) benzene (Citation13), sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester (Citation14), ethylenediammonium diacetate (Citation15), 2-hydroxy ethylammonium acetate (Citation16), N-(3-silicapropyl)-N-methyl imidazolium hydrogen sulfate (Citation17), benzyltriethylammonium chloride (Citation18), ceric ammonium nitrate (Citation19), silica-bonded S-sulfonic acid (Citation20), 1,3-disulfonic acid imidazolium tetrachloroaluminate (Citation21), and 1-sulfopyridinium chloride (Citation22) can be used as catalysts for this transformation. Hasaninejad et al. reported the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s by the condensation of aldehydes with two equivalents of 3-methyl-1-phenyl-5-pyrazolone in the absence of catalyst (Citation23–Citation25). Elinson et al. applied the electrocatalytic procedure to this transformation (Citation26). Niknam et al. reported the one-pot, multicomponent synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s by tandem Knoevenagel-Michael reaction of phenylhydrazine, ethyl acetoacetate, and aldehydes in the presence of silica-bonded N-propylpiperazine sulfamic acid (SBPPSA) as a recyclable solid acid catalyst (Citation27). However, most of these methods suffer from limitations such as moderate yields, long reaction times, the requirement of special apparatus, use of organic solvents or expensive catalysts, and tedious work-up procedures. So there is still a need to develop a new and convenient method for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s.
Ionic liquids have been widely used as environmentally benign reaction media and catalysts in organic synthesis because of their unique properties of nonflammability, nonvolatility, and recyclability (Citation28–Citation32). In recent years, the usage of hydroxyl ammonium ionic liquids has drawn considerable attention as an inexpensive, nontoxic, readily available catalyst for various organic transformations to afford the corresponding products in high yields (Citation33–Citation35).
Multicomponent reactions are generally one-pot reactions, in which three or more starting materials react together to form a product (Citation36). The one-pot character delivers fewer by-products compared to classical stepwise synthetic routes, with lower costs, time, and energy.
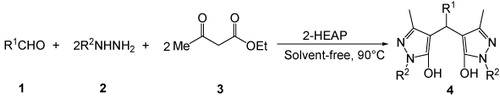
Recently, Sobhani et al. reported the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s by the reaction of aldehydes with 3-methyl-1-phenyl-5-pyrazolone in ethanol in the presence of 2-hydroxy ethylammonium acetate as a recyclable catalyst (Citation16). Here we wish to report the one-pot synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s, starting directly from aldehydes, phenylhydrazine/hydrazine hydrate, and ethyl acetoacetate using 2-hydroxy ethylammonium propionate as an efficient, cost-effective, and recyclable catalyst under solvent-free conditions ().
Results and discussion
In order to optimize the reaction conditions, the reaction of phenylhydrazine, ethyl acetoacetate, and 2,4-dichlorobenzaldehyde in the presence of a catalytic amount of catalyst under solvent-free conditions at 50°C was investigated. As shown in , the products were obtained in 40, 41, and 69% yields, respectively (, entries 1–3). It was obvious that 2-hydroxy ethylammonium propionate was an efficient catalyst for this transformation.
Table 1. Effect of different reaction conditions on ionic liquids catalyzed synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s.Footnotea
Furthermore, the effect of reaction temperature was examined. The mixture was stirred at different temperatures ranging from 50 to 100°C, with an increment of 10°C each time. The results show that the higher reaction temperature, the more efficiently the reaction could proceed, and 90°C was found to be the optimum temperature in terms of yield and reaction time. To determine the optimum concentration of catalyst, we have investigated the model reaction at 5, 10, and 20 mol% of 2-hydroxy ethylammonium propionate under solvent-free conditions at 90°C. The product was obtained in 71, 87, and 87% yield, respectively. This indicates that the use of 10 mol% of 2-hydroxy ethylammonium propionate is sufficient to promote the reaction forward. The catalyst plays a crucial role in the reaction. A blank experiment without catalyst gave very low yield after 210 min (, entry 7). While using 2-hydroxy ethylammonium propionate (10 mol%) as catalyst, the reaction gives the product in 87% yield at the same temperature in 45 min.
To evaluate the effect of solvent, we examined different solvents for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s in the presence of catalyst (, entries 12–17). The various solvents screened were tetrahydrofuran, acetonitrile, chloroform, benzene, ethanol, and water. It is observed that solvent-free conditions gave the best result for this transformation.
We further examined a wide variety of aromatic aldehydes with various substituents to establish the utility of this reaction. The results were summarized in . Ortho-, meta-, and para-substituted aromatic aldehydes containing electron-withdrawing groups (such as nitro group, halide) or electron-donating groups (such as hydroxyl group, alkoxyl group) undergo this one-pot multicomponent synthesis with phenylhydrazine and ethyl acetoacetate to afford 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s in good yields. When changing phenylhydrazine into hydrazine hydrate, a similar result was given; the reaction gave the corresponding products in good yields.
Table 2. 2-Hydroxy ethylammonium propionate catalyzed synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s.Footnotea
Recovery and reusability of the catalyst are demanded for an eco-friendly catalytic process. In this protocol, we used 2-hydroxy ethylammonium propionate as a recyclable catalyst. Progress of the reaction was monitored by thin-layer chromatography (TLC). After completion of the reaction, as indicated by the TLC, the reaction mixture was cooled to room temperature. The crude product was purified by recrystallization from ethanol (95%). The filtrate containing catalyst was evaporated under reduced pressure to give the catalyst which was directly used for the next run without further treatment under similar reaction conditions. As can be seen from , the catalyst was reused for successive reaction for at least five times without any loss of catalytic activity.
Table 3. Recycling of the 2-hydroxy ethylammonium propionate.Footnotea
In order to show the merit of this method, we have compared our results with results obtained by other group. The data listed in show that the 2-hydroxy ethylammonium propionate (2-HEAP) was relatively a good catalyst for this reaction. It is also noteworthy that 2-HEAP, which can be easily prepared from cheaply available acid and ethanolamine by a simple acid-base neutralization reaction, is very inexpensive compared with SBPPSA.
Table 4. Comparison of efficiency of various catalysts in synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s by one-pot three-component condensation reaction of 2,4-dichlorobenzaldehyde, ethyl acetoacetate, and phenylhydrazine.
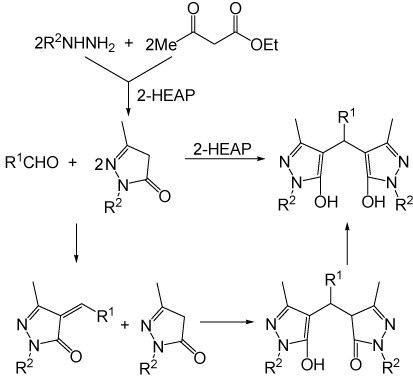
Sobhani et al. reported that 2-hydroxy ethylammonium acetate exhibited a high catalytic activity in the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s by the reaction of aldehydes with 3-methyl-1-phenyl-5-pyrazolone (Citation16). Herein we found that 2-hydroxy ethylammonium propionate exhibited a high catalytic activity not only in this transformation but also in the synthesis of 3-methyl-1-phenyl-5-pyrazolone by the condensation of phenylhydrazine with ethyl acetoacetate. Ethyl acetoacetate treated with phenylhydrazine under solvent-free conditions at 90°C for 50 min gave 3-methyl-1-phenyl-5-pyrazolone in 88% yield in the presence of 2-hydroxy ethylammonium propionate (10 mol%). The same reaction, carried out for 270 min in the absence of 2-hydroxy ethylammonium propionate, only gave 3-methyl-1-phenyl-5-pyrazolone in 79% yield. 2,4-Dichlorobenzaldehyde and 3-methyl-1-phenyl-5-pyrazolone treated with 2-hydroxy ethylammonium propionate (10 mol%) under solvent-free conditions at 90°C for 25 min provided 4,4′-[(2,4-dichlorophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) in 89% yield. The same reaction, carried out for 210 min in the absence of 2-hydroxy ethylammonium propionate, only gave the product in 9% yield. Based on our results and those reported in literature (Citation16), we proposed a mechanism for the one-pot synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s from aldehydes, phenylhydrazine/hydrazine hydrate and ethyl acetoacetate (). The first step involves the formation of pyrazolone by the condensation of phenylhydrazine/hydrazine hydrate with ethyl acetoacetate. Then, aldehydes reacted with 2 equiv. of pyrazolone resulted in the formation of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s via tandem Knoevenagel-Michael reaction.
Experimental
All chemicals were commercial products. 2-Hydroxy ethylammonium formate (2-HEAF), 2-hydroxy ethylammonium acetate (2-HEAA), and 2-hydroxy ethylammonium propionate (2-HEAP) were prepared according to the literature method (Citation37). Melting points were determined on an X-4 micro melting point apparatus and are uncorrected. FT-IR spectra were obtained as KBr pellets on a Nexus 470 spectrophotometer. 1H NMR spectra were recorded in deuterated dimethylsulfoxide on a Bruker Avance III 400 with TMS as internal standard.
General procedure for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s
A mixture of aldehyde (5 mmol), ethyl acetoacetate (10 mmol), phenylhydrazine (10 mmol), and 2-hydroxy ethylammonium propionate (0.5 mmol) was stirred at 90°C under solvent-free conditions. Progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature, where upon it solidified. The pure product was obtained from this solid by recrystallization in ethanol (95%). The filtrate containing catalyst was concentrated to recover catalyst, which was reused in subsequent reactions without further purification. All of the products are known, and physical data were found to be identical with those reported in the literature.
Representative spectral data
4,4′-(Phenylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (4a)
1H NMR (DMSO-d6, 400 MHz): δ = 2.32 (s, 6H, 2CH3), 4.96 (s, 1H, CH), 7.17–7.30 (m, 7H, ArH), 7.44 (t, J = 7.6 Hz, 4H, ArH), 7.71 (d, J = 8.0 Hz, 4H, ArH) ppm. IR (KBr) νmax/cm−1: 3358, 3061, 2965, 2582, 1596, 1495, 1414, 1272, 1074, 792, 734, 689.
4,4′-[(4-Bromophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (4j)
1H NMR (DMSO-d6, 400 MHz): δ = 2.32 (s, 6H, 2CH3), 4.95 (s, 1H, CH), 7.19–7.27 (m, 4H, ArH), 7.42–7.48 (m, 6H, ArH), 7.70 (d, J = 8.0 Hz, 4H, ArH) ppm. IR (KBr)νmax/cm−1: 3422, 3066, 2922, 2546, 1598, 1484, 1407, 1293, 1013, 809, 747, 687.
4,4′-[(2,4-Dichlorophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (4k)
1H NMR (DMSO-d6, 400 MHz): δ = 2.28 (s, 6H, 2CH3), 5.09 (s, 1H, CH), 7.25 (t, J = 7.2 Hz, 2H, ArH), 7.40–7.46 (m, 5H, ArH), 7.56 (d, J = 2.0 Hz, 1H, ArH), 7.69 (d, J = 8.0 Hz, 4H, ArH), 7.75 (d, J = 8.4 Hz,1H, ArH) ppm. IR (KBr) νmax/cm−1: 3425, 3060, 2919, 1595, 1573, 1498, 1471, 1380, 1295, 1105, 843, 754, 690.
4,4′-[(4-Hydroxyphenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) (4n)
1H NMR (DMSO-d6, 400 MHz): δ = 2.30 (s, 6H, 2CH3), 4.84 (s, 1H, CH), 6.66 (d, J = 8.8 Hz, 2H, ArH), 7.04 (d, J = 8.8 Hz, 2H, ArH), 7.22–7.26 (t, J = 7.2 Hz, 2H, ArH), 7.44 (t, J = 8.0 Hz, 4H, ArH), 7.71 (d, J = 8 Hz, 4H, ArH), 9.16 (s, 1H, OH) ppm. IR (KBr) νmax/cm−1: 3413, 3158, 2969, 1597, 1502, 1427, 1275, 819, 756, 692.
4,4′-(Phenylmethylene)bis(3-methyl-1H-pyrazol-5-ol) (4p)
1H NMR (DMSO-d6, 400 MHz): δ = 2.07 (s, 6H, 2CH3), 4.82 (s, 1H, CH), 7.09–7.14 (m, 3H, ArH), 7.19–7.22 (m, 2H, ArH) ppm. IR (KBr) νmax/cm−1: 3296, 2971, 1612, 1522, 1494, 1380, 1049, 825, 778, 717.
Conclusion
In summary, a simple, efficient and green procedure for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s using 2-hydroxy ethylammonium propionate as a readily available, inexpensive, and recyclable catalyst under solvent-free conditions has been developed. The advantages offered by this one-pot protocol are short reaction times, high yields, easy workup, recyclability of catalyst, and environmentally benignancy.
Acknowledgments
We thank the Natural Science Foundation of Hubei Province [Grant No. 2007ABA291] for financial support.
References
- Singh, Da.; Singh, De. J. Indian. Chem. Soc. 1991, 68, 165–167.
- Londershausen, M. Pestic. Sci. 1996, 48, 269–292. doi:10.1002/(SICI)1096-9063(199612)48:4<269::AID-PS478>3.0.CO;2-B.
- Lubs, H.A. The Chemistry of Synthetic Dyes and Pigments; American Chemical Society: Washington, DC, 1970.
- Kuarm, B.S.; Rajitha, B. Synth. Commun. 2012, 42, 2382–2387. doi:10.1080/00397911.2011.557516.
- Phatangare, K.R.; Padalkar, V.S.; Gupta, V.D.; Patil, V.S.; Umape, P.G.; Sekar, N. Synth. Commun. 2012, 42, 1349–1358. doi:10.1080/00397911.2010.539759.
- Niknam, K.; Mirzaee, S. Synth. Commun. 2011, 41, 2403–2413. doi:10.1080/00397911.2010.502999.
- Sobhani, S.; Hasaninejad, A.; Maleki, M.F.; Parizi, Z.P. Synth. Commun. 2012, 42, 2245–2255. doi:10.1080/00397911.2011.555589.
- Wang, W.; Wang, S.X.; Qin, X.Y.; Li, J.T. Synth. Commun. 2005, 35, 1263–1269. doi:10.1081/SCC-200054854.
- Sobhani, S.; Safaei, E.; Hasaninejad, A.; Rezazadeh, S. J. Organomet. Chem. 2009, 694, 3027–3031. doi:10.1016/j.jorganchem.2009.05.004.
- Hasaninejad, A.; Shekouhy, M.; Zare, A.; Hoseini Ghattali, S.M.S.; Golzar, N. J. Iran. Chem. Soc. 2011, 8, 411–423. doi:10.1007/BF03249075.
- Mosaddegh, E.; Hassankhani, A.; Baghizadeh, A. J. Chil. Chem. Soc. 2010, 55, 419–420. doi:10.4067/S0717-97072010000400001.
- Gouda, M.A.; Abu-Hashem, A.A. Green. Chem. Lett. Rev. 2012, 5, 203–209. doi:10.1080/17518253.2011.613858.
- Karimi-Jaberi, Z.; Pooladian, B.; Moradi, M.; Ghasemi, E. Chin. J. Catal. 2012, 33, 1945–1949. doi:10.1016/S1872-2067(11)60477-4.
- Tayebi, S.; Baghernejad, M.; Saberi, D.; Niknam, K. Chin. J. Catal. 2011, 32, 1477–1483. doi:10.1016/S1872-2067(10)60260-4.
- Hu, Y.; Wei, P.; Zhou, H.; OuYang, P.K.; Chen, Z.C. Chin. Chem. Lett. 2006, 17, 299–301.
- Sobhani, S.; Nasseri, R.; Honarmand, M. Can. J. Chem. 2012, 90, 798–804. doi:10.1139/v2012-059.
- Baghernejad, M.; Niknam, K. Int. J. Chem. 2012, 4, 52–60. doi:10.5539/ijc.v4n3p52.
- Shi, D.; Chen, J.; Wu, N.; Zhuang, Q.; Wang, X. Chin. J. Org. Chem. 2005, 25, 405–408.
- Sujatha, K.; Shanthi, G.; Selvam, N.P.; Manoharan, S.; Perumal, P.T.; Rajendran, M. Bioorg. Med. Chem. Lett. 2009, 19, 4501–4503. doi:10.1016/j.bmcl.2009.02.113.
- Niknam, K.; Saberi, D.; Sadegheyan, M.; Deris, A. Tetrahedron. Lett. 2010, 51, 692–694. doi:10.1016/j.tetlet.2009.11.114.
- Khazaei, A.; Zolfigol, M.A.; Moosavi-Zare, A.R.; Asgari, Z.; Shekouhy, M.; Zare, A.; Hasaninejad, A. RSC Adv. 2012, 2, 8010–8013. doi:10.1039/c2ra20988h.
- Moosavi-Zare, A.R.; Zolfigol, M.A.; Zarei, M.; Zare, A.; Khakyzadeh, V.; Hasaninejad, A. Appl. Catal. A: Gen. 2013, 467, 61–68. doi:10.1016/j.apcata.2013.07.004.
- Hasaninejad, A.; Zare, A.; Shekouhy, M.; Golzar, N. Org. Prep. Proced. Int. 2011, 43, 131–137. doi:10.1080/00304948.2010.526827.
- Tale, N.P.; Tiwari, G.B.; Karade, N.N. Chin. Chem. Lett. 2011, 22, 1415–1418. doi:10.1016/j.cclet.2011.09.004.
- Bai, Y.J.; Li, M.; Lu, J.; Wang, Z.J.; Shi, Z. Chin. J. Org. Chem. 2004, 24, 616–620.
- Elinson, M.N.; Dorofeev, A.S.; Nasybullin, R.F.; Nikishin, G.I. Synthesis 2008, 1933–1937. doi:10.1055/s-2008-1067079.
- Tayebi S.; Niknam K. Iran. J. Catal. 2012, 2, 69–74.
- Shi, F.; Gu, Y.; Zhang, Q.; Deng, Y. Catal. Surv. Asia. 2004, 8, 179–186. doi:10.1023/B:CATS.0000038536.55980.f3.
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Appl. Catal. A: Gen. 2010, 373, 1–56. doi:10.1016/j.apcata.2009.10.008.
- Zare, A.; Yousofi, T.; Moosavi-Zare, A.R. RSC Adv. 2012, 2, 7988–7991. doi:10.1039/c2ra20679j.
- Moosavi-Zare, A.R.; Zolfigol, M.A.; Zarei, M.; Zare, A.; Khakyzadeh, V. J. Mol. Liq. 2013, 186, 63–69. doi:10.1016/j.molliq.2013.05.009.
- Zare, A.; Abi, F.; Moosavi-Zare, A.R.; Beyzavi, M.H.; Zolfigol, M.A. J. Mol. Liq. 2013, 178, 113–121. doi:10.1016/j.molliq.2012.10.045.
- Sobhani, S.; Honarmand, M. J. Iran. Chem. Soc. 2012, 9, 661–669. doi:10.1007/s13738-012-0088-1.
- Shaterian, H.R.; Arman, M.; Rigi, F. J. Mol. Liq. 2011, 158, 145–150. doi:10.1016/j.molliq.2010.11.010.
- Alizadeh, A.; Khodaei, M.M.; Eshghi, A. Can. J. Chem. 2010, 88, 514–518. doi:10.1139/V10-011.
- Zhu, J.; Bienayme, H. Multicomponent reactions; Wiley-VCH: Weinheim, 2005.
- Cota, I.; Gonzalez-Olmos, R.; Iglesias, M.; Medina, F. J. Phys. Chem. B. 2007, 111, 12468–12477. doi:10.1021/jp073963u.