Abstract
A simple and efficient method has been developed for the synthesis of benzoxazoles from 2-aminophenol and substituted aldehydes in the presence of a catalytic amount of zinc triflate in ethanol solvent at reflux temperature.
Introduction
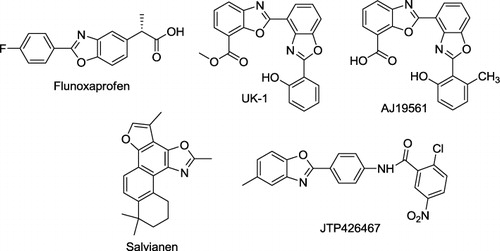
Benzoxazoles are an important class of heterocyclic compounds that have many applications in medicinal chemistry. Benzoxazole derivatives have been characterized as melatonin receptor agonists (Citation1), amyloidogenesis inhibitors (Citation2), Rho kinase inhibitors (Citation3), and antitumor agents (Citation4). In addition to their use in medicinal chemistry, benzoxazoles are recognized as an important scaffold in fluorescent probes such as anion and metal cation sensors (Citation5). Benzoxazoles are an important class of heterocycles that are encountered in a number of natural products and are used in drug and agrochemical discovery programs, as well as for a variety of other purposes (). For example, the benzoxazole core structure is found in a variety of cytotoxic natural products, such as the UK-1 (Citation6), AJI9561 (Citation7), and salvianen (Citation8). Recent medicinal chemistry applications of benzoxazoles include the cathepsin S inhibitor (Citation9) and selective peroxisome proliferator-activated receptor γ antagonist JTP-426467 (Citation10). Other applications of benzoxazoles include their use as herbicides, such as Fenoxaprop, and as fluorescent whitening agent dyes such as bisbenzoxazolyl ethylenes and arenes (Citation11).
Mainly there are two general methods for synthesizing 2-substituted benzoxazoles. One is the coupling of o-substituted aminoaromatics with carboxylic acid derivatives and acyl chlorides, which is either catalyzed by strong acids or microwave conditions. The other is the oxidative cyclization of Phenolic Schiff bases derived from the condensation of o-substituted aminoaromatics and aldehydes. In latter reactions various oxidants have been used. Different catalysts and different methods were also reported for the synthesis of these heterocycles like Pd (OAc)2 (Citation12), ZrOCl2 8H2O (Citation13), silica sulfuric acid (Citation14), silica-supported sodium hydrogen sulfate (Citation15), Indion 190 resin (Citation16), ([Hbim]BF4) (Citation17), methanesulphonic acid (Citation18), Cu(OTf)2 (Citation19), copper(II) oxide nanoparticles (Citation20), PCC-supported silica gel (Citation21), In(OTf)3 (Citation22), SnCl2 (Citation23), DDQ (Citation24), BF3.OEt2 (Citation25), Mn-(OAc)3 (Citation26), PhI(OAc)2 (Citation27), Th+.ClO4 −(Citation28), BaMnO4 (Citation29), NiO2 (Citation30), and Pb(OAc)4 (Citation31). However, many of these methods suffer from one or more of the drawbacks such as requirement of strong acidic conditions, long reaction times, low yields, tedious work-up procedures, requirement of excess amounts of reagent, and the use of toxic reagents, catalysts, or solvents. Therefore, there is a strong demand for a highly efficient and environmentally benign method for the synthesis of these heterocycles.
Results and discussions
In our preliminarily investigation on the model reaction of 2-amino phenol and benzaldehyde, it was found that the reaction could be finished under very simple reaction conditions in the presence of zinc (II) triflate (10 mol%) in reflux of ethanol solvent, which gives the desired benzoxazole product in good yield ().
The effect of solvent, catalyst, reaction temperature, and time on the reaction was systematically investigated and the results were summarized in . As can be seen from , the solvent plays an important role in the model reaction, it was found that ethanol is the best one among the solvents tested, and the reaction proceeded smoothly in ethanol and gave the desired product in 91% yield, while Toluene afforded the product only in 30%, use of methanol, THF, Dichloromethane, and 1,2-dichloroethane as solvents led to slower reactions and 70% yield of model product was isolated in solvent-free reaction condition. The optimized reaction conditions for the reaction were found to be Zn(OTf)2 under reflux in ethanol solvent for 5 h.
Table 1. Optimization of the reaction conditions.
Given these results, an array of aminophenol and substituted aldehydes was employed in order to investigate the scope of the reaction. The results are summarized in . Aldehydes were readily cyclized with 2-aminophenol and these preliminary results indicate that benzoxazole yield is affected by the position of the substituent on aromatic ring of the aldehydes. Ortho-substituted aryl aldehyde (Entry 2–6), the yield was lower than that when meta- and para-substituted aryl aldehydes were used. Also, the results found in (Entry 7–9) may indicate that the yield is dependent on the electronic nature of the substituent as well.
Table 2. Synthesis of 2-substituted benzoxazoles from 2-aminophenol and aldehydesFootnotea.
Conclusion
In conclusion, we have demonstrated that 2-substituted benzoxazoles can be synthesized from 2-aminophenols and aldehydes in the presence of Zn(OTf)2 in good yields. The present zinc triflate-mediated reaction is an alternative route to benzoxazole synthesis using 2-aminophenols and aldehydes.
Experimental
All 1H NMR spectra were recorded on 400 MHz Varian FT-NMR spectrometers. All chemical shifts are given as δ value with reference to Tetramethylsilane (TMS) as an internal standard. Products were purified by flash chromatography on 100–200 mesh silica gel. The chemicals and solvents were purchased from commercial suppliers either from Aldrich, Spectrochem and they were used without purification prior to use.
Zinc triflate-catalyzed synthesis of 2-substituted benzoxazoles from 2-aminophenols and aldehydes
A mixture of 2-amino phenol (1 mmol), benzaldehyde (1.2 mmol), and Zn(OTf)2 (10 mol%) in ethanol (5 ml) was placed in a 50-ml round bottom flask and stirred at reflux for 5 h. The progress of the reaction was monitored by thin layer chromatography (TLC) Hexane:EtOAc (9:1) after completion of the reaction, the reaction mixture was cooled and treated by dilution with 1N NaOH (5 mL). The solution was extracted with EtOAc (3 × 10 mL) Total organic layer was washed with water, brine solution and dried over Na2SO4 and evaporated under vacuum. Obtained crude residue was purified by column chromatography to give 2-substituted benzoxazoles.
2-Phenylbenzoxazole (Citation32): This compound was obtained as white solid, m.p. 102–104°C, Yield. 91%; 1H NMR (CDCl3): δ 8.27–8.24 (m, 2H), 7.79–7.76 (m, 1H), 7.60–7.57 (m, 1H), 7.54–7.51 (m, 3H), 7.38–7.32 (m, 2H); (LC-MS) m/z: 196.20 [M + H]+
2-(2-Methoxyphenyl)benzoxazole (Citation33): This compound was obtained as white solid, m.p. 54–56°C, Yield. 79%; 1H NMR (CDCl3): δ 8.13 (d, J = 8.8 Hz, 1H), 7.83–7.80 (m, 1H), 7.60–7.57 (m, 1H), 7.51–7.47 (m, 1H), 7.35–7.32 (m, 2H), 7.13–7.07 (m, 2H), 4.02 (s, 3H); (LC-MS) m/z: 226.10 [M + H]+
2-(2-Chlorophenyl)benzoxazole (Citation22): This compound was obtained as white solid, m.p. 70–73°C, Yield. 68%; 1H NMR (CDCl3): δ 8.15 (dd, J = 1.6, 5.6 Hz, 1H), 7.87–7.83 (m, 1H), 7.64–7.61 (m, 1H), 7.59–7.56 (m, 1H), 7.48–7.37 (m, 4H); (LC-MS) m/z: 230.12 [M + H]+
2-(2-Bromophenyl)benzoxazole (Citation33): This compound was obtained as white solid, m.p. 53–56°C, Yield. 62%; 1H NMR (CDCl3): δ 8.06 (d, J = 8 Hz, 1H), 7.86–7.83 (m, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.63–7.60 (m, 1H), 7.48–7.32 (m, 4H); (LC-MS) m/z: 273.95, 275.90 [M + H]+
2-(2-Fluorophenyl)benzoxazole (Citation34): This compound was obtained as white solid, m.p. 92–94°C, Yield. 72%; 1H NMR (CDCl3): δ 8.24–8.22 (m, 1H), 7.85–7.82 (m, 1H), 7.63–7.61 (m, 1H), 7.54–7.52 (m, 1H), 7.39–7.37 (m, 2H), 7.33–7.26 (m, 2H); (LC-MS) m/z: 214.16 [M + H]+
2-o-tolylbenzoxazole (Citation35): This compound was obtained as white solid, m.p. 63–66°C, Yield. 82%; 1H NMR (CDCl3): δ 8.18–8.16 (m, 1H), 7.81–7.79 (m, 1H), 7.60–7.58 (m, 1H), 7.33–7.41 (m, 5H), 2.81 (s, 3H); (LC-MS) m/z: 210.14 [M + H]+
2-(3-Methoxyphenyl)benzoxazole (Citation34): This compound was obtained as white solid, m.p. 70–73°C, Yield. 87%; 1H NMR (CDCl3): δ 7.86–7.76 (m, 3H), 7.60–7.57 (m, 1H), 7.43 (t, J = 8 Hz, 1H), 7.36–7.34 (m, 2H), 7.10–7.07 (m, 1H), 3.92 (s, 3H); (LC-MS) m/z: 226.23 [M + H]+
2-(3-Chlorophenyl)benzoxazole (Citation22): This compound was obtained as white solid, m.p. 131–133°C, Yield. 89%; 1H NMR (CDCl3): δ 8.26 (s, 1H), 8.16–8.13 (m, 1H), 7.80–7.77 (m, 1H), 7.61–7.58 (m, 1H), 7.52–7.44 (m, 2H), 7.39–7.37 (m, 2H); (LC-MS) m/z: 230.12 [M + H]+
2-(4-Methoxyphenyl)benzoxazole (Citation32): This compound was obtained as white solid, m.p. 97–99°C, Yield. 90%; 1H NMR (CDCl3): δ 8.20 (d, J = 9.2 Hz, 2H), 7.74–7.72 (m, 1H), 7.56–7.54 (m, 1H), 7.35–7.31 (m, 2H), 7.03 (d, J = 9.2 Hz, 2H), 3.89 (s, 3H); (LC-MS) m/z: 226.23 [M + H]+
2-p-tolylbenzoxazole (Citation32): This compound was obtained as white solid, m.p. 114–116°C, Yield. 91%; 1H NMR (CDCl3): δ 8.15 (d, J = 8 Hz, 2H), 7.77–7.75 (m, 1H), 7.58–7.56 (m, 1H), 7.35–7.32 (m, 4H), 2.44 (s, 3H); (LC-MS) m/z: 210.20 [M + H]+
2-(furan-2-yl)benzoxazole (Citation32): This compound was obtained as white solid, m.p. 85–87°C, Yield. 90%; 1H NMR (CDCl3): δ 7.77–7.75 (m, 1H), 7.68–7.67 (m, 1H), 7.58–7.55 (m, 1H), 7.37–7.35 (m, 2H), 7.28 (d, J = 3.6 Hz, 1H), 6.62 (dd, J = 3.2, 2 Hz, 1H); (LC-MS) m/z: 186.02 [M + H]+
2-(thiophen-2-yl)benzoxazole (Citation32): This compound was obtained as white solid, m.p. 104–107°C, Yield. 92%; 1H NMR (CDCl3): δ 7.92–7.91 (m, 1H), 7.75–7.72 (m, 1H), 7.57–7.54 (m, 2H), 7.36–7.33 (m, 2H), 7.21–7.19 (m, 1H); (LC-MS) m/z: 202.06 [M + H]+
Acknowledgments
The authors are very much grateful to the management of Chalapathi Institute of Engineering and Technology, Guntur, AP, India, for providing moral support in carrying out this work.
References
- Sun, L.Q.; Chen, J.; Takaki, K.; Johnson, G.; Iben, L.; Mahle, C. D.; Ryan, E.; Xu, C. Bioorg. Med. Chem. Lett. 2004, 14, 1197. 10.1016/j.bmcl.2003.12.052
- Johnson, S.M.; Connelly, S.; Wilson, I.A.; Kelly, J.W. J. Med. Chem. 2008, 51, 260. 10.1021/jm0708735
- Sessions, E.H.; Yin, Y.; Bannister, T.D.; Weiser, A.; Griffin, E.; Pocas, J.; Cameron, M.D.; Ruiz, C.; Lin, L.; Schuerer, S.C.; Schroeter, T.; LoGrasso, P.; Feng, Y. Bioorg. Med. Chem. Lett. 2008, 18, 6390. 10.1016/j.bmcl.2008.10.095
- Rida Samia, M.; Ashour Fawzia, A.; El-Hawash Soad, A.M.; ElSemary Mona, M.; Badr Mona, H.; Shalaby Manal, A. Eur. J. Med. Chem. 2005, 40, 949. 10.1016/j.ejmech.2005.03.023
- Taki, M.; Wolford, J.L.; O Halloran, T.V. J. Am. Chem. Soc. 2004, 126, 712. 10.1021/ja039073j
- Ueki, M.; Ueno, K.; Miyadoh, S.; Abe, K.; Shibata, K.; Taniguchi, M.; Oi, S. J. Antibiot. 1993, 46, 1089. 10.7164/antibiotics.46.1089
- Sato, S.; Kajiura, T.; Noguchi, M.; Takehana, K.; Kobayashi, T.; Tsuji, T. J. Antibiot. 2001, 54, 102. 10.7164/antibiotics.54.102
- Don, M.J.; Shen, C.C.; Lin, Y.L.; Syu Jr, W.; Ding, Y.H.; Sun, C.M. J. Nat. Prod. 2005, 68, 1066. 10.1021/np0500934
- Tully, D.C.; Liu, H.; Alper, P.B.; Chatterjee, A.K.; Epple, R.; Roberts, M.J.; Williams, J.A.; Nguyen, K.T.; Woodmansee, D.H.; Tumanut, C.; Li, J.; Spraggon, G.; Chang, J.; Tuntland, T.; Harris, J.L.; Karanewsky, D.S. Bioorg. Med. Chem. Lett. 2006, 16, 1975. 10.1016/j.bmcl.2005.12.095
- Nishiu, J.; Ito, M.; Ishida, Y.; Kakutani, M.; Shibata, T.; Matsushita, M.; Shindo, M. Diabetes Obes. Metab. 2006, 8, 508. 10.1111/j.1463-1326.2005.00536.x
- Leaver, I.H.; Milligan, B. Dyes Pigm. 1984, 5, 109. 10.1016/0143-7208(84)80008-X
- Pang, Y.; Hua, W. Tetrahedron Lett. 2009, 50, 6680–6683. 10.1016/j.tetlet.2009.09.084
- Baltork, I.M.; Khosropour, A.R.; Hojati, S.F. Catal. Commun. 2007, 8, 1865–1870. 10.1016/j.catcom.2007.02.020
- Baltork, I.M.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Zolfigol, M.A.; Hojati, S.F. J. Iran. Chem. Soc. 2008, 5, 65–70. 10.1007/BF03246491
- Ravi Kumar, K.; Satyanarayana, P.V.V.; Srinivasareddy, B. Der Pharma. Chemica. 2012, 4, 761–766.
- Padalkar, V.S.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Green Chem. Lett. Rev. 2012, 5 (2), 139–145. 10.1080/17518253.2011.585666
- Nadaf, R.N.; Siddiqui, S.A.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. J. Mol. Catal. A Chem. 2004, 214, 155–159. 10.1016/j.molcata.2003.10.064
- Kumar, D.; Rudrawar, S.; Chakraborti, A.K. Aust. J. Chem. 2008, 61, 881–887. 10.1071/CH08193
- Guru, M.M.; Ali, M.A.; Punniyamurthy, T. Org. Lett. 2011, 13, 1194–1197. 10.1021/ol2000809
- Saha, P.; Ramana, T.; Purkait, N.; Ali, M.A.; Paul, R.; Punniyamurthy, T. J. Org. Chem. 2009, 74, 8719–8725. 10.1021/jo901813g
- Praveen, C.; Kumar, K.H.; Muralidharan, D.; Perumal, P.T. Tetrahedron. 2008, 64, 2369–2374. 10.1016/j.tet.2008.01.004
- Wang, Bo.; Zhang, Y.; Li, P.; Wang, L. Chin. J. Chem. 2010, 28, 1697–1703. 10.1002/cjoc.201090287
- Cho, C.S.; Kim, D.T.; Zhang, J.Q.; Ho, S.L.; Kim, T.J.; Shim, S.C. J. Heterocyclic Chem. 2002, 39, 421–423. 10.1002/jhet.5570390229
- Chang, J.; Zhao, K.; Pan, S. Tetrahedron Lett. 2002, 43, 951–954. 10.1016/S0040-4039(01)02302-4
- Nagawade, R.R.; Shinde, D.B. Chin. Chem. Lett. 2006, 17, 453–456.
- Varma, R.S.; Kumar, D. J. Heterocycl. Chem. 1998, 35, 1539–1540. 10.1002/jhet.5570350656
- Varma, R.S.; Saini, R.K.; Prakash, O. Tetrahedron Lett. 1997, 38, 2621–2622. 10.1016/S0040-4039(97)00444-9
- Park, K.H.; Jun, K.; Shin, S.R.; Oh, S.W. Tetrahedron Lett. 1996, 37, 8869–8870. 10.1016/S0040-4039(96)02070-9
- Srivastava, R.G.; Venkataramani, P.S. Synth. Commun. 1988, 18, 1537–1544. 10.1080/00397918808081311
- Nakagawa, K.; Onoue, H.; Sugita, J. Chem. Pharm. Bull. 1964, 12, 1135–1138. 10.1248/cpb.12.1135
- Stephens, F.F.; Bower, J.D. J. Chem. Soc. 1949, 2971–2972. 10.1039/jr9490002971
- Cho, C.S.; Kim, D.T.; Zhang, J.Q.; Ho, S.L.; Kim, T.J.; Shim, S.C. J. Heterocyclic Chem. 2002, 39, 421–423. 10.1002/jhet.5570390229
- Guru, M.M.; Ashif Ali, Md.; Punniyamurthy, T. J. Org. Chem. 2011, 76, 5295–5308. 10.1021/jo2005632
- Bonnamour, J.; Bolm, C. Org. Lett. 2008, 10, 2665–2667. 10.1021/ol800744y
- Zhang, M.; Zhang, S.; Liu, M.; Cheng, J. Chem. Commun. 2011, 47, 11522–11524. 10.1039/c1cc14718h