Abstract
An atom-efficient, catalyst-free, and one-pot multicomponent condensation of 8-hydroxyquinoline, aromatic aldehyde, acetoacetanilide, and ammonium acetate under solvent-free conditions is reported. This eco-friendly protocol offers several advantages such as a green and cost-effective procedure with excellent yield, shorter reaction time, simpler work-up, convergence, and facile automation.
Introduction
Recent challenges in modern organic chemistry encompass the development of methodologies that afford assembly of molecular complexity and diversity in a fewer steps from readily available starting materials, under green conditions and with high yields (Citation1–Citation1). For this purpose, multicomponent domino reactions (MDRs) play an increasingly important role for the synthesis of chemically and biologically important compounds because of their high degree of atom economy, convergence, productivity, ease of execution, excellent yields, and green chemistry characteristic (Citation6–Citation12). These reactions can avoid time-consuming and costly processes for purification of various precursors and tedious steps of protection and deprotection of functional groups (Citation13, Citation14). Therefore, the development of new regio- and stereo-selective domino reactions is a continuing challenge at the forefront of modern organic chemistry.
Our interest in the synthesis of analogues with 1,10-phenantholine pharmacophores is based on their various biological activities, such as antibacterial (Citation15, Citation17), antifungal (Citation15), and antitumor (Citation16, Citation18) activities. They are also important heterocyclic systems because of being applicable to chelating ligands forming stable complexes with transition metals. In particular, the ability of metal-binding ligands such as heteroatom-bridged macrocycles to stabilize the G-quadruplex structure of telomeric DNA and interfere with the action of telomerase. Porphyrins (tetradentate nitrogen ligands) have been shown to act as telomerase inhibitors (Citation19), and a platinum 1,10-phenanthroline complex has demonstrated telomerase inhibition (Citation20). Moreover quinoline core is of great interest in the field of medicinal chemistry as it is present in a wide range of biologically active heterocyclic compounds inclusive of most of the phenanthroline derivatives (Citation21–Citation24).
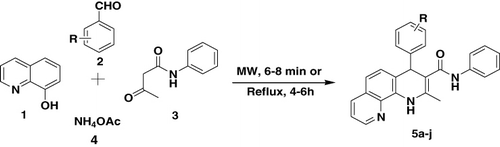
In view of the importance of phenanthroline derivatives and as a continuation of our research devoted to the development of new methods for the preparation of heterocycles via multicomponent reactions, herein we report a solvent- and catalyst-free, green, and rapid procedure for the one-pot synthesis of new 1,10-phenanthroline derivatives by the reaction of 8-hydroxyquinoline, aromatic aldehyde, acetoacetanilide, and ammonium acetate under the microwave (MW) method as well as the classical method.
Results and discussion
Growing emphasis on following the “green chemistry” protocols while developing a synthetic technology has contributed in popularizing and extending the scope of microwave-assisted organic synthesis (MAOS) (Citation25, Citation26). Compared to the conventional method, the MW-assisted reactions in solvent-free conditions have gained popularity because of rapid reaction rate, cleaner reactions, higher atom economy, ease of manipulation, reducing the hazard, etc. Therefore, MAOS methodology when coupled with dry reaction media presents a green protocol to carry out organic reactions.
Initially, we investigated solvent- and metal-free Hantzsch-type reactions of 8-hydroxyquinoline, benzaldehyde, acetoacetanilide, and ammonium acetate to heating under reflux, whereupon the reaction could be completed in 6 h affording 5a in 70% yield (, entry 1). The similar reaction mixture was irradiated at 100 W and 110 °C under the MW method; the reaction went to completion in 10 min affording a higher yield of 5a (88%; , entry 1) than thermal reaction. This reaction does not proceed with aliphatic aldehydes.
Table 1. Physical data of 1,10-phenanthroline derivatives (5a–j).
To optimize the reaction conditions, the influences of catalyst, solvent, and absence of both on the reaction were investigated by the reflux method as well as the MW method; these results are summarized in . Initially, the reaction of substituted 1,10 phenanthroline (5c) via four-component cyclocondensation of 8-hydroxyquinoline, p-nitrobenzaldehyde, acetoacetanilide, and ammonium acetate was examined. Among various conditions tested, p-TSA in ethanol, K2CO3 in toluene, absence of catalyst in ethanol, and p-TSA in absence of solvent gave poor results of the expected products (, entries 1–4). The solvent- and catalyst-free technique proved to be the best condition to reducing the reaction time and giving excellent yield (, entry 5).
Table 2. Optimization of reaction conditions for the synthesis of 5c.
A large number of optimized procedures for the Hantzsch reaction (Citation27–29) and for the synthesis of multi-substituted 1,4-dihydropyridines (1,4-DHPs) employ catalysts such as L-proline (Citation30), S-Valin (Citation31), Yb(OTf)3(Citation32), I2(Citation33), TBAHS (Citation34), HOAc (Citation35), Et3N (Citation36), DBU (Citation37, Citation38), Py (Citation39), and so on. Furthermore, along with the development of environmental consciousness in chemical research and industries, solvent-free reaction, MW method (Citation37, Citation38), “grindstone chemistry” technique (Citation39), and some efficient attempts have been used to build dihydropyridine scaffold (Citation40–Citation42). However, some drawbacks still exist, such as long reaction times, low yields, and tedious work-up processes. Further, the use of toxic organic solvents, formation of side products, and the use of corrosive, expensive, and nonreusable catalysts limit the usefulness of some of these methods, especially for large-scale operation and lead to serious environmental and safety problems. Consequently, the present Hantzsch-type reaction for the synthesis of 1,4-dihydro-2-methyl-N,4-diphenyl-1,10-phenanthroline-3-carboxamide derivatives was performed under catalyst- and solvent-free conditions; these result in short reaction time and in high yield, considering the number of steps involved under metal-free conditions in the presence of ammonium acetate, a soft Bronsted acid, which could serve as a reactant as well as a catalyst. From these experiments, it is clearly demonstrated that the synthesis of 1,10-phenanthroline derivatives in the absence of both solvent and catalyst under MW irradiation is indeed an effective method and is undoubtedly superior to other procedures with respect to reaction time, economical, safe handling, work-up procedure, high yields, and environmental compatibility. In view of the ever-mounting ecological concern in the field of chemistry, the proposed methods meet the Green Chemistry criterion for the synthesis of 1,10-phenanthrolines very well.
Investigations of the reaction scope revealed that electronic factors play a limited role and steric factors play a major role in the product formation. For example, when the para or meta position of aromatic aldehyde was substituted, whether it is an electron-withdrawing group or electron-donating group, the reactions were performed smoothly (, entries 5b–f). However, electron-withdrawing groups observed better yields than electron-donating groups. In the case of ortho-substituted aldehydes, the yields are slightly lower (probably due to steric hindrance) than para-substituted ones. A possible explanation for better yield in solvent-free conditions is that the eutectic mixture having uniform distribution of the reactants brings the reacting species in close proximity to react than in the solvent.
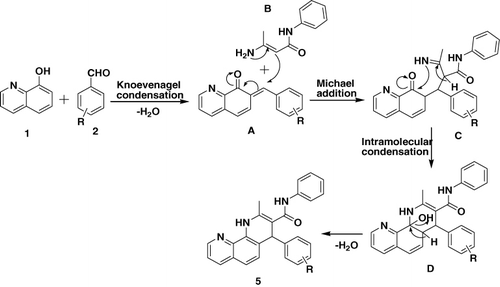
A plausible reaction mechanism for the formation of 1,10-phenanthroline derivatives (Citation5) is depicted in (Citation43). Presumably, the first step is the formation of the Knoevenagel product (A) generated from 8-hydroxyquinoline (Citation1) and aldehyde (Citation2). The second key intermediate is substituted 3-amino-N-phenylbut-2-enamide (B) produced by the condensation of acetoacetanilide with ammonia. Condensation of these two fragments gives the acyclic Michael adduct intermediate (C), which undergoes intramolecular cyclization with participation of the amino function and the carbonyl group of the substituted 2-((7,8-dihydro-8-oxoquinolin-7-yl)(phenyl)methyl)-3-imino-N- phenylbutanamide to form the substituted 1,4-dihydro-2-methyl-N,4-diphenyl-1,10-phenanthroline-3-carboxamide derivatives (Citation5).
Experimental
Materials
All the chemicals and solvents were obtained from AR grade and were used without further purification. Melting points were taken in an open capillary tube. The MW-assisted syntheses of title compounds were carried out in a CEM – 908010, bench mate model, 300 watts laboratory MW reactor. Elemental analyses were carried out using a Perkin Elmer, CHN elemental analyser model 2400. 1H NMR and 13C NMR spectra were recorded at 25°C at 400 MHz and 100 MHz (Bruker Avance instrument), respectively, using tetraMethylSilane (TMS) as the internal standard. EI-MS spectra were determined on an liquid chromatography quadrupole (LCQ) ion trap mass spectrometer (Thermo Fisher, San Jose, CA, USA) equipped with an EI source.
Synthesis of substituted 1,4-dihydro-2-methyl-N,4-diphenyl-1,10-phenanthroline-3-carboxamide derivatives by the MW method
8-hydroxyquinoline (Citation1), aromatic aldehyde (Citation2), acetoacetanilide (Citation3), and NH4OAc (Citation4) (1:1:1:2.5 mmol) were thoroughly mixed in a glass tube which was loosely closed. The reaction mixture was irradiated for 6–8 min with 100 W MWs at 110°C in the MW oven in temperature control mode. The completion of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the resulting mixture was cooled to room temperature and poured in ice water. The precipitated solid was filtered and purified by column chromatography from ethyl acetate and n-hexane.
Synthesis of substituted 1,4-dihydro-2-methyl-N,4-diphenyl-1,10-phenanthroline-3-carboxamide derivatives by the classical method
A mixture of 8-hydroxyquinoline (Citation1), aromatic aldehyde (Citation2), acetoacetanilide (Citation3), and NH4OAc (Citation4) (1:1:1:2.5 mmol) were refluxed for 4–6 h. The completion of the reaction was monitored by TLC. After completion of the reaction, the resulting mixture was cooled to room temperature and poured in ice water. The precipitated solid was filtered and purified by column chromatography from ethyl acetate and n-hexane.
1,4-dihydro-2-methyl-N,4-diphenyl-1,10-phenanthroline-3-carboxamide (5a): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.24 (s, 3H, CH3), 4.82 (s, 1H), 5.89 (br s, 1H, Ar-NH), 7.11–8.81 (m, 15H, Ar-H), 8.43 (br s, 1H, -NH); 13C NMR: δ 19.2 (-CH3), 46.6 (-CH), 104.8, 119.1, 120.6, 122.7, 123.4, 125.1, 126.8, 127.4, 128.8, 130.6, 132.1, 136.8, 137.3, 139.6, 141.1, 143.2, 150.4 for aromatic carbons, 166.1 (C=O); Mass spectra, m/z = 391 (M+, 100%), Elemental analysis: Calcd (found): C, 79.77 (79.74); H, 5.41 (5.39); N, 10.73 (7.76).
1,4-dihydro-4-(4-methoxyphenyl)-2-methyl-N-phenyl-1,10-phenanthroline-3-carboxamide (5b): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.31 (s, 3H, CH3), 3.91 (s, 3H, -OCH3), 4.92 (s, 1H), 5.92 (br s, 1H, Ar-NH), 6.62–8.82 (m, 14H, Ar-H), 8.52 (br s, 1H, -NH); 13C NMR: δ 18.6 (-CH3), 47.8 (-CH), 58.2 (-CH3), 104.4, 116.4, 119.6, 121.1, 122.9, 124.2, 125.8, 128.1, 131.2, 133.1, 134.8, 136.4, 138.1, 139.8, 143.8, 151.4, 161.5 for aromatic carbons, 165.7 (C=O); Mass spectra, m/z = 421 (M+, 100%), Elemental analysis: Calcd (found): C, 76.94 (76.88); H, 5.50 (5.49); N, 9.97 (9.94).
1,4-dihydro-2-methyl-4-(4-nitrophenyl)-N-phenyl-1,10-phenanthroline-3-carboxamide (5c): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.35 (s, 3H, CH3), 4.93 (s, 1H), 5.95 (br s, 1H, Ar-NH), 7.03–8.81 (m, 14H, Ar-H), 8.49 (br s, 1H, -NH); 13C NMR: δ 19.5 (-CH3), 46.8 (-CH), 103.9, 120.6, 121.4, 122.9, 123.4, 124.1, 125.8, 128.1, 130.2, 131.2, 136.5, 138.2, 140.3, 144.1, 147.3, 148.8, 150.9 for aromatic carbons, 166.2 (C=O); Mass spectra, m/z = 436 (M+, 100%), Elemental analysis: Calcd (found): C, 71.55 (71.60); H, 4.62 (4.59); N, 12.84 (12.85).
4-(4-(dimethylamino)phenyl)-1,4-dihydro-2-methyl-N-phenyl-1,10-phenanthroline-3-carboxamide (5d): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.31 (s, 3H, CH3), 3.22 (s, 6H, -N(CH3)2), 4.93 (s, 1H), 5.78 (br s, 1H, Ar-NH), 6.41–8.81 (m, 14H, Ar-H), 8.52 (br s, 1H, -NH); 13C NMR: δ 18.8 (-CH3), 42.6 (-N(CH3)2), 47.1 (-CH), 104.4, 116.4, 119.7, 121.4, 123.1, 124.2, 126.5, 128.5, 129.9, 131.9, 137.1, 138.5, 139.4, 144.6, 149.4, 151.8 for aromatic carbons, 165.3 (C=O); Mass spectra, m/z = 434 (M+, 100%), Elemental analysis: Calcd (found): C, 77.39 (77.41); H, 6.03 (6.09); N, 12.89 (12.86).
1,4-dihydro-4-(3-hydroxyphenyl)-2-methyl-N-phenyl-1,10-phenanthroline-3-carboxamide (5e): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.27 (s, 3H, CH3), 4.86 (s, 1H), 5.72 (br s, 1H, Ar-NH), 6.54–8.79 (m, 14H, Ar-H), 8.57 (br s, 1H, -NH), 10.92 (br s, 1H, Ar-OH); 13C NMR: δ 19.1 (-CH3), 46.8 (-CH), 105.2, 115.3, 116.4, 118.6, 120.1, 121.6, 123.4, 123.8, 126.4, 127.8, 130.3, 131.8, 137.7, 138.1, 139.8, 142.4, 144.6, 150.9, 161.6 for aromatic carbons, 166.4 (C=O); Mass spectra, m/z = 407 (M+, 100%), Elemental analysis: Calcd (found): C, 76.64 (76.61); H, 5.19 (5.19); N, 10.31 (10.35).
1,4-dihydro-2-methyl-4-(3-nitrophenyl)-N-phenyl-1,10-phenanthroline-3-carboxamide (5f): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.29 (s, 3H, CH3), 4.78 (s, 1H), 5.86 (br s, 1H, Ar-NH), 7.08–8.83 (m, 14H, Ar-H), 8.51 (br s, 1H, -NH); 13C NMR: δ 18.7 (-CH3), 47.5 (-CH), 104.9, 119.6, 120.1, 121.7, 122.9, 123.7, 124.4, 126.3, 127.6, 129.8, 131.6, 135.8, 137.2, 138.4, 140.2, 142.6, 144.7, 149.8, 151.4 for aromatic carbons, 165.9 (C=O); Mass spectra, m/z = 436 (M+, 100%), Elemental analysis: Calcd (found): C, 71.55 (71.56); H, 4.62 (4.69); N, 12.84 (12.86).
1,4-dihydro-2-methyl-4-(2-nitrophenyl)-N-phenyl-1,10-phenanthroline-3-carboxamide (5g): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.30 (s, 3H, CH3), 4.79 (s, 1H), 5.95 (br s, 1H, Ar-NH), 7.11–8.81 (m, 14H, Ar-H), 8.51 (br s, 1H, -NH); 13C NMR: δ 19.4 (-CH3), 47.5 (-CH), 104.5, 120.3, 121.3, 123.5, 124.6, 126.4, 128.3, 131.2, 136.1, 138.3, 140.8, 144.6, 152.8 for aromatic carbons, 166.8 (C=O); Mass spectra, m/z = 436 (M+, 100%), Elemental analysis: Calcd (found): C, 71.55 (71.54); H, 4.62 (4.59); N, 12.84 (12.81).
4-(2-chlorophenyl)-1,4-dihydro-2-methyl-N-phenyl-1,10-phenanthroline-3-carboxamide (5h): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.28 (s, 3H, CH3), 4.81 (s, 1H), 5.91 (br s, 1H, Ar-NH), 7.01–8.81 (m, 14H, Ar-H), 8.48 (br s, 1H, -NH); 13C NMR: δ 18.6 (-CH3), 46.9 (-CH), 105.4, 119.6, 121.3, 122.9, 124.3, 126.2, 127.1, 128.1, 129.8, 130.3, 131.1, 131.8, 134.6, 137.4, 138.7, 140.5, 143.8, 150.2 152.2 for aromatic carbons, 166.3 (C=O); Mass spectra, m/z = 425 (M+, 100%), Elemental analysis: Calcd (found): C, 73.32 (73.34); H, 4.73 (4.79); N, 8.32 (8.31).
1,4-dihydro-2-methyl-N-phenyl-4-o-tolyl-1,10-phenanthroline-3-carboxamide (5i): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.36 (s, 3H, CH3), 3.12 (s, 3H, -CH3), 4.93 (s, 1H), 5.89 (br s, 1H, Ar-NH), 6.91–8.82 (m, 14H, Ar-H), 8.48 (br s, 1H, -NH); 13C NMR: δ 19.2 (-CH3), 22.2 (-CH3), 47.1 (-CH), 104.9, 120.3, 121.6, 122.7, 124.2, 125.3, 126.8, 127.1, 128.8, 130.1, 131.4, 137.2, 138.7, 140.6, 144.5, 146.4, 152.1 for aromatic carbons, 166.6 (C=O); Mass spectra, m/z = 405 (M+, 100%), Elemental analysis: Calcd (found): C, 79.97 (79.94); H, 5.72 (5.79); N, 10.36 (7.36).
4-(furan-2-yl)-1,4-dihydro-2-methyl-N-phenyl-1,10-phenanthroline-3-carboxamide (5j): 1H NMR (400 MHz, CDCl3) δ (ppm) = 2.34 (s, 3H, CH3), 4.88 (s, 1H), 5.84 (br s, 1H, Ar-NH), 6.01–8.81 (m, 13H, Ar-H), 8.56 (br s, 1H, -NH); 13C NMR: δ 19.1 (-CH3), 46.8 (-CH), 104.4, 108.4, 112.4, 119.7, 120.9, 122.2, 123.8, 125.7, 127.1, 130.9, 136.4, 137.8, 139.5, 143.7, 145.1, 150.8, 154.4 for aromatic carbons, 166.5 (C=O); Mass spectra, m/z = 381 (M+, 100%), Elemental analysis: Calcd (found): C, 75.57 (75.54); H, 5.02 (5.06); N, 11.02 (11.06).
Conclusion
In conclusion, we have developed a facile and efficient one-pot, four-component Hantzsch-type reaction for the synthesis of 1,4-dihydro-2-methyl-N,4-diphenyl-1,10-phenanthroline-3-carboxamide derivatives under solvent- and catalyst-free conditions by using the MW method. The advantages of this method over other existing methods are reduced milder conditions, reaction times, low costs, higher yields, easy purification, and environmental safety.
Acknowledgements
We greatly acknowledge the head of the Department of Chemistry, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur (India) for laboratory facilities, and the director of SAIF Chandigarh (India) for providing necessary spectral data of compounds.
References
- Parenty, A.D.C.; Smith, L.V.; Pickering, A.L.; Long, D.L.; Cronin, L. J.Org. Chem. 2004, 69, 5934–5946.10.1021/jo0495440
- Tietze, L. F. Chem. Rev. 1996, 96, 115–136.10.1021/cr950027e
- Winker, J. D. Chem. Rev. 1996, 96, 167–176.10.1021/cr950029z
- Ryu, I.; Sonoda, N.; Curran, D.P. Chem. Rev. 1996, 96, 177–194.10.1021/cr9400626
- Ramakanth, P.; Suresh, M.; Surjyakanta, R.; Sreekantha, B. J. RSC Adv. 2014, 4, 6602–6607. doi:10.1039/c3ra47145d
- Huang, Y.; Waljji, A.M.; Larsen, C.H.; MacMillan, D.W.C. J. Am. Chem. Soc. 2005, 127, 15051–15053. doi:10.1021/ja055545d
- Yang, J.W.; Fonseca, H.M.T.; List, B.J. Am. Chem. Soc. 2005, 127, 15036–15037.10.1021/ja055735o
- Enders, D.; Huttl, M.R.M.; Grondal, C.; Raab, G. Nature, 2006, 441, 861–863.10.1038/nature04820
- Lu, M.; Zhu, D.; Lu, Y.; Hou, B.; Tan, B.; Zhong, G. Angew. Chem. Int. Ed. 2008, 47, 10187–10191. doi:10.1002/anie.200803731
- Wang, J.; Xie, H.; Li, H.; Zu, L.; Wang, W. Angew. Chem. Int. Ed. 2008, 47, 4177–4179.10.1002/anie.200800381
- AndreyIvanov, S. Chem. Soc. Rev. 2008, 37, 789–811.10.1039/b716020h
- Ramakanth, P.; Suresh, M.; Sreekantha, B.J. Ultrasonics Sonochem. 2014, 21, 472–477. doi:10.1016/j.ultsonch.2013.08.024
- Tietze, L.F. Chem. Rev. 1996, 96, 115–136.10.1021/cr950027e
- Enders, D.; Huttl, M.R.M.; Grondal, C.; Raabe, G. Nature. 2006, 441, 861–863.10.1038/nature04820
- Kumar, R.S.; Arunachalam, S. Polyhedron. 2007, 26, 3255–3262.
- Liu, J.; Zheng, W.; Shi, S.; Tan, C.; Chen, J.; Zheng, K.; Ji, L.J. Inorg. Biochem. 2008, 102, 193–202.10.1016/j.jinorgbio.2007.07.035
- Efthimiadou, E.K.; Katsarou, M.E.; Karaliota, A.; Psomas, G.J. Inorg. Biochem. 2008, 102, 910–920.10.1016/j.jinorgbio.2007.12.011
- Mei, W.J.; Wang, N.; Liu, Y.J.; Ma, Y.Z.; Wang, D.Y.; Liang, B.X. Trans. Met. Chem. 2008, 33, 499–503.10.1007/s11243-008-9071-1
- Maraval, A.; Franco, S.; Vialas, C.; Pratviel, G.; Blasco, M.A.; Meunier, B. Org. Biomol. Chem. 2003, 1, 921–927.10.1039/b211634k
- Reed, J.E.; Neidle, S.; Vilar, R. Chem. Commun. 2007, 42, 4366–4368.10.1039/b709898g
- Chen, Y.L.; Hung, H.M.; Lu, C.M.; Li, K.C.; Tzeng, C.C. Bioorg. Med. Chem. 2004, 12, 6539–6546.10.1016/j.bmc.2004.09.025
- Zarghi, A.; Ghodsi, R.; Azizi, E.; Daraie, B.; Hedayati, M.; Dadrass, O.G. Bioorg. Med. Chem. 2009, 17, 5312–5317.10.1016/j.bmc.2009.05.084
- Shi, A.; Nguyen, T.A.; Battina, S.K.; Rana, S.; Takemoto, D.J.; Chiang, P.K.; Hua, D.H. Bioorg. Med. Chem. Lett. 2008, 18, 3364–3368.10.1016/j.bmcl.2008.04.024
- Tseng, C.H.; Tzeng, C.C.; Yang, C.L.; Lu, P.J.; Chen, H.L.; Li, H.Y.; Chuang, Y.C.; Yang, C.N.; Chen, Y.L. J. Med. Chem. 2010, 53, 6164–6179.10.1021/jm1005447
- Moseley, J.D.; Kappe, C.O. Green Chem. 2011, 13, 794–806.10.1039/c0gc00823k
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Tetrahedron. 2001, 57, 9225–9283.10.1016/S0040-4020(01)00906-1
- Hantzsch, A.Chem. Ber. 1881, 14, 1637–1638.
- Ramakanth, P.; Suresh, M.; Venkata, D.B.C.D.; Sreekantha, B.J. Cat. Comm. 2014, 45, 148–152.10.1016/j.catcom.2013.11.012
- Malek, T.M.; Ghasem, M.; Nourollah, H.; Ali, A.; Roya, K. Tetrahedron Lett. 2007, 48, 3197–3199.10.1016/j.tetlet.2007.03.048
- Shi, C.L.; Chen, H.; Shi, D.Q. J. Heterocycl. Chem. 2012, 49, 125–129.10.1002/jhet.782
- Jiang, H.F.; Mai, R.H.; Cao, H.; Zhu, Q.H.; Liu, X.H. Org. Biomol. Chem. 2009, 7, 4943–4953.10.1039/b914659h
- Wang, L.M.; Sheng, J.; Zhang, L.; Han, J.W.; Fan, Z.Y.; Tian, H.; Qian, C.T. Tetrahedron. 2005, 61, 1539–1543.10.1016/j.tet.2004.11.079
- Ko, S.; Sastry, M.N.V.; Lin, C.; Yao, C.F. Tetrahedron Lett. 2005, 46, 5771–5774.10.1016/j.tetlet.2005.05.148
- Tewari, N.; Dwivedi, N.; Tripathi, R.P. Tetrahedron Lett. 2004, 45, 9011–9014.10.1016/j.tetlet.2004.10.057
- Wang, X.H.; Hao, W.J.; Tu, S.J.; Zhang, X.H. J. Heterocycl. Chem. 2009, 46, 742–747.10.1002/jhet.143
- Wen, L.R.; Sun, J.H.; Li, M.; Sun, E.T.; Zhang, S.S. J. Org. Chem. 2008, 73, 1852–1863.10.1021/jo7024702
- Singh, S.K.; Singh, K.N. Monatsh. Chem. 2012, 143, 805–808.10.1007/s00706-011-0651-y
- Shi, F.; Zhang, G.; Zhang, Y.; Ma, N.; Jiang, B.; Tu, S.J. J. Heterocycl. Chem. 2009, 46, 965–970.10.1002/jhet.187
- Ramin, G.; Somayeh, A.; Ghazaleh, I. Tetrahedron Lett. 2010, 51, 499–502.10.1016/j.tetlet.2009.11.041
- Javad, S.; Sayed, H.; Shiva, D.J. Mol. Catal. A: Chem. 2011, 335, 46–50.10.1016/j.molcata.2010.11.012
- Majid, M.; Hamideh, A.; Khadijeh, B. Synth. Commun. 2010, 40, 2191–2200.10.1080/00397910903219591
- Zonouz, A.M.; Hosseini, S.B. Synth. Commun. 2008, 3, 290–296.10.1080/00397910701750003
- Kundu, D.; Majee, A.; Hajra, A. Cat. Comm. 2010, 11, 1157–1159.10.1016/j.catcom.2010.06.001