Abstract
The zinc(II) and nickel(II) complexes of glycine–vanillin Schiff base were synthesized by one-step solid–solid reaction at room temperature. The composition and structure of the complexes were characterized by elemental analyses, Fourier transform infrared spectra (FTIR), X-ray powder diffraction (XRD), and thermogravimetry and differential scanning calorimetry (TG–DSC). The crystal structure of the complexes belongs to monoclinic system with the lattice parameters: a = 0.6807 nm, b = 1.3818 nm, c = 1.2011 nm, β = 95.80° for [Zn(C10H9O4N)(H2O)3], and a = 0.7457 nm, b = 1.3331 nm, c = 1.2560 nm, β = 91.89° for [Ni(C10H9O4N)(H2O)3]·1.5H2O. The experimental results indicate that the zinc and nickel ions are all six-coordinated by imino nitrogen, carboxylic oxygen, and phenolic oxygen from the Schiff base ligand, and oxygen from three coordinated water molecules, respectively. The possible pyrolysis reactions in the thermal decomposition processes of the complexes and the experimental and calculated percentage mass loss are also given. The two complexes have the most intense antibacterial activities against Escherichia coli.
1. Introduction
Schiff bases are a series of organic compounds which contain the azomethine group (–C=N–). These compounds are easily synthesized by the reaction of a primary amine and an active carbonyl compound (aldehyde or ketone). Because of some special functions and a wide range of biological activities and industrial application, Schiff bases continue to occupy an important position as ligands in metal coordination chemistry (Citation1–Citation10). Schiff bases and their complexes have been found to possess the pharmacological activities, such as antibacterial (Citation11–Citation14), antifungal (Citation14, Citation15), antitumor (Citation16, Citation17), antimalarial (Citation18), anticonvulsant (Citation19), and antiviral agents (Citation20). Amino acid is not only a kind of aminocarboxylic acids but also a kind of important biological compounds for the body. Studying Schiff bases of amino acids and their complexes has important significance to bioinorganic chemistry and medicine field. The use of Schiff bases as ligands in the formation of transition-metal complexes had been extensively studied, but the Schiff base ligands and its complexes were previously synthesized in some solvent, and most synthetic methods were performed in ethanol or methanol solution (Citation21–Citation32). This method had the disadvantages of long response time, the use of a large amount of solvents, environmental pollution, and low yield. Solid–solid chemical reaction at room temperature is the method used in a wide range of methods for the preparation of some compounds, and the method has a high yield rate and selectivity (Citation33–Citation35).
Amino acids constitute the building blocks of proteins and are chemical species indispensable for performing a huge number of biological functions. The complexes of the amino acid Schiff base synthesized by the one-step solid–solid reaction at room temperature have not been previously reported in literatures. Based on the concept of green chemistry, in this work we have synthesized the zinc(II) and nickel(II) complexes of the glycine–vanillin Schiff base ligand, and studied their crystal structure, infrared spectra, thermal decomposition process, and antibacterial activity.
2. Results and discussion
2.1. The composition and properties of the complexes
The results of elemental analyses and the melting point of the ligand and the complexes are listed in . The composition formulae of the zinc(II) and nickel(II) complexes are ZnC10H15O7N and NiC10H18O8.5N, respectively. The calculated results of mass fraction of each element in the complexes are very close to the measured ones. Therefore, combined with the results of the infrared spectra and the thermal analysis, the possible molecular formulae of the complexes are [Zn(C10H9O4N)(H2O)3] and [Ni(C10H9O4N)(H2O)3]·1.5H2O, or [Zn(gly-van)(H2O)3] and [Ni(gly-van)(H2O)3]·1.5H2O. The molar ratio of the Schiff base ligand and metal ions of the complexes is 1:1. The decomposition of the complexes occurs near the melting point. The complexes are all stable at room temperature, non-hygroscopic, and partially soluble in water and ethanol.
Table 1. Elemental analyses results and the melting point of the ligand and the complexes.
2.2. Analysis of X-ray powder diffraction
The X-ray powder diffraction (XRD) patterns of the complexes are shown in . Compared with the raw material, the strong peak locations of the resultants are changed obviously. The main strong peaks, which come from the reactants glycine, vanillin, and zinc acetate or nickel acetate, are disappeared in X-ray powder diffraction patterns of the complexes. At the same time, the new strong peaks show the new compounds formation rather than the mixture of the reactants. There is the small background, and the high and intense of diffraction peaks in the XRD patterns of the complexes, which indicate that the complexes have a fine crystalline state. All the diffraction data can be very well indexed by a set of cell parameters according to monoclinic system, and the index results of the powder X-ray diffraction patterns are shown in and , respectively. As and show, the calculated spacing d cal are consistent with the experimental spacing d exp, and the largest relative deviation is less than 0.5%. The results indicate that the complexes are all single phase, and the crystal structures of the complexes belong to monoclinic system. The lattice parameters of the glycine–vanillin Schiff base complexes are: a = 0.6807 nm, b = 1.3818 nm, c = 1.2011 nm, and β = 95.80° for [Zn(C10H9O4N)(H2O)3], and a = 0.7457 nm, b = 1.3331 nm, c = 1.2560 nm, and β = 91.89° for [Ni(C10H9O4N)(H2O)3]·1.5H2O.
![Figure 1. X-ray powder diffraction patterns of the complexes [Zn(C10H9O4N)(H2O)3] (a) and [Ni(C10H9O4N)(H2O)3]·1.5H2O (b).](/cms/asset/324c9102-33a0-4109-8e59-7809090fe306/tgcl_a_927008_f0001_b.jpg)
Table 2. Experimental data and calculated results for powder X-ray diffraction pattern of the complex [Zn(gly-van)(H2O)3] (monoclinic system: a = 0.6807 nm, b = 1.3818 nm, c = 1.2011 nm, and β = 95.80°).
Table 3. Experimental data and calculated results for powder X-ray diffraction pattern of the complex [Ni(gly-van)(H2O)3]·1.5H2O (monoclinic system: a = 0.7457 nm, b = 1.3331 nm, c = 1.2560 nm, and β = 91.89°).
2.3. FTIR spectra
The infrared spectra of the complexes are shown in . The absorption peaks from the stretching vibration of the bonds in the water molecule are in the range of 3550–3200 cm−1(Citation36). As shows, there are some strong peaks in the range of 3550–3200 cm−1 in the infrared spectra of the complexes. This indicates that there are water molecules in the crystals of the complexes. Therefore, the infrared spectra demonstrate the existence of the coordinated water or lattice water molecules in the complexes and support the molecular formulae of the complexes. As shows, the infrared spectrum of [Zn(C10H9O4N)(H2O)3] makes out broad peaks at 3460 cm−1 and 3268 cm−1 due to the v(O–H) bond in a water molecule. The absorption peaks at 913 cm−1 and 718 cm−1 are assigned to the rocking and wagging vibrations of the hydroxyl, and which indicate that there are the coordinated water molecules in the complexes (Citation28). This is in accord with the result of thermal analysis. The absorption peak at the range of 1700–1740 cm−1 of carbonyl in the glycine–vanillin Schiff base ligand disappears in the complexes. The broad absorption peaks at 1608 cm−1 and 1394 cm−1 can be assigned to the absorption peak overlap of the νas(COO) and v(C=N) stretching vibration (Citation13), and the νs(COO) stretching vibration, respectively. The difference value of 214 cm−1 between νas(COO) and νs(COO) is in line with a monodentate type of coordination (Citation23). The absorption peak at 1305 cm−1 is assigned to the phenolic v(ph–O) stretching vibration. Compared with the ligand, the peak in the complex shifts obviously toward higher wavenumber, and this indicates that the phenolic oxygen is coordinated with Zn2+ ion. In the low-frequency region, the absorption peak at 596 cm−1 is assigned to the stretching vibration of the Zn−N bond, and the absorption peaks at 547 cm−1 and 478 cm−1 arise from the stretching vibration of the Zn−O bond (Citation36).
![Figure 2. Infrared spectra of the complexes [Zn(C10H9O4N)(H2O)3] (a) and [Ni(C10H9O4N)(H2O)3]·1.5H2O (b).](/cms/asset/ee5dd1f5-7002-4679-b127-c57a848533bb/tgcl_a_927008_f0002_b.jpg)
As shows, a broad band at 3313 cm−1 in the infrared spectrum of [Ni(C10H9O4N)(H2O)3]·1.5H2O is assigned to the v(O–H) in a water molecule, and the absorption peaks at 940 cm−1 and 731 cm−1 arise from the rocking and wagging vibrations of the coordinated water molecules. The broad absorption peaks at 1628 cm−1 and 1421 cm−1 can be assigned to the absorption peak overlap of the νas(COO) and v(C=N) stretching vibration, and the νs(COO) stretching vibration, respectively. The difference value of 207 cm−1 between νas(COO) and νs(COO) is in line with a monodentate type of coordination. The absorption peak at 1351 cm−1 arises from the v(ph–O) stretching vibration. The band at 627 cm−1 is assigned to the v(Zn N) bond, and the peaks at 592 cm−1 and 519 cm−1 arise from the v(Ni–O) bond (Citation36). From the IR results, it may be concluded that the Schiff base ligand is tridentate and coordinates with the metal ion through the azomethine nitrogen, phenolic oxygen, and carboxylic oxygen atoms.
2.4. Thermal analysis
Studying the thermal decomposition process of various Schiff base complexes is helpful to the understanding of the coordination structure of the complexes (Citation37–Citation41). The thermogravimetry and differential scanning calorimetry (TG–DSC) curves of the complexes from room temperature to 650°C are shown in . The possible thermal decomposition processes and the experimental and calculated results for the thermal analysis of the complexes are summarized in . shows that there are one endothermic peak and two exothermic peaks in the DSC curve. First, the endothermic peak at 148°C accompanies evidently mass loss, and the sample will gradually lose 3H2O molecules. The experimental percentage mass loss (15.72%) closes to the calculated one (16.55%). This is consistent with the results of elemental analyses and infrared spectra of the zinc(II) complex. The coordinated water molecules are eliminated at higher temperatures than the water molecules of hydration. The water of coordination is usually eliminated in the temperature range 100–316°C (Citation42). Because of the high temperature of loss water, the three water molecules should be of coordinated water. The organic part of the complexes may decompose in one or more steps with the possibility of the formation of one or two intermediates. These intermediates may finally decompose to stable metal oxides. Thereafter, sequential exothermic peaks at 296°C and 367°C correspond to step-by-step oxidation and decomposition of the ligand and loss of CH3CN, CO2, and –CH3OC6H3 group, the mass loss of 59.78% in thermogravimetry (TG) curve is in agreement with the calculated result of 58.53%. The complex is decomposed completely at about 450°C. The final residue is zinc oxide, and the experimental result (24.50%) is in agreement with the calculated result (24.92%). shows that there are two obvious endothermic peaks and one exothermic peak in the DSC curve. First the endothermic peak at 100°C accompanies 8.31% of mass loss from 1.5 water molecules. Because of the low temperature of loss water, the water molecules should be of crystal water. Thereafter, the endothermic peak at 293°C accompanies 15.28% of mass loss from three coordinated water molecules. Finally the exothermic peak at 424°C corresponds to oxidation and decomposition of the ligand and loss of CH3CN, CO2, and –CH3OC6H3 group. The final remnant mass from the TG curve is 22.09%, and it is in agreement with the calculated result (21.53%) of the residue nickel oxide.
![Figure 3. TG–DSC curves of the complexes [Zn(C10H9O4N)(H2O)3] (a) and [Ni(C10H9O4N)(H2O)3]·1.5H2O (b).](/cms/asset/b1b6c6e2-d41d-41b4-93fe-c6ff4ee22bd7/tgcl_a_927008_f0003_b.jpg)
Table 4. Thermal decomposition data of the Schiff base complexes.
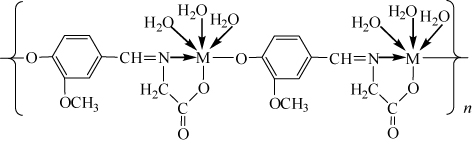
In summary, the two complexes have similar space structure, and the zinc and nickel ions are coordinated by imino nitrogen, carboxylic oxygen, and phenolic oxygen from the Schiff base ligand, and oxygen from three coordinated water molecules, respectively. Based on the results gained from elemental analyses, IR, and TG–DSC, the possible structural formula of the formed complexes is shown in .
2.5. Qualitative antimicrobial activity
Preliminary screening for antimicrobial activities of the stock solutions of the complexes was performed qualitatively using the disk diffusion assay in . Moxifloxacin was used as a standard drug. The complexes yielded clear inhibition zones around the disks. Each of the compounds was tested three times and recorded the average data. The results showed that the complexes had significant antibacterial activities against four bacteria; the antibacterial activities of the sequence were Escherichia coli, Bacillus subtilis, Staphylococcus epidermidis, and Staphylococcus aureus, respectively. The two complexes had the most effective antibacterial activity against E. coli, and the average diameter of inhibition zone of the zinc(II) and nickel(II) complexes was 16.6 mm and 18.7 mm with the concentration of 1.0 mg mL−1.
Table 5. Antibacterial activity of the Schiff base and the complexes.
3. Conclusion
The nickel(II) and zinc(II) complexes of glycine–vanillin Schiff base had been synthesized by one-step solid–solid reaction at room temperature. The complexes were characterized by elemental analyses, XRD, FTIR, and TG–DSC. The results of indexing to data of the XRD indicate that the complexes belong to monoclinic system with the lattice parameters: a = 0.6807 nm, b = 1.3818 nm, c = 1.2011 nm, and β = 95.80° for [Zn(C10H9O4N)(H2O)3], and a = 0.7457 nm, b = 1.3331 nm, c = 1.2560 nm, and β = 91.89° for [Ni(C10H9O4N)(H2O)3]·1.5H2O. The zinc and nickel ions are all six-coordinated by imino nitrogen, carboxylic oxygen, and phenolic oxygen from the Schiff base ligand, and oxygen from the three coordinated water molecules, respectively. The thermal decomposition processes of the complexes include dehydration, and pyrolysis of the ligand, and the final residue is metallic oxide. The complexes displayed a strong activity against E. coli. The advantages of the synthetic method are simple and convenient operation, solvent-free, high yield, energy-saving, and being environmental friendly, and it is in accordance with the requirements of green chemistry.
4. Experimental
4.1. Materials and physical measurements
All chemicals used in the experiments were analytical reagents as received from commercial sources with no further purification. Vanillin and glycine were purchased from Sinopharm Chemical Reagent Co., Ltd., and zinc acetate, nickel acetate, and potassium hydroxide were received from Chengdu Kelong Chemical Reagent Company. S. aureus, E. coli, B. subtilis, and S. epidermidis were provided by the 404 hospital of Sichuan Mianyang.
The content of carbon, hydrogen, nitrogen, and oxygen in the complexes was obtained by an Elementar Vario EL CUBE elemental analyzer. Zinc and nickel were determined by ethylenediaminetetraacetic acid (EDTA) complexometric titration. The X-ray powder diffraction patterns of the complexes were recorded by a Rigaku D/Max-RB X-ray diffractometer, Cu Kα radiation at room temperature (λ = 0.154056 nm, step width: 2θ = 0.2°, scan speed: 8°/min). The infrared spectra of the complexes were measured by a Nicolet 5700 Fourier Transform Infrared spectrometer using potassium bromide pellets in the region 400–4000 cm−1. Thermal analyses of the complexes were performed by a TA Q500 thermal analyzer, and the heating rates were suitably controlled at 10°C min−1 under nitrogen atmosphere, and the weight loss was measured from ambient temperature up to 650°C.
4.2. Synthesis of the complexes
Glycine (5 mmol) and potassium hydroxide (5 mmol) were weighed and placed in an agate mortar, and continually grinded until they became sticky. Then, vanillin (5 mmol) was added and grinded continuously, the color of the reactants turned into yellow quickly, and the loose yellow powder solid was obtained after about 30 minutes. Afterward zinc acetate or nickel acetate (5 mmol) was added to the agate mortar and grinded continuously about 30 minutes. The reaction was carried out at room temperature. The resultants were washed repeatedly with distilled water and a little dehydrated ethanol. Lastly, the resultants were filtered and dried about 24 h in a vacuum drying oven at 40°C. The zinc complex of the glycine–vanillin Schiff base was pale–yellow powder, and the yield was about 82%. The nickel complex of the glycine–vanillin Schiff base was pale–blue powder, and the yield was about 86%.
4.3. Qualitative antimicrobial assay
Four pathogenic bacteria were used to test the biological potential of the zinc(II) and nickel(II) complexes of glycine–vanillin Schiff base ligand. They were S. aureus, E. coli, B. subtilis, and S. epidermidis. The culture maintenance and preparation of inoculum were referenced by the literature method (Citation43). The antimicrobial activity of these compounds was determined qualitatively by Oxford cup diffusion method (Citation43, Citation44). A lawn of microorganisms was prepared by pipetting and evenly spreading inoculums (106–107 CFU cm−3; CFU = colony forming units) onto agar set in petri dishes, using nutrient agar for the bacteria. The Oxford cups were sticked on the previously inoculated agar surface and injected solution of the complex (0.15 mL) under sterile conditions. The plates were incubated for 24 h at 37°C. The antimicrobial activity was indicated by the presence of clear inhibition zones around the disks.
Acknowledgments
This work was supported by the Scientific Research Funds of Sichuan Provincial Education Department in China (10ZA016). The authors are very grateful to State Key Laboratory Cultivation Base for Nonmetal Composite and Functional Materials, and Engineering Research Center of Biomass Materials of Education Ministry for the testing of elemental analyses, XRD, FTIR, and TG–DSC.
References
- Hazra, S.; Koner, R.; Lemoine, P.; Sañudo, E.C.; Mohanta, S. Eur. J. Inorg. Chem. 2009, 2009, 3458. 10.1002/ejic.200900353
- Li, B.Y.; Yao, Y.M.; Wang, Y.R.; Zhang, Y.; Shen, Q. Inorg. Chem. Commun. 2008, 11, 349. 10.1016/j.inoche.2007.12.035
- Shang, P.; Zhang, L. J. Chem. 2013, 2013, Article ID 206847.
- Ahmed, R.M.; Yousif, E.I.; Hasan, H.A.; Al-Jeboori, M.J. Sci. World J. 2013, 2013, Article ID 289805.
- Montazerozohori, M.; Nozarian, K.; Ebrahimi, H.R. J. Spectrosc. 2013, 2013, Article ID 718149.
- Ganguly, R.; Sreenivasulu, B.; Vittal, J.J. Coord. Chem. Rev. 2008, 252, 1027. 10.1016/j.ccr.2008.01.005
- Maxim, C.; Pasatoiu, T.D.; Kravtsov, V.Ch.; Shova, S.; Muryn, C.A.; Winpenny, R.E.P.; Tuna, F.; Andruh, M. Inorg. Chim. Acta. 2008, 361, 3903. 10.1016/j.ica.2008.03.013
- Gürten, T.; Serindağ, O. Acta Phy.-Chim. Sin. 2009, 25, 2218.
- Roy, G.B. Inorg. Chim. Acta. 2009, 362, 1709. 10.1016/j.ica.2008.08.009
- Begum, M.S.A.; Saha, S.; Nethaji, M.; Chakravarty, A.R. J. Inorg. Biochem. 2010, 104, 477. 10.1016/j.jinorgbio.2010.01.001
- Rosu, T.; Pahontu, E.; Maxim, C.; Georgescu, R.; Stanica, N.; Gulea, A. Polyhedron. 2011, 30, 154. 10.1016/j.poly.2010.10.001
- Singh, H.L.; Singh, J.; Mukherjee, A. Bioinorg. Chem. Appl. 2013, 2013, Article ID 425832.
- Singh, H.L. Spectrochim. Acta Part A. 2010, 76, 253. 10.1016/j.saa.2010.03.029
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Eur. J. Med. Chem. 2010, 45, 651. 10.1016/j.ejmech.2009.11.008
- Modh, P.H.; Pandya, D.R. Der Chem. Sin. 2012, 3, 663.
- Sathisha, M.P.; Revankar, V.K.; Pai, K.S.R. Metal-based Drugs. 2008, 2008, Article ID 362105. 10.1155/2008/362105
- Wang, M.Z.; Meng, Z.X.; Liu, B.L.; Cai, G.L.; Zhang, C.L.; Wang, X.Y. Inorg. Chem. Commun. 2005, 8, 368. 10.1016/j.inoche.2005.01.023
- Harpstrite, S.E.; Collins, S.D.; Oksman, A.; Goldberg, D.E.; Sharma, V. Med. Chem. 2008, 4, 392. 10.2174/157340608784872280
- Kurdekar, G.S.; Sathisha, M.P.; Budagumpi, S.; Kulkarni, N.V.; Revankar, V.K.; Suresh, D.K. Med. Chem. Res. 2012, 21, 2273. 10.1007/s00044-011-9749-3
- Bagihalli, G.B.; Avaji, P.G.; Patil, S.A.; Badami, P.S. Eur. J. Med. Chem. 2008, 43, 2639. 10.1016/j.ejmech.2008.02.013
- Han, J.; Xing, Y.H.; Zhang, X.J.; Zhou, G.H.; An, Y.; Ge, M.F. Chem. J. Chin. U. 2007, 28, 1431.
- Pawlica, D.; Marszalek, M.; Mynarczuk, G.; Sieroń, L.; Eilmes, J. New J. Chem. 2004, 28, 1615.
- Neelakantan, M.A.; Rusalraj, F.; Dharmaraja, J.; Johnsonraja, S.; Jeyakumar, T.; Sankaranarayana Pillai, M. Spectrochim. Acta Part A. 2008, 71, 1599. 10.1016/j.saa.2008.06.008
- Nair, M.S.; Joseyphus, R.S. Spectrochim. Acta Part A. 2008, 70, 749. 10.1016/j.saa.2007.09.006
- Al-Garawi, Z.S.M.; Tomi, I.H.R.; Al-Daraji, A.H.R. E-J. Chem. 2012, 9, 962. 10.1155/2012/218675
- Hosny, N.M.; El-Dossoki, F.I. J. Chem. Eng. Data. 2008, 53, 2567. 10.1021/je800415n
- Doğan, F.; Ulusoy, M.; Öztürk, Ö.F.; Kaya, İ.; Salih, B.J. Therm. Anal. Calorim. 2009, 98, 785. 10.1007/s10973-009-0205-2
- Zhong, G.Q.; Chen, Y.R.; Zang, X.S.; Luan, S.R. Chin. J. Inorg. Chem. 2001, 17, 597.
- Sain, S.; Saha, R.; Mostafa, G.; Fleck, M.; Bandyopadhyay, D. Polyhedron. 2012, 31, 82. 10.1016/j.poly.2011.08.040
- Zhong, G.Q.; Luan, S.R. Chin. J. Synth. Chem. 2002, 10, 30.
- Abdel-Latif, S.A.; Hassib, H.B.; Issa, Y.M. Spectrochim. Acta Part A. 2007, 67, 950. 10.1016/j.saa.2006.09.013
- Aman, R.; Matela, G. J. Chem. 2013, 2013, Article ID 637290. 10.1155/2013/637290
- Zhong, G.Q.; Zhong, W.W.; Jia, R.R.; Jia, Y.Q. J. Chem. 2013, 2013, Article ID 217947. 10.1155/2013/217947
- Zhong, G.Q.; Jia, R.R.; Jia, Y.Q. Adv. Mater. Res. 2012, 549, 292. 10.4028/www.scientific.net/AMR.549.292
- Min, C.Y.; Yang, X.F.; Zhang, R.X.; Yao, F.; Ouyang, W.M. J. Coord. Chem. 2011, 64, 1617. 10.1080/00958972.2011.577425
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; John Wiley & Sons: New York, 1986.
- Fan, Y.H.; Gao, Z.X.; Bi, C.F.; Xie, S.T.; Zhang, X. J. Therm. Anal. Calorim. 2008, 91, 919. 10.1007/s10973-006-8239-1
- Luan, S.R.; Zhu, Y.H.; Jia, Y.Q. J. Therm. Anal. Calorim. 2009, 95, 951. 10.1007/s10973-008-8926-1
- Parekh, H.M.; Panchal, P.K.; Patel, M.N. J. Therm. Anal. Calorim. 2006, 86, 803. 10.1007/s10973-005-7284-5
- Avsar, G.; Altinel, H.; Yilmaz, M.K.; Guzel, B. J. Therm. Anal. Calorim. 2010, 101, 199. 10.1007/s10973-009-0453-1
- Olar, R.; Badea, M.; Marinescu, D.; Lazar, V.; Chifiriuc, C. J. Therm. Anal. Calorim. 2009, 97, 721. 10.1007/s10973-009-0280-4
- Abdel-Ghani, N.T.; Sherif, O.E. Thermochim. Acta. 1989, 156, 69. 10.1016/0040-6031(89)87172-2
- Zhao, S.M.; Zheng, L.J.; Du, B. Sci. Technol. Rev. 2009, 27, 37.
- Ali, A.M.; Mackeen, M.M.; Intan-Safinar, I.; Hamid, M.; Lajis, N.H.; El-Sharkawy, S.H.; Murakoshi, M. J. Ethnopharmacol. 1996, 53, 165. 10.1016/0378-8741(96)01434-1