Abstract
A simple, effective, and economic method for determination of nine triazines (ametryn, atrazine, cyanazine, prometryn, propazine, simazine, simetryn, terbuthylazine, and terbutryn) in drinking water based on solid-phase extraction (SPE) followed by high-performance liquid chromatography-diode array detection (HPLC-DAD) was developed. A specialized solid phase (Oasis HLB) was used, and the parameters that may affect the efficiency of SPE were optimized. The limits of detection (ranged from 0.010 to 0.023 µg L−1) were satisfactory and allow the determination of triazines at the levels required by European Union legislation. Repeatability (2.4–7.6%) and intermediate precision (0.9–11.0%) calculated at 0.1 µg L−1 (legislation level) were adequate. The accuracy calculated as the average recovery of spiked tap and mineral waters was higher than 86% for all compounds. The developed method also could be used for undergraduate laboratory experiments because it acquaints students with solution preparation, solid phase extraction procedure, and HPLC-DAD technique.
Keywords:
1. Introduction
Triazines are well-known herbicides that are worldwide applied to soil for the control of weeds in many agricultural crops, as well as, railways, roadside, and golf courses. Their mechanism of action is via photosynthetic inhibition, and for this reason, they should be toxic only for plants; however, there are experimental evidences that these compounds can affect the ecosystem and can be dangerous for human health (Citation1). The high persistence of these compounds has required rigorous control of environmental contamination, especially for drinking water sources. Therefore, the presence of pesticides in waters for human consumption is regulated by the European Directive 98/83/EC (Citation2) that establishes the maximum admissible concentration of each pesticide at 0.1 µg L−1 and the total amount of pesticides at 0.5 µg L−1. It is important to take into account that atrazine and simazine have been included in the list of “priority hazardous substances” (Decision no. 2455/2001/EC (Citation3) and that amends the Directive 2000/60/EC (Citation4)) due to their persistence, toxicity, moderate leaching capacity, and potential to adsorb onto soils and sediments. Furthermore, atrazine, ametryn, prometryn, terbutryn, simazine, and propazine are considered as a group to be endocrine-disrupting chemicals by the US Environmental Protection Agency (Citation5).
Due to the low limits established by the legislation for these compounds in water samples, a preconcentration step previous to their determination is necessary. In the last few years, the trends for simplifying the analytical procedures have driven to the development of new analytical approaches which enable the determination of pollutants with improved capabilities, reduced clean-up, and preconcentration steps, and reduction of toxic reagents, solvent wastes, and energy consumption according to principles of green analytical chemistry. With this purpose, solid-phase extraction (SPE) using different sorbents such as Oasis HLB (Citation6–13), C18 (Citation14–17), molecular imprinted polymers (MIP) (Citation18–21), and others sorbents (Citation22–33) is the preconcentration technique most commonly used for the determination of triazines in water samples. Recently, microextraction techniques have become important procedures in environmental analysis. So solid-phase microextraction (SPME) (Citation34), stir bar sorptive extraction (SBSE) (Citation35–37), micro-liquid-liquid extraction (micro-LLE) (Citation38), liquid-phase microextraction (LPME), and liquid-liquid-solid microextraction (LLSME) (Citation39–41), dispersive liquid-phase microextraction (DLPME) (Citation42, Citation43), and solid-phase membrane tip extraction (SPMTE) (Citation44) have been developed for the extraction of triazines in water samples as alternative to the SPE techniques. These new techniques have some advantages over SPE, but they also have drawbacks because some of them have shown low sensitivity for the triazines studied (Citation34, Citation36, Citation38, Citation43, Citation44), others are very laborious (Citation38–41), and in many cases, poor recoveries were obtained (Citation36, Citation42, Citation43). Triazine herbicides have been analyzed using chromatographic techniques (gas chromatography (GC) and high-performance liquid chromatography (HPLC)). In the studies employing HPLC for the determination of triazines, various detectors have been used as diode array detection (DAD) (Citation6–8, Citation14, Citation15, Citation18, Citation20, Citation22, Citation24, Citation25, Citation36, Citation43), ultraviolet detection (UV) (Citation19, Citation23, Citation28, Citation38, Citation41, Citation42, Citation44), or mass spectrometry (MS) detection (Citation9, Citation10, Citation12, Citation13, Citation16, Citation21, Citation27, Citation30, Citation33, Citation40). It is important to note the advantages relating to the cost when using detection by DAD or ultraviolet (UV) compared to techniques that use MS as detector.
This work has a double purpose: (Citation1) development of a simple, effective, and low-cost method for the simultaneous determination of nine triazine herbicides in drinking water samples based on SPE followed by HPLC-DAD and (Citation2) employment of the method as a laboratory experiment for undergraduate students. It should be noted that although many papers about analysis of triazines in water using SPE have been published, but none of them presented the nine herbicide compounds included in this study. On the other hand, the developed method is suitable for both analytical chemistry and environmental sciences, and the use of this method as undergraduate experiment permits the introduction of the concept of green chemistry. This experiment would acquaint students on preparation of solutions, SPE of herbicide residues from liquid samples, employment of concentration steps, utilization of HPLC-DAD technique, and data analysis. Therefore, it would allow students gaining experience on a number of essential techniques in the analytical chemistry laboratory. Furthermore, the students would get experience on laboratory safety procedures (including the use of goggles and gloves) and on waste disposal (all solutions are discarded in an organic waste container and cartridges in a solid residue container).
2. Experimental
2.1. Chemical and materials
All herbicides analytical standards (ametryn, atrazine, cyanazine, prometryn, propazine, simazine, simetryn, terbuthylazine, and terbutryn) were supplied by Sigma-Aldrich (Inc. St. Louis, MO, USA). The individual stock standard solutions of 1000 mg L−1 were prepared in methanol (MeOH) by exact weighing of high-purity substances and stored at −18°C in the dark. A solution containing all the studied triazines at a concentration of 10 mg L−1 was obtained by convenient dilution of individual standard solutions in MeOH and stored at −18°C. All working solutions were prepared daily from the mixed solution by appropriate dilution in MeOH:water (50:50, v/v).
Reverse phase polymeric cartridges Oasis HLB (6 mL, 200 mg) were supplied by Waters (Milford, MA, USA). Acetonitrile (ACN) was purchased from Panreac (Barcelona, Spain) and MeOH and acetone from Romil (Cambridge, UK). All chemicals were HPLC grade. Ultra-pure Milli-Q water was obtained using a Millipore Milli-Q system (Millipore, Bedford, MA, USA).
2.2. Apparatus
Chromatographic analyses were carried out using a HPLC-DAD. The system consisted of a 2695 pump with a 996 DAD from Waters (Milford, MA, USA). The column was a stainless steel column (150 mm × 4.6 mm ID, particle size 5 µm) packed with Hypersil GOLD C18 chemical-bonded phase from Thermo Scientific (Austin, TX, USA).
A Visiprep vacuum system from Supelco (Bellefonte, PA, USA), a rotary evaporator (Büchi, Labortechnic AG, Flawil, Switzerland), and an ultrasonic bath (Branson 3200, Energieweg, The Netherlands) were used.
2.3. SPE procedure
A Visiprep system was employed for the SPE procedure. Prior to their use, SPE cartridges were conditioned by washing with 10 mL MeOH and 10 mL Milli-Q water. Water sample (500 mL) was pumped through the cartridge at a flow rate of 10 mL min−1 and then the cartridge was washed with 20 mL Milli-Q water. Once the retention step had been completed, the cartridges were partially dried under a vacuum system for 5 min, and then it was totally dried using a nitrogen stream for 30 min. The elution of retained compounds was done with 3 mL of acetone, and the organic extract was brought to dryness under a combination of rotary evaporator at 40°C and a gentle nitrogen stream. Finally, the sample was reconstituted in MeOH /water (50:50, v/v) to a final volume of 1 mL, and 20 µL was injected into the HPLC.
2.4. Chromatographic conditions
The analysis was performed using the following ACN:H2O gradient conditions: ACN initial percentage of 30% (8 min), increased linearly to 40% in 5 min; and increased to 50% in 5 min, after which the percentage was returned to the initial conditions in 9 min. A constant mobile phase flow rate of 1 mL min−1 and 20 µL of sample volume was used. The column temperature was kept at 25°C employing the oven of the system.
The absorbance was measured continuously in the 200–400 nm range, and peaks areas quantification were carried out at 222.7 nm in order to achieve maximum sensitivity. All triazine herbicides were identified initially by retention time, and then by applying spectral contrast techniques (incorporated in Millenium32 software) the homogeneity of the spectral peak is confirmed. Finally, a spectral identification was carried out contrasting the spectrum with a standard library created in the wavelength interval of 200–400 nm.
3. Results and discussion
3.1. HPLC-DAD optimization
Elution (isocratic or gradient) and the solvent make-up of the mobile phase were studied to achieve optimal separation. A standard solution of 1 mg L−1 and a flow rate of 1 mL min−1 were employed to optimize the liquid chromatography conditions. Initially, a study using isocratic elution with a ratio 50% ACN-50% H2O was carried out. This ratio did not resolve adequately the pairs of ametryn/propazine and atrazine/simetryn, and the cyanazine and simazine peaks were overlapped. Then, three ratios of ACN-H2O at different levels (40:60 (v/v), 35:65 (v/v), and 30:70 (v/v)) were evaluated. For the first two ratios, the peaks of ametryn, propazine, atrazine, and simetryn were adequately resolved, but cyanazine and simazine remained partially overlapped. Good resolution of the nine triazines was achieved with a ratio 30:70 (v/v); however, it was at the expense of a longer elution time (50 min). Therefore, it was necessary to assay several gradients of elution to allow adequate resolution of all the compounds in a reasonably short time. Gradient elution was studied by increasing the phase mobile strength from 30% up to 40 or 50% of ACN. The results have shown an adequate separation for all compounds, but a shorter time was achieved when 50% ACN was employed (22 min). It can be concluded that the use of an ACN-water gradient as mobile phase allowed the adequate separation with a good resolution of the target compounds. The gradient elution chosen is described in the Section 2.4.
3.2. SPE procedure optimization
In this work, Oasis HLB was chosen because it showed a remarkable retention of polar compounds and its relative hydrophobic retention capacity is three times higher than that of silica-based sorbents such as C18. Furthermore, Oasis HLB sorbent is wettable and maintains its capability for high retention and excellent recoveries even if the sorbent runs dry during conditioning or sample loading.
To obtain appropriate extraction performance of the herbicides in the preconcentration step, several parameters that may affect efficiency of the SPE procedure were optimized including elution solvent, elution volume, sample volume, and redissolution solvent.
Three different solvents (ACN, MeOH, and acetone) at three different volumes (1, 3, and 5 mL) were compared for elution of selected herbicides. For this experiment, 100 mL of a standard solution at a concentration level of 500 µg L−1 was employed. The results showed loss of extraction efficiency when 1 mL of an elution solvent was used (recoveries between 45–67%, 48–68%, and 68–76% for ACN, MeOH, and acetone respectively). For all of the tested herbicides, complete recovery was obtained using 3 mL of the assayed solvents with recoveries between 90–100%, 94–98% and 89–99% for ACN, MeOH, and acetone, respectively. Nevertheless, the time consumed in the evaporation step is much lower with acetone than with the other sorbents. Therefore, 3 mL of acetone was selected as elution solvent.
After selecting the elution conditions, the sample volume was optimized. The study was carried out using standard solutions spiked at the concentration level required by the legislation (0.1 µg L−1) and different sample volumes between 250 and 1000 mL. For the studied herbicides, the recoveries obtained for the tested volumes of 500 and 1000 mL were adequate (83–105% and 86–101% respectively and with relative standard deviation [RSD] < 6), indicating that there was no analyte loss from the column. However, low recoveries with high standard deviation were achieved for simetryn (49 ± 21%), atrazine (69 ± 23), terbuthylazine (61 ± 10), and terbutryn (76 ± 6) when a sample volume of 250 mL was used. These results are due to the lower concentration present in the final extract when 250 mL was employed. To reach a high preconcentration factor as well as good recoveries with the minimal volume of the sample, 500 mL of water sample was chosen.
To improve the sensitivity of the method, the redissolution of the extract was studied. For this purpose, standard solutions at a concentration of 0.1 µg L−1 and different solvents to reconstitute the extract (MeOH/H2O (1:1) and ACN/H2O (1:1)) at two different volumes (0.5 and 1 mL) were employed. The results have shown inadequate recoveries for propazine (62 ± 10%) and terbuthylazine (66 ± 7) using 0.5 mL ACN/H2O and very high relative standard deviation (up to ± 50) when 0.5 mL MeOH/H2O was used. Moreover, the results obtained for both the solvents when using a volume of 1 mL were similar (82–99% and 89–104% for ACN/H2O and MeOH/H2O, respectively), but a lower relative standard deviation was achieved using MeOH/H2O (RSD < 7). For this reason, MeOH/H2O was chosen as a solvent for extract reconstitution.
3.3. Method validation
The analytical characteristics of the developed SPE-HPLC method were evaluated. The linearity was studied by using tap water spiked with the target compounds at five concentration levels (0.02, 0.04, 0.06, 0.08, and 0.1 µg L−1). As it can be seen in , determination coefficients (r2) were higher than 0.99 for all herbicides at concentrations within the interval tested except for terbuthylazine. Repeatability (n = 8) and reproducibility (n = 5) were evaluated using tap water spiked at 0.1 µg L−1 concentration level. As it can be seen in , the results obtained have shown good precision of the method with RSD values below 10% for repeatability and between 0.9 and 11.0% for reproducibility.
Table 1. Analytical characteristics of the SPE-HPLC-DAD method.
The limits of detection (LODs) were determined as 3 × Sy/x/b and the limits of quantification (LOQs) as 10 × Sy/x/b, where Sy/x is the residual standard deviation and b is the slope of the calibration curves. As it can be seen in , the LODs varied from 0.010 to 0.023 µg L−1 being in all cases below 30% of the legislated parametric value (Citation2). Furthermore, the LOQs values were later confirmed by the analysis of five tap water samples spiked at a concentration level of 0.050 µg L−1 for all herbicides, except for propazine and terbuthylazine, for which a concentration level of 0.080 µg L−1 was used. This different concentration was chosen because the LOQs obtained for both the herbicides were higher than those of the remaining compounds. The values obtained were in good agreement with the ones resulting from theoretical calculation using standard solutions (RSD lower than 10%, see ) except for ametryn (11.7%). Therefore, the LOD and LOQ values were satisfactory and allow the determination of these triazines at the levels required by the legislation in water for human consumption. The LODs obtained with the proposed method are similar (Citation6, Citation7) or even better (Citation8) to those reported in other studies using Oasis HLB in which standard solutions or mains water have been used for their calculation. Most studies using different sorbents and HPLC with DAD or UV detection have achieved worse LODs to those obtained with the developed method (Citation14, Citation22, Citation23, Citation28).
The accuracy (expressed as percentage recovery) was studied using 500 mL of unpolluted tap and mineral water samples spiked with 0.1 µg L−1 of a standard mixture of the compounds. Tap water was collected from our laboratory, and mineral water was purchased in a supermarket. The spiked samples were mixed in an ultrasonic bath for 5 min to ensure efficient distribution of the herbicides and allowed to equilibrate for 5 min prior to extraction. Then samples were subjected to the SPE method and analyzed. As an example, chromatograms corresponding to tap water sample () and tap water sample spiked at a concentration level of 0.1 µg L−1 () are presented in . As it can be seen in , a clear chromatogram without interferents was obtained.
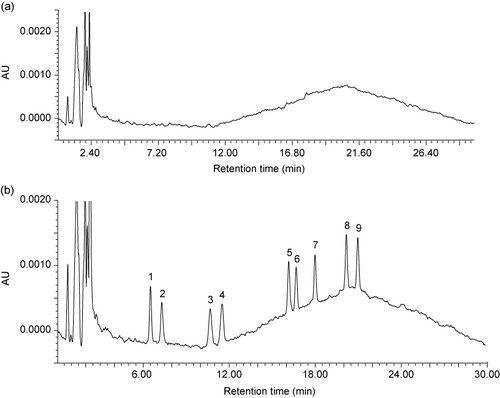
Analytical recoveries were assessed by comparing the results obtained for spiked water samples with those obtained for standard solution made up in Milli-Q water. The results are presented in . As it can be seen, the mean recoveries for each compound in the tap water sample, ranged from 86 to 110% and in the mineral water sample, ranged from 97 to 109%. It should be pointed out that the free chlorine present in tap water does not significantly affect herbicides detection and quantification. However, the recoveries reached on tap water were worse than those obtained on mineral water probably due to its presence. Therefore, it can be concluded that the proposed method is useful to determine triazines in drinking water samples.
Table 2. Analytical recoveries for 2 µg L−1 concentration of analytes (n = 5).
3.4. Laboratory experience for undergraduate education
Our main aim was to design a laboratory experiment that allows students to participate actively in learning of concepts through practical experience. Furthermore, the procedure described is meant to be used as a demonstration whereby the students can understand the overlap among different fields of the chemistry. On the other hand, this experiment works well for groups of three–four students, which led to generate stimulants discussions between the members of the group during laboratory sessions.
The four pedagogic goals for this experiment were (Citation1) utilization of HPLC-DAD technique (components of HPLC system and developing of HPLC-DAD methods); (Citation2) learning of the SPE procedure (selection of elution solvent, volume of solvent, volume of sample) as well as the concentration step (combination of rotary evaporator and nitrogen stream); (Citation3) study and evaluation of analytical characteristics (linearity, accuracy, precision, LODs, …); and (Citation4) evaluation of the process taking into account the principles of Green Chemistry (waste generation, safety of processing steps, health, and environmental impact of the reagents).
After completing the laboratory experiment, the students will be required to present their results in a classroom session. It will inspire interesting discussions because the students will have to compare the results obtained and formulate explanations about the experimental procedure carried out and also propose solutions to undesirable obstacles.
The didactic experience with the developed method is being carried out in the current academic year. However, it can be said that the views of students over other similar but simpler didactic experiences were very satisfactory. If we consider the answers of students in surveys and during the laboratory sessions as well as their participation in the classroom activities, the teaching approach allowed them to learn actively. Students were very interested in the subject and responded positively to the challenge of carrying out the entire procedure and evaluating their own results.
4. Conclusions
An effective, accurate, simple, and low-cost method based on SPE combined with HPLC-DAD for simultaneous analysis of nine triazines in water for human consumption was developed. Oasis HLB sorbent has demonstrated to be appropriate since it favorably extracts the nine triazine herbicides in tap and mineral water samples with an adequate accuracy for all compounds. Furthermore, the SPE–HPLC–DAD method employing ACN–water as mobile phase allowed the separation with a good resolution and the quantification of the target compounds at the levels required by European legislation for drinking waters. The proposed method showed good sensitivity with LODs (between 0.010 and 0.023 µg L−1) lower than 30% of parametric value requested by the Directive 98/83/EC (Citation2), concerning the quality of water for human consumption. For all the above mentioned, the proposed method could be established as an adequate method for the analysis of triazines in drinking waters in compliance with EU directives. Regarding the employment of this method for laboratory experiments for undergraduate students, the developed SPE–HPLC–DAD method provides students a good opportunity to understand the connection between analytical chemistry and environmental sciences. Furthermore, the students would gain knowledge and practice about laboratory techniques such as solution preparation, extraction, and concentration of pollutants from water samples and chromatographic techniques; they would also get experience on the study of the analytical characteristics of a method and the on laboratory safety procedures.
Acknowledgments
The authors acknowledge the financial support of the “Ministerio de Educación y Ciencia” [Project CTQ2007-63949/BQU] and the “Xunta de Galicia” [Projects 09MDS038103PR and QANAP 2010/52]. R. Pouso-Blanco also thanks the “Xunta de Galicia” for the concession of her award of Lucas Labrada Program.
References
- LeBaron, H.M.; McFarland, J.E.; Burnside, O.C. The Triazine Herbicides 50 Years Revolutionizing Agriculture; Elsevier: Oxford, 2008; pp 399–411.
- European Commission Directive. Off. J. Eur. Union 1998, L 330, 32.
- European Commission, Decision No. 2455/2001. Off. J. Eur. Union 2001, L 331, 1.
- European Commision Directive. Off. J. Eur. Union 2000, L 327, 1.
- Environmental Protection Agency Federal Register 2009, 74, 17579–17585.
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Fernández-Laespada, M.E.; Sánchez-San Roman, F.J. J. Chromatogr. A 2000, 869, 471–480. 10.1016/S0021-9673(99)01188-7
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Fernández-Laespada, M.E.; Calvo-Seronero, L.; Sánchez-San Roman, F.J. Water Res. 2003, 37, 928–938. 10.1016/S0043-1354(02)00366-4
- Tran, A.T.K.; Hyne, R.V.: Pablo, F.; Day, W.R.; Doble, P. Talanta 2007, 71, 1268–1275. 10.1016/j.talanta.2006.06.031
- Gervais, G.; Brosillon, S.; Laplanche, A.; Helen, C. J. Chromatogr. A 2008, 1202, 163–172. 10.1016/j.chroma.2008.07.006
- Mazzella, N.; Delmas, F.; Delest, B.; Mechin, B.; Madigou, C.; Allenou, J. P.; Gabellec, R.; Caquet, T. J. Environ. Monitor, 2009, 11, 108–115. 10.1039/b805160g
- Navarro, A.; Tauler, R.; Lacorte, S.; Barceló, D. J. Hydrol. 2010, 383, 18–29. 10.1016/j.jhydrol.2009.06.039
- Dujakovic, N.; Grujic, S.; Radisic, M.; Vasiljevic, T.; Lausevic, M. Anal. Chim. Acta 2010, 678, 63–72. 10.1016/j.aca.2010.08.016
- Benvenuto, F.; Marín, J.M.; Sancho, J.V.; Canobbio, S.; Mezzanotte, V.; Hernández, F. Anal. Bioanal. Chem. 2010, 397, 2791–2805. 10.1007/s00216-010-3712-x
- Lekkas, T.; Kolokythas, G.; Nikolaou, A.; Kostopoulou, M.; Kotrikla, A.; Gatidou, G.; Thomaidis, N.S.; Golfinopoulos, S.; Makri, C.; Babos, D.; Vagi, M.; Stasinakis, A.; Petsas, A.; Lekkas, D.F. Environ. Int. 2004, 30, 995–1007. 10.1016/j.envint.2004.04.001
- Kotrikla, A.; Gatidou, G.; Lekkas, T. J. Environ. Sci. Heal. B 2006, 41, 135–144. 10.1080/03601230500364336
- Van Pinxteren, M.; Bauer, C.; Popp, P. J. Chromatogr. A 2009, 1216, 5800–5806. 10.1016/j.chroma.2009.06.027
- Portolés, T.; Pitarch, E.; López, F. J.; Hernández, F. J. Chromatogr. A 2011, 1218, 303–315. 10.1016/j.chroma.2010.11.010
- Ferrer, I.; Lanza, F.; Tolokan, A.; Horvath, V.; Sellergren, B.; Horvai, G.; Barceló, D. Anal. Chem. 2000, 72, 3934–3941. 10.1021/ac000015f
- Sambe, H.; Hoshina, K.; Haginaka, J. J. Chromatogr. A 2007, 1152, 130–137. 10.1016/j.chroma.2006.09.003
- Kueseng, P.; Noir, M.L.; Mattiasson, B.; Thavarungkul, P.; Kanatharana, P. J. Environ. Sci. Heal. B 2009, 44, 772–780. 10.1080/03601230903238319
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. J. Hydrol. 2010, 383, 30–38. 10.1016/j.jhydrol.2009.09.025
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Herrero-Hernández, E.; Sánchez-San Roman, F.J.; Prado-Flores, M.G. J. Chromatogr. A 2002, 950, 157–166. 10.1016/S0021-9673(01)01613-2
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Miranda-Cruz, E.M.; Domínguez-Alvarez, J.; Hernández-Méndez, J. J. Chromatogr. A 2006, 1122, 194–201. 10.1016/j.chroma.2006.04.017
- Zhou, Q.X.; Xiao, J.P.; Wang, W.D.; Liu, G.G.; Shi, Q.Z.; Wang, J. H. Talanta 2006, 68, 1309–1315. 10.1016/j.talanta.2005.07.050
- Papadopoulos, N.; Gikas, E.; Zalidis, G.; Tsarbopoulos, A. J. Agr. Food Chem. 2007, 55, 7270–7277. 10.1021/jf0706777
- Berzas-Nevado, J.J.; Guiberteau-Cabanillas, C.; Villaseñor-Llerena, M.J.; Rodríguez-Robledo, V. Microchem. J. 2007, 87, 62–71. 10.1016/j.microc.2007.05.004
- Song, Y.; Zhao, S.; Tchounwou, P.; Liu, Y.M. J. Chromatogr. A 2007, 1166, 79–84. 10.1016/j.chroma.2007.07.074
- Zhao, R.S.; Yuan, J.P.; Jiang, T.; Shi, J.B.; Cheng, C.G. Talanta 2008, 76, 956–959. 10.1016/j.talanta.2008.04.029
- Bagheri, H.; Khalilian, F.; Naderi, M.; Babanezhad, E. J. Sep. Sci. 2010, 33, 1132–1138.
- Postigo, C.; de Alda, M.J.L.; Barceló, D.; Ginebreda, A.; Garrido, T.; Fraile, J. J. Hydrol. 2010, 383, 83–92. 10.1016/j.jhydrol.2009.07.036
- Katsumata, H.; Kojima, H.; Kaneco, S.; Suzuki, T.; Ohta, K. Microchem. J. 2010, 96, 348–351. 10.1016/j.microc.2010.06.005
- Matamoros, V.; Jover, E.; Bayona, J. M. Anal. Chem. 2010, 82, 699–706. 10.1021/ac902340e
- Lissalde, S.; Mazzella, N.; Fauvelle, V.; Delmas, F.; Mazellier, P.; Legube, B. J. Chromatogr. A 2011, 1218, 1492–1502. 10.1016/j.chroma.2011.01.040
- Mohammadi, A.; Ameli, A.; Alizadeh, N. Talanta 2009, 78, 1107–1114. 10.1016/j.talanta.2009.01.025
- León, V.M.; Alvarez, B.; Cobollo, M.A.; Muñoz, S.; Valor, I. J. Chromatogr. A 2003, 999, 91–101. 10.1016/S0021-9673(03)00600-9
- Portugal, F.C.M.; Pinto, M.L.; Nogueira, J.M.F. Talanta 2008, 77, 765–773. 10.1016/j.talanta.2008.07.026
- Sánchez-Ortega, A.; Unceta, N.; Gómez-Caballero, A.; Sampedro, M.C.; Akesolo, U.; Goicolea, M.A.; Barrio, R.J. Anal. Chim. Acta 2009, 641, 110–116. 10.1016/j.aca.2009.03.044
- Hu, Y.L.; Liu, R.J.; Zhang, Y.; Li, G.K. Talanta 2009, 79, 576–582. 10.1016/j.talanta.2009.04.029
- Shen, G.; Lee, H.K. Anal. Chem. 2002, 74, 648–654. 10.1021/ac010561o
- Trtic-Petrovic, T.; Dordevic, J.; Dujakovic, N.; Kumric, K.; Vasiljevic, T.; Lausevic, M. Anal. Bioanal. Chem. 2010, 397, 2233–2243. 10.1007/s00216-010-3725-5
- Hu, Y.L.; Wang, Y.Y.; Hu, Y.F.; Li, G.K. J. Chromatogr. A 2009, 1216, 8304–8311. 10.1016/j.chroma.2009.09.063
- Zhou, Q.X.; Pang, L.; Xie, G.H.; Xiao, J.P.; Bai, H.H. Anal. Sci. 2009, 25, 73–76. 10.2116/analsci.25.73
- Wang, S.L.; Ren, L.P.; Liu, C.Y.; Ge, J.; Liu, F.M. Anal. Bioanal. Chem. 2010, 397, 3089–3095. 10.1007/s00216-010-3841-2
- See, H.H.; Sanagi, M.M.; Ibrahim, W.A.W.; Naim, A.A J. Chromatogr. A 2010, 1217, 1767–1772. 10.1016/j.chroma.2010.01.053
