Abstract
The intriguing research toward the exploitation of zeolite-Y-based hybrid nanocatalysts for catalytic oxidation reactions has been growing significantly. In the present investigation, we describe the synthesis of zeolite-Y entrapped transition metal complexes of the general formulae [M(SFCH)·xH2O]-Y (where, M = Mn, Fe, Co, Ni (x = 3) and Cu (x = 1)); H2SFCH = (E)-N′-(2-hydroxybenzylidene)furan-2-carbohydrazide]. These nanocatalysts have been characterized by various physicochemical techniques. Density functional theory calculations are performed to address the relaxed geometry, bond angle, bond length, dihedral angle, highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) energy gap, and electronic density of states of H2SFCH ligand and their neat transition metal complexes. The observed HOMO–LUMO gap and the Fermi energy is higher for Cu(II) complexes, which demonstrates the better catalytic activity of this nanocatalyst. The catalytic activity was performed in liquid-phase oxidation of cyclohexane using hydrogen peroxide as oxidant to give cyclohexanone (CyONE) and cyclohexanol (CyOL). Among them, [Cu(SFCH)·H2O]-Y catalyst has the highest selectivity toward CyONE (84.5%).
1. Introduction
The selective oxidation is an industrially momentous reaction for the synthesis of chemical intermediates in the manufacture of high tonnage commodities, high-value fine chemicals, and pharmaceutically important ingredients but still is an ineffective process. The heterogeneous catalysts offer great advantages such as effortless separation of product, catalyst revival, and make it suitable for continuous processing. As a result, heterogenization of homogeneous catalysts has become a significant approach for obtaining supported catalysts that sustain the active catalytic sites of the homogeneous analog (Citation1, Citation2). The encapsulation of the transition metal complexes inside the nanopores of zeolite is one of the heterogenization methods and also is a theme of contemporary research (Citation3–Citation7). Heterogenization is accomplished either by entrapping the metal complex within the zeolite nanocavities or by anchoring and/or tethering them to inert supports (Citation8). The structural design of zeolite-Y-based hybrid nanocatalysts via flexible ligand approach is convenient and ideal because the complex, once formed inside the cages of the zeolite is fitted suitably and not easily to diffuse out during the catalytic reaction (Citation9, Citation10).
In particular, the selective oxidation of cyclohexane is an industrially important chemical reaction because of its oxidized products, such as cyclohexanol (CyOL) and cyclohexanone (CyONE), which are important intermediates in the production of adipic acid and caprolactam. Caprolactam is used in the manufacture of Naylon-6 and Nylon-66 polymers. In a recent industrial process, cyclohexane is oxidized at a temperature range of 150–170°C and pressure of 115–175 psi in the presence of homogeneous cobalt salt, where the conversion is very less (∼4%), and the process is environmentally hazardous (Citation11, Citation12). With the emphasis on environmentally benign catalytic oxidation of cyclohexane, several research groups have been designing various catalytic systems (Citation13–Citation20).
The use of computational techniques in catalysis, homogeneous as well as heterogeneous, has become explored mainly because of the sophistication of several density functional theory software. Scientifically, many known catalytic materials are expected to gain better exploitations because of their new properties and possible high catalytic activities in the nanometer regime (density functional theory (DFT) approach) (Citation21, Citation22). With these newer approaches and knowledge, our laboratory has been exploring the chemistry of this class of hybrid nanocatalysts (Citation23, Citation24). Continuing our study, herein we report the synthesis, characterization, and catalytic activity of zeolite-Y entrapped bivalent transition metal complexes containing salicylaldehyde furoic-2-carboxylic hydrazone ligand (H2SFCH) in combination with the DFT studies. Results of the DFT for Schiff base ligand (H2SFCH), [Cu(H2SFCH)·H2O] and [Mn(H2SFCH)·3H2O] were used to explore the energetics of the metal–ligand interactions and the possibility for the compound to adopt different conformations.
2. Results and discussion
represents the chemical compositions which confirmed the purity and stoichiometry of the host zeolite-Y and zeolite-Y entrapped nanocatalysts. The chemical analyses of compounds reveal the presence of organic matter within the nanocavities of zeolite-Y. The mole ratio Si/Al obtained by chemical analysis for neat zeolite-Y and zeolite-Y entrapped nanocatalysts are shown in . The Si and Al contents in Na-Y and [M(SFCH)·xH2O]-Y (where, M = Mn, Fe, Co, Ni (x = 3) and Cu (x = 1)) are almost in the same ratio, which indicates no dealumination during the metal ion exchange. The nitrogen adsorption–desorption isotherms for Na-Y, MII-Y, and [M(SFCH)·xH2O]-Y are analyzed and shown in . The results showed a decrease in the amount of N2 adsorbed in case of Na-Y to zeolite-Y entrapped complexes, which indicates the filling of the nanocavities of zeolite-Y by complexes (Citation25, Citation26).
Table 1. Analytical and physical data of compounds
Table 2. Surface area and pore volume data of compounds.a
The SEM images before and after Soxhlet extraction of [Ni(SFCH)·3H2O]-Y are shown in . The encapsulation of the complexes inside the zeolite-Y nanocavities can be confirmed by the absence of extraneous material by SEM image after Soxhlet extraction ().
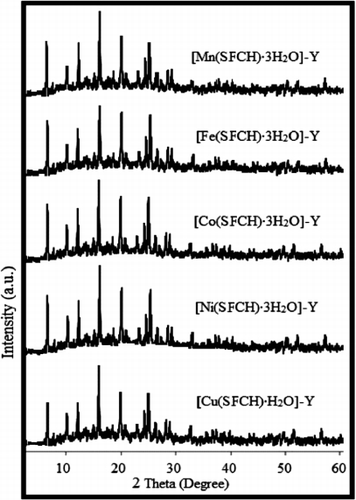
shows the powder X-ray diffraction (XRD) patterns of zeolite-Y entrapped nanocatalysts. It is clear that the XRD pattern of [M(SFCH)·xH2O]-Y (where, M = Mn, Fe, Co, Ni (x = 3) and Cu (x = 1)) was not rigorously affected by the introduction of the metal complex into the zeolitic structure. The entrapped complexes exhibit similar peaks to those of parent zeolite-Y and except for a slight change in the intensity of the peaks, no new crystalline pattern emerges. These facts confirmed that the framework and crystallinity of zeolite were not destroyed during the flexible ligand synthesis and that the complexes were well entrapped in the supercages of zeolite-Y.
The data obtained by vibrational spectroscopy can provide information on the integrity of the Schiff base ligand (H2SFCH), zeolite-Y entrapped transition metal complexes, as well as the crystallinity of the host framework. The intensity of the bands of the entrapped complex is weak due to their low concentration in the zeolite matrix. The main Fourier transform-infrared spectroscopy (FT-IR) bands of the Schiff base ligand (H2SFCH) and their entrapped transition metal complexes are tabulated in . Comparison between the spectra of H2SFCH and their entrapped transition metal complexes provides evidence for the coordinating mode of Schiff base ligand in entrapped complexes. The spectra of H2SFCH shows a sharp and strong band at 1603 cm−1 due to ν(C = N) of the azomethine group. This band undergoes hypsochromic effect in the spectra of entrapped complexes, indicating coordination of the azomethine nitrogen (Citation27). The ν(N–H) and ν(C = O) modes of the lateral chain in the uncoordinated Schiff base appearing at 3216 and 1680 cm−1, respectively (Citation28). The reaction of the enolic ligand with M(II) ion is revealed by the presence of a new band in the spectra of 1275–1281 cm−1 due to the ν(C–O) (enolic) (Citation29). In addition, the phenolic-OH vibration of the Schiff base ligand (3380 cm−1) disappeared in the entrapped complexes. From these observations, it is concluded that the ligand reacts in enol form with prototropy, which incorporates into proton transfer through oxygen atoms of the ligand, forming two bonds with the metal ion. Furthermore, all zeolite-Y entrapped complexes exhibit bands around 1135, 1020, 790, and 720 cm−1 due to host zeolite framework (Citation30–Citation32).
Table 3. Selected FT-IR assignments of H2SFCH and zeolite-Y entrapped nanocatalysts (cm−1)
The electronic spectral bands of Schiff base ligand (H2SFCH) and their entrapped metal complexes are discussed in . The H2SFCH ligand exhibits three bands at 213, 339, and 382 nm due to ϕ→ϕ*, π→π*, and n→π* transitions, respectively. The former band (ϕ→ϕ*) undergoes hypsochromic shift in Cu(II) and bathochromic shifts in Mn(II) and Co(II) entrapped complexes, resulting from chelation of the ligand with the transition metal. In the electronic spectrum of entrapped complex [Mn(SFCH)·3H2O]-Y, the characteristic bands appeared at 322 and 252 nm are assignable to 6A1g→4A1g, 4Eg (ν 3), and metal to ligand charge transfer (MLCT) transitions, respectively, due to distorted octahedral geometry around the metal ion. The electronic spectrum of [Fe(SFCH)·3H2O]-Y exhibits band at 289 nm may be assignable to MLCT transition. Absence of d-d transitions in the spectra of entrapped Fe(II) complex may be due to the lower concentration inside the nanopores of zeolite-Y. [Co(SFCH)·3H2O]-Y displays bands at 708, 319, and 257 nm are attributed to 4T1g(F)→4A2g(F), 4T1g(F)→4T1g(P), and MLCT transitions of an octahedral geometry around the metal ion, respectively. The absorption spectrum of [Ni(SFCH)·3H2O]-Y exhibits two bands at 680 and 242 nm that may be attributed to 3A2g→3T1g(P) (ν3) and 3A2g→3T1g(F) (ν2) transitions, respectively (Citation33). The electronic spectra of [Cu(SFCH)·H2O]-Y consists of a band at 253 nm and a shoulder at 321 nm, which can be assigned to the dxz,yz → dxy and MLCT transitions, respectively, for tetrahedrally distorted (D2h) mononuclear copper(II) complexes (Citation34).
Table 4. Electronic spectral data of H2SFCH and zeolite-Y entrapped nanocatalysts
We have performed first-principles total energy calculations within the DFT using the plane-wave self-consistent field (PWSCF) implementation (Citation35, Citation36). The wave functions describe only the valence and the conduction electrons, while the core electrons are taken into account for pseudo-potentials. For the exchange–correlation functional, we have employed the generalized-gradient approximation (GGA) functional developed by Perdew, Burke and Ernzerhof (PBE) (Citation37) since it is known that GGA gives better results than the simpler local density approximation (LDA) when describing the structural properties of transition metal complexes. A set of convergence tests has been performed in order to choose correctly the mesh of k-points and the cut-off kinetic energy plane waves to start the ground-state and linear-response calculations. The kinetic energy cut-off for the plane wave basis is set to 40 Ryd for all structures. The electronic configurations of atoms are as follows: C[He]2s22p2; N[He]2s22p3; O[He]2s22p4; Mn[Ar]3d54s2; and Cu[Ar]3d104s1. The self-consistent calculations were considered to be converged using the conjugate–gradient algorithm until the absolute value of the forces on unconstrained atoms was less than 0.03 eV/Å2 and up to a precision of 10−4 eV in total energy difference. The Brillouin zone sampling was restricted to the gamma point (Citation38). Marzari–Vanderbilt (Citation39) Gaussian smearing with a width of 0.05 Ryd was used to accelerate the convergence of the total energy calculations. For density of states (DOS) calculations, we increased the sampling of the Brillouin zone.
In our calculations, Broyden–Fletcher–Goldfarb–Shanno (BFGS) method is used to construct the geometry of the model structures for the ligand and their Mn(II) and Cu(II) neat transition metal complexes and then allowed to relax. The optimized structure of [Mn(SFCH)·3H2O] complex shows a distorted octahedral geometry, whereas in the case of [Cu(SFCH)·H2O] shows tetrahedrally distorted geometry along with one water molecule, two oxygen, and one azomethine nitrogen atoms of H2SFCH, as shown in . However, the [Mn(SFCH)·3H2O] complex has shown some distortion in the relaxed structure (), recognized by the dihedral angles O(8)-N(9)-O(17)-Mn and O(20)-O(19)-O(21)-O(8) with 30° and 105°, respectively. Further, dihedral angles of the Mn(II) complex O(8)-O(19)-O(17)-N(9) and C(11)-C(12)-O(17)-Mn are 56° and 29°, respectively, and confirm a distortion in geometry. In the case of Cu(II) complex, the dihedral angle C(11)-C(10)-N(9)-Cu is 65° and reveals a very small distortion for azomethine nitrogen of the lateral chain.
![Figure 3. Representative structures for H2SFCH ligand (a), neat distorted octahedral [Mn(SFCH)·3H2O] (b), tetrahedrally distorted [Cu(SFCH)·H2O] (c), zeolite-Y nanocavity (d), encapsulated [Mn(SFCH)·3H2O]-Y (e) and [Cu(SFCH)·H2O]-Y (f).](/cms/asset/4e5aa905-7d31-4965-87ae-a467c18ed198/tgcl_a_946101_f0003_oc.jpg)
Table 5. Selected bond lengths, bond angles, and dihedral angles of neat Mn(II) and Cu(II) complexes
The bond lengths of Mn-N(9) and Mn-O(20) (∼2.0 Å) are consistent with the characteristic lengths in the octahedral coordinate Mn(II) complex. Similarly, the lengths of resultant bonds for the tetrahedral coordinate Cu(II) complexes are 1.6–1.7 Å [19].The electronic DOS of Cu(II) and Mn(II) complexes and the Schiff base ligand (H2SFCH) are shown in . The most significant difference is observed at Fermi level where the density and shape of the spectra changes going from ligand to complex. An apparent gap just above the Fermi level in the case of ligand vanishes which is indicating a metallic behavior of complexes. A sharp high peak with two shoulders turns to the less-intense peak shifted to a lower energy side. Another, interesting remark is that the opening of the gap in between −22.0 and −20.8 eV is wider in the case of Cu(II) complex. Similar gap opening is observed in between −17.0 and −16.0 eV. The appearance of complex induced hybrid states near to Fermi energy in the present study in explains the spectral shift observed in Schiff base ligand and in neat complexes during the experiments. Furthermore, the main contribution to highest occupied molecular orbital (HOMO) is from ligand orbitals, while the d-orbitals of the metal ion contribute to the lowest unoccupied molecular orbital (LUMO). The shift in HOMO and LUMO energy difference from ligand (H2SFCH) may be due to the columbic and coordination effects and Van Der Waals' interactions. The columbic effects are produced by the charge distribution which might alter the energy level of metal complexes.
![Figure 4. Electronic density of states (DOS) of (a) neat [Cu(SFCH)·H2O] (b) neat [Mn(SFCH)·3H2O] and (c) Schiff base ligand (H2SFCH).](/cms/asset/721253cd-82a7-4e58-a98c-6f176148d530/tgcl_a_946101_f0004_b.jpg)
In , the relaxed parameters and the difference in HOMO and LUMO are shown. The HOMO–LUMO gap is −3.617, −2.924, and −1.992 eV for Cu(II) complex, Mn(II) complex, and Schiff base ligand (H2SFCH), respectively. In the DOS, the complex producing the bonding and anti-bonding like hybrid orbitals below and above the middle of metal d-band is as interpreted by Newns–Anderson model (Citation 40 ). It is clear that the electronic structure and the gap width are very sensitive to the changes in complexes. The Fermi energy for ligand is more than that of the complexes. This might be due to structural changes in relaxation. Further, it is also seen that the higher Fermi energy (3.611 eV) in the case of [Cu(SFCH)·H2O] may enhance the catalytic properties of the nanocatalysts and lower Fermi energy (2.828 eV) lower down the catalytic properties in the case of [Mn(SFCH)·3H2O].
Table 6. Relaxed parameters and HOMO and LUMO energy difference of H2SFCH and their neat Mn(II) and Cu(II) complexes
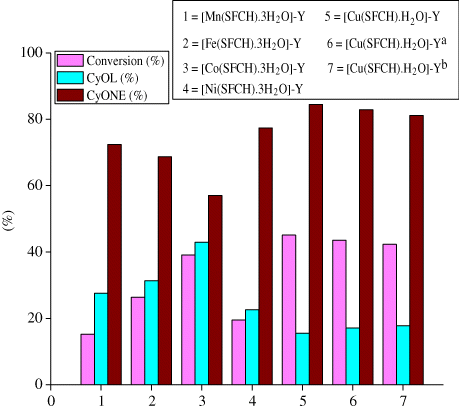
In a typical reaction of cyclohexane oxidation, 30% H2O2 (20 mmol), cyclohexane (10 mmol), and catalyst (45 mg) were mixed in 2 mL acetonitrile in an oil bath at 80°C with continuous stirring for 2 h. The progress of the reaction was checked as a function of time by withdrawing portions of the sample at fixed time intervals and analyzing them by gas chromatography. The host–guest catalyzed oxidation of cyclohexane gives mainly CyOL and CyONE as given in . The catalyst was filtered, thoroughly washed with different solvents like acetone, methanol, water, and dried under similar conditions.
Table 7. Catalyzed oxidation of cyclohexane to CyOL and CyONE by zeolite-Y entrapped nanocatalysts (temperature, 80°C; H2O2/cyclohexane molar ratio, 2:1; time, 2 h; amount of catalyst, 45 mg)
The catalytic conversion of cyclohexane by various zeolite-Y entrapped nanocatalysts is illustrated in . This shows the hydrocarbon yield 15.2, 19.5, 26.4, 39.1, and 45.1% conversion corresponding to [Mn(SFCH)·3H2O]-Y, [Ni(SFCH)·3H2O]-Y, [Fe(SFCH)·3H2O]-Y, [Co(SFCH)·3H2O]-Y and [Cu(SFCH)·H2O]-Y, respectively, at the same reaction condition.
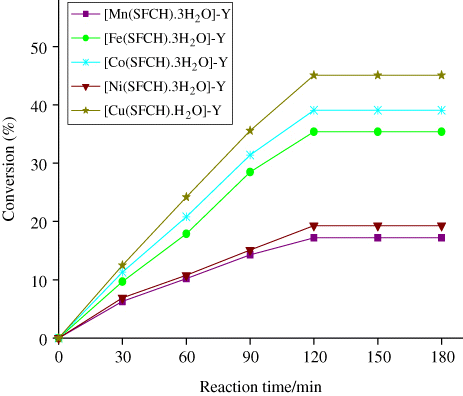
The effect of reaction time at 80°C, with various zeolite-Y entrapped nanocatalysts over the oxidation of cyclohexane was investigated and summarized in by keeping the other parameters fixed. It was found that the conversion of cyclohexane gradually increases and reaches a maximum of 45.1% after 2 h.
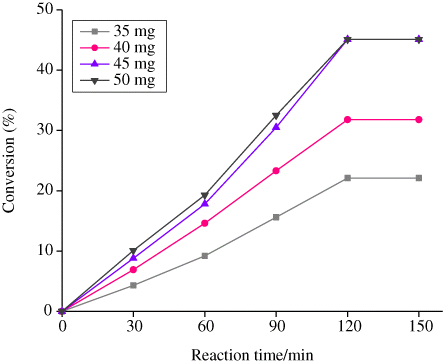
The effect of amount of zeolite-Y entrapped nanocatalysts for the cyclohexane oxidation using [Cu(SFCH)·H2O]-Y as a representative catalyst was investigated at four different amounts namely, 35, 40, 45, and 50 mg, keeping with all other reaction parameters fixed. The results are shown in , indicating 22.1, 31.8, 45.1, and 45.2% conversion corresponding to 35, 40, 45, and 50 mg catalyst, respectively. The maximum percentage conversion was observed with 45 mg catalyst, but there was no remarkable difference in the progress of reaction when 45 or 50 mg of catalyst was employed. Therefore, 45 mg amount of catalyst was taken to be optimal.
The test for recyclability using [Cu(SFCH)·H2O]-Y as a representative catalyst has been carried out. This nanocatalyst was recycled for the oxidation of cyclohexane with 30% H2O2 with a view to finding the effect of encapsulation on stability. It was observed that the conversion was nearly the same as for the first cycle, and there was only a minor loss in catalytic activity for the second cycle may be due to some blocking of zeolite channels during the first cycle. The initial run showed a conversion of 45.1%; this is marginally reduced to 43.6% on first reuse and 42.3% on second reuse of the catalyst, respectively. This revealed that [Cu(SFCH)·H2O]-Y catalyst is almost stable to be recycled for cyclohexane oxidation. Thus, the zeolite-Y entrapped complexes are found to increase the life of the catalyst by a reduction of leaching due to the host zeolite framework.
3. Conclusions
The results obtained in this study allow the following conclusions:
A series of [M(SFCH)·xH2O]-Y complexes (where, M = Mn, Fe, Co, Ni (x = 3) and Cu (x = 1)) have been successfully synthesized by flexible ligand method as evidenced by inductively coupled plasma/optical emission spectrometry (ICP-OES), elemental analyses, (FT-IR and electronic) spectral studies, BET, SEMs, and X-ray diffraction pattern results.
The geometries of neat Mn(II) and Cu(II) complexes with the Schiff base ligand (H2SFCH) are characterized by a quantum mechanical method based on the DFT. From the DFT calculations, it was observed that the complexes are suitable in size for the zeolite channels, which confined the complex and restricted it from coming out of the nanocavity of zeolite-Y.
The HOMO–LUMO gap and the Fermi energy is higher in the case of Cu(II) complexes, which demonstrates the better catalytic activity of this nanocatalyst.
The catalytic behavior of these zeolite-Y entrapped nanocatalysts was performed over the oxidation of cyclohexane affording CyOL and CyONE, with good CyONE selectivity.
Among them, [Cu(SFCH)·H2O]-Y catalyst has the highest percentage of selectivity toward CyONE (84.5%).
To summarize, zeolite-Y entrapped transition metal complexes have interesting catalytic potential particularly with respect to the activity for the oxidation of cyclohexane selectively and offer an open field to design efficient catalyst systems.
4. Experimental
4.1. Materials and methods
Furoic-2-carboxylic acid was obtained from Spectrochem (India). Salicylaldehyde was purchased from Loba Chemie (India). Thirty percent H2O2 was purchased from Rankem (India). Sodium form of zeolite-Y (Si/Al = 2.60) was procured from Hi-media, India. The carbon, hydrogen, and nitrogen were analyzed with a Perkin Elmer, USA 2400-II CHN analyzer. The Si, Al, Na, and transition metal ions were determined by ICP-OES (Model: Perkin Elmer optima 2000 DV). UV-vis spectra were recorded on Spectrophotometer Make/model Varian Cary 500, Shimadzu. FT-IR spectra of compounds were recorded on a Thermo Nicolet IR 200 FT-IR spectrometer. The crystallinity of compounds was ensured by XRD using a Bruker AXS D8 Advance X-ray powder diffractometer with a Cu Kα target. The surface area of entrapped nanocatalysts was measured by multipoint BET method, using Micromeritics, ASAP 2010 surface area analyzer. The scanning electron micrographs of entrapped nanocatalysts were recorded using a SEM instrument (Model: LEO 1430 VP).
4.2. Synthesis of salicylaldehyde furoic-2-carboxylic hydrazone (H2SFCH)
Salicylaldehyde furoic-2-carboxylic hydrazone (H2SFCH) was prepared by following the procedure reported earlier (Citation24).
4.3. Synthesis of zeolite-Y entrapped transition metal complexes
An amount of 5.0 g of Na-Y zeolite was suspended in 300 mL of deionized water containing 12 mmol metal salts (Mn(CH3COO)2·4H2O, FeSO4·7H2O, Co(CH3COO)2·4H2O, Ni(CH3COO)2·4H2O, and Cu(CH3COO)2·H2O, respectively) and heated at 90°C with constant stirring (24 h) for the synthesis of metal exchanged zeolite-Y. Then the solid was filtered, washed with hot deionized water until the filtrate was free from any metal ion content, and dried for 12 h at 120°C. In the next step, 1.0 g of MII-Y was uniformly mixed with an excessive amount of H2SFCH ligand (nligand/nmetal = 3) in ethanol and sealed into a round-bottom flask. The reaction mixture was refluxed (∼24 h) in an oil bath with stirring followed by Soxhlet extraction with ethanol, acetone, and finally with acetonitrile (6 h) to remove uncomplexed ligand and the complex adsorbed on the exterior surface of the zeolite-Y. The extracted sample was ion-exchanged with 0.01 M NaCl aqueous solution for 24 h, followed by washing with deionized water until no Cl− ion could be detected with AgNO3 solution. The product [M(SFCH)·xH2O]-Y (where, M = Mn, Fe, Co, Ni (x = 3) and Cu (x = 1)) was collected and dried in air.
Acknowledgments
We express our gratitude to the Head, Department of Chemistry, M. K. Bhavnagar University, Bhavnagar, India, for providing the necessary laboratory facilities. Mr. Parthiv M. Trivedi would like to acknowledge UGC, Delhi, for providing meritorious fellowship. One of us (SKG) is indebted to the Ministry of New and Renewable Energy (MNRE) for awarding the post-doc fellowship. An analytical facility provided by CSMCRI, Bhavnagar and Computations were carried out on the computer cluster PAWAN at the Department of Physics, M.K. Bhavnagar University, Bhavnagar, financed by the Department of Science and Technology, Govt. of India are highly acknowledged.
References
- Thomas, J.M.; Raja, R. Stud. Surf. Sci. Catal. 2004, 148, 163.
- Corma, A.; Garcia, H. Eur. J. Inorg. Chem. 2004, 2004, 1143.10.1002/ejic.200300831
- Salavati-Niasari, M.; Shakouri-Arani, M.; Davar, F. Micropor. Mesopor. Mater. 2008, 116, 77.10.1016/j.micromeso.2008.03.015
- Chavan, S.A.; Srinivas, D.; Ratnasamy, P.J. Catalysis. 2002, 212, 39.10.1006/jcat.2002.3756
- Zhang, N.; Zhou, X. J. Mol. Catal. A: Chem. 2012, 365, 66.
- Salavati-Niasari, M. Polyhedron. 2009, 28, 2321.10.1016/j.poly.2009.04.018
- Maurya, M.R.; Bishta, M.; Avecillab, F. J. Mol. Catal. A: Chem. 2011, 344, 18.10.1016/j.molcata.2011.04.017
- Salavati-Niasari, M.; Sobhani, A. J. Mol. Catal. A: Chem. 2008, 285, 58.10.1016/j.molcata.2008.01.030
- Abbo, H.S.; Titinchi, S.J.J. Top. Catal. 2010, 53, 254.10.1007/s11244-009-9408-9
- Srinivas, D.; Sivasanker, S. Catal. Surv. Asia. 2003, 7, 121.10.1023/A:1025381423820
- Ingold, K.U. Aldrichim Acta. 1989, 22, 69.
- Saji, P.V.; Ratnasamy, R.; Gopinath, S.; Ciric, J. US 6392, 093B1.US Patent Mobil Oil Corporation 1976, pp 972–983.
- Maurya, M.R.; Chandrakar, A.K.; Chand, S. J. Mol. Catal. A: Chem. 2007, 274, 192.10.1016/j.molcata.2007.05.018
- De Vos, D.E.; Dams, M.; Sels, B.F.; Jacobs, P.A. Chem. Rev. 2002, 102, 3615.10.1021/cr010368u
- Armengol, E.; Corma, A.; Fornés, V.; García, H.; Primo, J. Appl. Catal. A: Gen. 1999, 181, 305.10.1016/S0926-860X(98)00402-5
- Jin, C.; Fan, W.; Jia, Y.; Fan, B.; Ma, J.; Li, R. J. Mol. Catal. A: Chem. 2006, 249, 23.10.1016/j.molcata.2005.12.035
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Appl. Catal. A: Gen. 2013, 43, 464–465.
- Pal, N.; Pramanik, M.; Bhaumik, A.; Ali, M. J. Mol. Catal. A: Chem. 2014, 392, 299.10.1016/j.molcata.2014.05.027
- Wu, K.; Li, B.; Han, C.; Liu. J. Appl. Catal. A: Gen. 2014, 479, 70.10.1016/j.apcata.2014.04.004
- Silva, T.F.S.; Mac Leod, T.C.O.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Schiavon, M.A.; Pombeiro, A.J.L. J. Mol. Catal. A: Chem. 2013, 367, 52.10.1016/j.molcata.2012.10.024
- Cuenya, B.R. Thin Solid Films. 2010, 518, 3127.10.1016/j.tsf.2010.01.018
- Park, J.B.; Graciani, J.; Evans, J.; Stacchiola, D.; Ma, S.; Liu, P.; Nambu, A.; Sanz, J.F.; Hrbek, J.; Rodriguez, J.A. Proc. Natl. Acad. Sci. USA. 2009, 106, 4975.10.1073/pnas.0812604106
- Modi, C.K.; Trivedi, P.M. Micropor. Mesopor. Mater. 2012, 155, 227.10.1016/j.micromeso.2012.02.008
- Modi, C.K.; Trivedi, P.M. J. Coord. Chem. 2012, 65, 525.10.1080/00958972.2012.656616
- Biernacka, I.K.; Biernacka, K.; Magalhães, A.L.; Fonseca, A.M.; Neves, I.C. J. Catal. 2011, 278, 102.10.1016/j.jcat.2010.11.022
- Ahmed, A.H.; Thabet, M.S. J. Mol. Struct. 2011, 1006, 527.10.1016/j.molstruc.2011.09.061
- Das, S.; Pal, S. J. Organomet. Chem. 2006, 691, 2575.10.1016/j.jorganchem.2006.01.052
- Modi, C.K.; Jani, D.H.; Patel, H.S.; Pandya, H.M. Spectrochim. Acta A. 2010, 75, 1321.10.1016/j.saa.2009.12.076
- Modi, C.K.; Jani, D.H. Appl. Organomet. Chem. 2011, 25, 429.10.1002/aoc.1782
- Alcón, M.J.; Corma, A.; Iglesias, M.; Sánchez, F.J. Organomet. Chem. 2002, 655, 134.10.1016/S0022-328X(02)01469-9
- Li, N.; Zhang, X.; Wang, Q.; Wang, F.; Shen, P. RSC Adv. 2012, 2, 3288.10.1039/c2ra00019a
- Gai, S.; Yang, P.; Wang, D.; Li, C.; Niu, N.; He, F.; Zhang, M.; Lin, J. RSC Adv. 2012, 2, 3281.10.1039/c2ra00862a
- El-Metwaly, N.M. Tran. Met. Chem. 2007, 32, 88.10.1007/s11243-006-0135-9
- Maki, A.H.; McGarvey, B.R. J. Chem. Phys. 1958, 29, 35.10.1063/1.1744457
- Baroni, S.; Corso, A.D.; de Gironcoli, S.; Giannozzi, P. http://www.pwscf.org
- Baroni, S.; Gironcoli, S.; De, A.; Dal, C.; Giannozzi, P. Rev. Moder. Phys. 2001, 73, 515.10.1103/RevModPhys.73.515
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.10.1103/PhysRevLett.77.3865
- Monkhorst, H.J.; Pack, J.D. Phys. Rev. B. 1976, 13, 5188.10.1103/PhysRevB.13.5188
- Marzari, N.; Vanderbilt, D.; Payne, M.C. Phys. Rev. Lett. 1997, 79, 1337.10.1103/PhysRevLett.79.1337
- Joshi, P.; Shewale, V.; Pandey, R. J. Phys. Chem. C. 2011, 115, 22818.10.1021/jp2070437

![Figure 1. SEM images of [Ni(SFCH)·3H2O]-Y (a) before and (b) after Soxhlet extraction.](/cms/asset/41e353cf-ea70-48da-b9eb-709be69b86dc/tgcl_a_946101_f0001_oc.jpg)