Abstract
We report an efficient electrolytic procedure for the reduction of aldehydes to primary alcohols in the absence of dimeric products. In this promising approach, based on environmentally friendly chemical synthetic methods, a simple electrolytic undivided cell was designed. Two copper plates served as electrodes and recycled water as the medium. A 6-V battery provided the required energy. Although initially the method was intended for the preparation of 2-aryl-3-hydroxymethyl imidazo[1,2-a]azines, it was successfully extended to reduce other aliphatic and aromatic aldehydes. The attractiveness of this procedure includes its operational simplicity, practical viability, inexpensiveness and good yields. In most cases the results were better than those obtained with the standard reduction procedure using sodium borohydride.
Introduction
Electrochemistry has been considered as an alternative to polluting methodologies. Among the many synthetic procedures using electrochemistry is the selective hydrogenation of unsaturated carbonyl compounds, which has received a growing interest because of being an important step in the preparation of fine chemicals (Citation1).
In particular, the electrochemical conversion of aldehydes to the corresponding primary alcohols has for many years been described in the literature. As long ago as 1913, the electrolysis of 4-hydroxy-3-methoxybenzaldehyde (vanillin) was carried out (Citation2). Although this first attempt delivered a low yield (28%) of the corresponding benzyl alcohol, it was later improved by modifying the electrolytic conditions (Citation3). Vanillin was also reduced (82%) in aqueous methyl alcohol by applying a constant direct current of 8 Amp for 96 h, using stainless steel plates as electrodes and sodium acetate as the supporting electrolyte in a buffer solution (Citation4). Reduction of 4-nitrobenzaldehyde to 4,4’-bis(hydroxymethyl)hydrazobenzene has been profusely investigated in alkaline media and by employing different metals as cathodes (Citation5).
On the other hand, numerous efforts have been made to avoid the formation of dimeric products (pinacols) during the electro-reduction of benzaldehydes. For instance, the process has been carried out using a solid polymer as electrolyte and Pt-Pb/Nafion® as electrode (Citation6). Using low concentrations of benzaldehyde and high concentrations of potassium salts in buffer solutions of pH 6.0–6.5 at 2.0 V (relative to the saturated calomel electrode employed) provided an excellent yield of benzyl alcohol (Citation7). A method has been reported for the conversion of aldehydes and ketones to their corresponding alcohols based on an indirect, selective electro-reduction with alcohol as the supporting electrolyte and tri-alkoxide aluminum as the interface (Citation8).
Copper electrodes and instruments have been designed for the electrochemical reduction of carbon dioxide and aldehydes to corresponding hydrocarbons and alcohols, respectively, using solar energy to avoid global warming (Citation9). Copper or copper alloys, in aqueous solutions containing electrolytes and with a heterocyclic catalyst (under pH and electric voltage control), have been used in the electro-reduction of aldehydes (Citation10). Recently the electro-reduction of aldehydes and imines was achieved through the use of a microreactor system based on a carbon nanofiber membrane that separates an aqueous electrolyte and an immiscible organic phase (toluene or acetonitrile) (Citation11). Additionally, very interesting electrochemical oxidations have recently been described, using an electrochemical reaction setup with a simple 6-V photovoltaic power (Citation12).
In the present contribution, we describe the successful selective hydrogenation of aldehydes to their corresponding primary alcohols by means of an easily accessible electrolytic process using an undivided cell containing copper electrodes in recycled water. Although the copper electrodes are connected to a 6-V battery, this may be eventually replaced by a solar cell (Citation12), making the process fully green.
Results and discussion
Reduction of aldehydes to primary alcohols is usually achieved by the use of sodium borohydride (NaBH4) in a protic solvent, under neutral, basic or acidic reaction media (Citation13). Aldehydes derived from imidazo[1,2-a]pyridines 3 and pyrimidines 4 were prepared from the corresponding 2-arylimidazo[1,2-a]pyridines 1 and pyrimidines 2 by treatment with anhydrous dimethylformamide and phosphorous oxychloride (POCl3) through a procedure reported elsewhere(Citation14). Due to the luminescence exhibited by the reduction products (alcohols 5 and 6) of these molecules, there has been a great interest in obtaining them. In the chemical reduction, the mechanism involved considers addition of a hydride ion (H–), provided by NaBH4, to the activated carbonyl. On the other hand, in the electrochemical process electrons reduce the carbonyl functional group to the corresponding alcohol ().
![Scheme 1. Synthesis and reduction of 2-aryl imidazo[1,2-a]azines-3-carbaldehyde 3 and 4. Reaction conditions: (a) N2, anh DMF/POCl3/0–100°C, 1 h then aq. KOH, (b) ethyl alcohol, NaBH4/0.1 N NaOH/rt, then 0.1 N HCL, and (c) electrochemical reduction, copper electrodes, H2O/electric current, e-/rt](/cms/asset/d9f4f546-522a-4850-89b6-ccac111e52d6/tgcl_a_946973_f0001_b.jpg)
Therefore, in an effort to demonstrate the utility of recycled (also known as reclaimed) water in simple electrochemical processes and to add a sustainable character to the procedure, it was decided to explore the reduction of 2-aryl-3-carbaldehydes imidazo[1,2-a]pyridines and pyrimidines 3 and 4 to the corresponding 2-aryl 3-hydroxymethyl imidazo[1,2-a] pyridines and pyrimidines 5 and 6 under electrolytic conditions. Since compounds 5 and 6 develop intense fluorescence in solution, the idea of using an electrolytic process was an attractive alternative to monitoring the chemical transformation through fluorescence emission. The fluorescence of 5 and 6 has been previously described (Citation14).
The first problem encountered was the poor solubility of the compounds in water. To overcome this problem in an electrochemical process, the standard recommendation is the addition of a suitable transfer catalyst, solvent, and supporting electrolyte (Citation15). Nevertheless, these modifications may trigger a complex procedure, and therefore we decided to carry out the electro-reduction in a heterogeneous environment without the addition of a supporting electrolyte or solvent. The electrolysis proceeded smoothly to give the expected alcohols in the yields shown in and , with no dimeric alcohols (pinacols) being formed. In general, the yields obtained from the electrolytic process were better than those using NaBH4. In the case of 5 (R = Cl, R = MeO, R = 3,4-diMeO) and 6 (R = Cl, R = F, R = Me, R = 3,4-diMeO), yields were considerably improved (although the Faraday yield was not calculated).
Table 1. Yields of 2-aryl-3-hydroxymethyl imidazo[1,2-a]pyridines 5 by reduction with NaBH4 and by electrolysis of corresponding 2-aryl imidazo[1,2-a]pyridine-3-carbaldehyde 3.
Table 2. Yields of 2-aryl-3-hydroxymethyl imidazo[1,2-a]pyrimidines 6 by reduction with NaBH4 and by electrolysis of corresponding 2-aryl imidazo[1,2-a]pyrimidine-3-carbaldehyde 4.
The voltage of solution within the electrolytic cell was measured during electrolysis, by means of a multimeter ( and ), and the values obtained were interpreted as a decay of the battery voltage applied to a small value as it has occured in similar electroreduction processes Citation(11). During the electrolysis process, copper sulfate formed and the copper anodic stripe darkened. With higher voltage, the electrolytic process was shortened. Unfortunately, the fluorescence emitted by the alcohols was circumscribed to the area beneath the product.
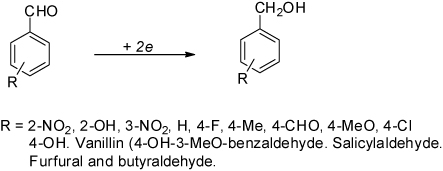
Encouraged by these results, we decided to extend the procedure to the reduction of other randomly chosen aromatic aldehydes, butyraldehyde, and as well as to salicylaldehyde and vanillin because of the interesting biological applications of their reduction products (Citation16, Citation17). Once again, the reaction was performed under heterogeneous conditions, resulting in a process with high yields and without diols (dimeric products) being formed (, ). Characterization of the compounds was carried out by comparison of their physical constants to those reported in the literature. Liquid alcohols were also identified by comparative HPLC.
Table 3. Synthesis of primary alcohols from diverse aldehydes by electrolysis.
Derivatives of 2-arylimidazo[2,1-b]thiazole-3-carbaldehyde 8 were prepared from imidazothiazoles 7 by a similar synthetic sequence as the one followed for the imidazo[1,2-a]azine system. Unfortunately, when the products 8 were subjected to the electrolysis conditions described above, there was no reduction of the carbaldehyde at C-3 of the fused heterocycle to give the corresponding alcohols 9, which represents a limitation of the process. During this reaction, electric current was detected within the electrolytic cell, but no hydrogenation took place and extensive metal corrosion was observed at the anionic cathode. This is interesting, since a sulfur-containing compound (benzothiazole) is usually effective for protecting copper from corrosion (Citation18) ().
![Scheme 3. Synthesis and attempted electrolytic reduction of 2-arylimidazo[2,1-b]thiazole-3-carbaldehyde derivatives.](/cms/asset/406470e1-ca0a-4720-bf2d-fb13c1253abb/tgcl_a_946973_f0003_b.jpg)
In conclusion, a simple, economic, and efficient electrolytic process for the reduction of aldehydes to primary alcohols was developed, using an undivided cell with copper electrodes. The products were readily isolated in good yields and purity, with no dimeric products being formed. However, the reduction procedure was not successful when applied to aldehyde derivatives of imidazo[2,1-b]thiazole.
Experimental
Materials
2-Aryl-3-carboxaldehyde imidazo[1,2-a]pyridines and pyrimidines were prepared in-house and characterized by comparison of their physical constants with those already reported in the literature. Recycled water (treated sewage water) was used as the reaction medium. The electrolytic cell designed to perform electrolysis consisted of a 250-mL cylinder glass beaker, equipped with a magnetic stirrer and adapted with two copper strips immersed in water with a distance of 5 cm between them. Each strip was connected to a 6-V battery through plugs (a schematic diagram of the apparatus used may be found in the Supplementary material). Melting points were determined in an electrothermal melting point apparatus. Infrared spectra were measured in an IR spectrophotometer (Fourier transform infrared spectroscopy [FTIR]), model Spectrum BX, Perkin Elmer. HPLC analysis of anion concentration in the water medium showed the presence of chlorides in a concentration of 360.30 ppm and sulfates at 254.03 ppm.
Electrolysis
The corresponding 2-aryl-3-carboxaldehyde imidazo[1,2-a]pyridine or pyrimidine (1.45 mmol) was suspended in water (50 mL) in a beaker. Stirring was started and the battery was switched on. The heterogeneous mixture was stirred at room temperature for 6–24 h until the starting material disappeared. Progress of the reaction was monitored by thin layer chromatography. After this time, stirring was suspended and the solid formed collected by filtration, washed with water to eliminate inorganic salts and rinsed with n-hexane. Solid products were further re-crystallized from ethyl alcohol. The other non-heterocyclic aldehydes underwent a similar treatment to obtain the corresponding alcohols. The products were identified by comparison of the IR spectrum of the isolated alcohol with that of an authentic sample, and by mixed melting point determination. The two series of primary alcohols 5 and 6 were prepared in accordance with .
Supplemental data
The supplemental data for this article can be accessed at http://dx.doi.org/10.1080/17518253.2014.946973.
Supplementary Material.jpg
Download JPEG Image (328.9 KB)Acknowledgments
We thank the Consejo Nacional de Ciencia y Tecnología (Mexico) for financial support through project 49937. We are grateful to Dr Hernado Romero Paredes Rubio for all his comments. S.V-.O. thanks the Consejo Nacional de Ciencia y Tecnología for graduate scholarship No. 257526.
References
- Gallezot, P.; Giroir-Fender, A.; Richard, D. In Catalysis of Organic Reactions; Pascoe, W., Ed.; Dekker: New York, 1991, p 1.
- Schepss, W. Chem. Ind. 1913, 46, 2566.
- Sharma, P.L.; Gaur, J.N. J. Appl. Electrochem. 1981, 11, 173. 10.1007/BF00610977
- Saharan, R.; Verma, P.S.; Sharma, I.K.; Joshi, J. Int. Appl. Biol. Pharm. Tech. 2011, 2, 503.
- Sharma, S.; Kumari, M.; Sharma, D.K. Nat. Sci. 2009, 7, 84.
- Chen, Y.L.; Chou, T.C. J. Appl. Electrochem. 1994, 24, 434.
- Mairanovskii, S.G.; Rubinskaya, T.Ya.; Gul'tyai, V.P.; Proskurovskaya, I.V. UDC 541.138, 3, 547–571.
- Wei-Dong, Y.; Chi, Y.; An-Xing, W. Synth. Commun. 1998, 28, 2827. 10.1080/00397919808004859
- Toshikatsu, M.; Ryota, D.; Hiroshi, H.; Hiroshi, M. Electrode for electrochemical reduction and device for utilizing the same. JP5311476 (A).
- Cole, E.B.; Bocarsly, A. Heterocycle Catalyzed Electrochemical Process. US 2011/0226632 (A1).
- Watkins, J.D.; Taylor, J.E.; Bull, S.D.; Marken, F. Tetrahedron Lett. 2012, 53, 3357. 10.1016/j.tetlet.2012.04.092
- Nguyen, B.H.; Redden, A.; Moeller, K.D. Green Chem. 2014, 16, 69. 10.1039/c3gc41650j
- Chaikin, S.W.; Brown, W.G. J. Am. Chem. Soc. 1949, 71, 122. 10.1021/ja01169a033
- Velazquez-Olvera, S.; Salgado-Zamora, H.; Velazquez-Ponce, M.; Campos-Aldrete, E.; Reyes-Arellano, A.; Perez-Gonzalez, C. Chem. Cent. J. 2012, 6, 8. 10.1186/1752-153X-6-83
- Do, J.S.; Chou, T.C. J. Appl. Electrochem. 1990, 20, 998.
- Fraaije, M.W.; Veeger, C.; Van Berkel, W.J.H. Eur. J. Biochem. 1995, 234, 271. 10.1111/j.1432-1033.1995.271_c.x
- Kim, I.S.; Choi, D.-K.; Jung, H.J. Molecules. 2011, 16, 5349–5361. 10.3390/molecules16075349
- Incralac Protection for Copper. Metal industry, London, 1964, 104, 696.

![Scheme 4. Electrosynthesis of 2-aryl-3-hydroxymethyl imidazo[1,2-a]azines.](/cms/asset/eed9dac4-bf80-4142-bdee-68c6bbbfc2d9/tgcl_a_946973_f0004_b.jpg)