Abstract
Elemental analysis of organic compounds is a traditional component in identification of substances. The technique is traditionally performed with molten sodium in test tubes that are later broken by plunging the hot tube into cold water. This article deals with the detection of several elements (Cl, Br, I, S, and N) using a nonbreakable sodium ignition apparatus (NOSIA) in place of breakable ignition tubes. In the method, an organic compound and sodium metal are loaded in fusion zone of the NOSIA for heating until they melt (100–150°C), then water (80–90°C) is added. The resultant mixture was directly used for element detection. In the traditional method, the sodium, on heating, often jumps out of the ignition tube and frequently catches fire posing a significant hazard in the laboratory. NOSIA is a safer and resource-saving approach that has been used by many instructors, as well as undergraduate, postgraduate, and research students. The NOSIA method does not require breaking of glass ignition tubes nor filtration of the subsequent solution. Thus, the NOSIA method provides element detection and is a single-step process for functional group detection.
Keywords:
Introduction
Green chemistry leads to the development of new experimental methods for applications to chemistry students with innovative laboratory approaches. Traditional methods of elemental analysis lead to hazards that can be addressed by the principles of green chemistry. The nonbreakable sodium ignition apparatus (NOSIA) that we describe is a novel apparatus for detection of a number of additional elements in an organic compound, that reduces use of chemicals and eliminates breaking of glass ignition tubes, reducing the amount of waste that is discharged when doing a more traditional method of elements detection.
In organic chemistry, for identification of compounds, element detection is a common technique and thus, efforts have been made to make it a safer and less wasteful exercise. For example, compounds that are volatile, flammable, toxic, or carcinogenic may escape from a traditional breakable ignition tube (Citation1, Citation2), but the NOSIA prevents their escape (Citation3–Citation8). The driving force in developing NOSIA has been to attract students to the green chemistry approach, while making element detection an interesting learning experience. In general, creative approaches in laboratory experiments tend to inspire both students and researchers. Hundreds of undergraduate students have performed several experiments with NOSIA and have found it to be a safer and resource-saving method. Several parallel experiments were conducted with NOSIA and traditional breakable ignition tubes and the former was found to be far safer while up to 97% of the resources as compared to the traditional method. The fusion monitoring unit of NOSIA is assembled with a high-quality glass socket and cone arrangement (Citation9, Citation10). The glass is a valuable piece of equipment and involves sophisticated technology and special conditions for its manufacturing and quality control. Therefore, the glass must be used with care in routine laboratory experiments (Citation11).
In the traditional method, a small piece of sodium metal is heated with an organic compound in a breakable ignition tube until it melts and the tube becomes red hot at about 200–300°C. Often the sodium jumps out of the tube which presents a hazardous situation for the students. In general, about eight red hot tubes are broken in a porcelain dish holding hot water and the resultant mixture is further heated to reduce the volume to about one-third. The solution is then filtered and analyzed for element detection. Contrary to traditional method, the NOSIA completely removes the need for breaking ignition tubes because the fusion zone works effectively without breaking it.
On fusion with sodium, nitrogen-containing organic compounds produce water-soluble sodium cyanide (NaCN). The NaCN is detected by treatment with iron (II) sulfate (FeSO4) forming sodium ferrocyanide, Na4[Fe(CN)6]. Next ferric ions react with ferrocyanide to yield a blue precipitate of ferric ferrocyanide, Fe4[Fe(CN)6] ().
The FeSO4 reacts with NaOH and forms a dirty green color precipitate.
On fusion with sodium, halide elements are transformed into sodium halide, NaX, which is detected using aqueous AgNO3 to form precipitates of silver halide (AgX).
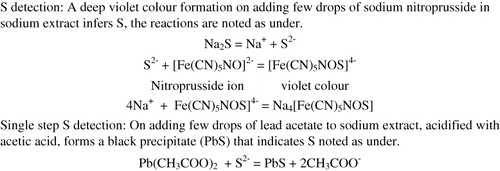
On fusion with sodium, sulfur in organic compounds is converted into aqueous sodium sulfide. The sulfide ions are detected with sodium nitroprusside forming sodium sulphonitroprusside of violet color ().
Clean and dry the unit, especially, the fusion zone so that no contaminants remain there.
Vertically mount the NOSIA on ordinary laboratory stainless steel stand firmly.
Add sodium granule through socket followed by organic compound.
Heat the contents smoothly and carefully till the sodium and the compound melt and then stop heating.
Slightly cool to about 80–100°C and then add hot water (60–80°C) in the melt held in fusion zone so that no damage to NOSIA takes place.
Continue cooling to about 30–45°C and use solution for chemical tests for detection of elements.
NOSIA efficiently detects nitrogen because of a partial oxidation of Fe(OH)2 into Fe(OH)3 on a passage of air in fusion zone through its side capillary.
A thermodynamic mismatch caused on plunging a red hot ignition tube in water of 30°C is completely removed.
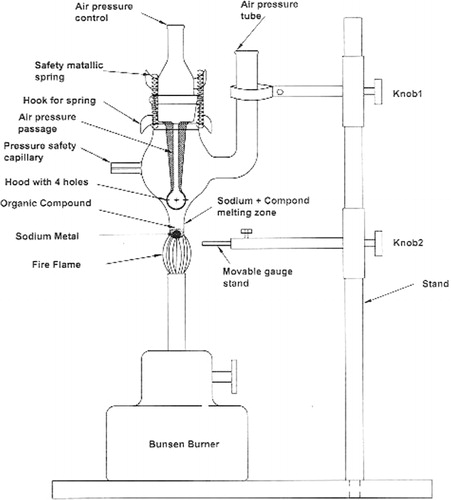
The NOSIA is clamped with LST on normal laboratory stand at some height above a base of the experimental table. A Bunsen burner was kept below the ELT for heating () and after adding hot water through the open top end of LST, the contents were further heated for 2–3 min producing the sodium extract. The extract, when cooled to 40–70°C, was removed through the SC into a test tube or by a glass dropper via LST without any further filtration as no glass part was smashed during the process.
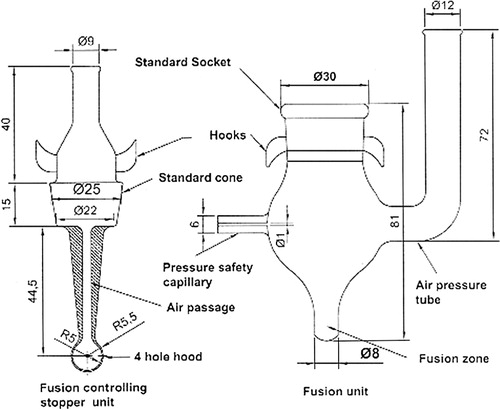
The complete fusion unit has a central bulb B of 17 mm inner diameter with LST of 6 mm inner diameter and 72 mm in height with the nonbreakable fusion tube at bottom. The fusion tube is 12 mm in height. The top end of B is in a shape of standard socket for holding the fusion vessel stopper via standard glassware connectors. The contents are taken in a fusion unit and a ring stand. The Bunsen burner is brought near the fusion unit to melt the contents. After a few minutes of heating, the sodium swells and bumps but the perforated hood of cone does not allow its escape. The pressure generated on heating is exchanged via the air pressure tube and pressure SC (). For safety, the cone and socket are fixed with springs.
The hot sodium and organic compound mixture is cooled and diluted with hot distilled water added to B via the side tube of the fusion unit, the mixture is stirred and the extract is used without filtration. In traditional methods, many ignition tubes are purposely broken and their contents are filtered using funnel and filter paper. This leads to increased cost and experimental steps.
Experimental details
Chemicals were purchased from Sigma-Aldrich and were used as received without further purification.
The NOSIA unit was made using glass blowing methods with high-quality borosilicate glass. Initially, the NOSIA unit is cleaned and dried at 110°C for 3–4 hr.
To use the unit, small pieces of solid sodium metal (ca. 50 mg) are added in the fusion zone and an organic compound (ca. 50 mg solid or 100 µL liquid) is added over the sodium metal and then the fusion controlling stopper unit is fitted into the cone of the NOSIA.
After adding the contents to the fusion unit, the cone stopper is fitted in socket (), and the NOSIA as a whole is clamped on a ring stand and Bunsen burner is brought near the unit to melt the contents.
The hot mixture obtained in fusion zone is allowed to cool for 2–4 min and then about 15 mL hot distilled water is added to B through its side tube. After addition of the hot water the cone stopper is withdrawn from the NOSIA main unit clamped on the stand. The mixture contained in the fusion zone is stirred with a clean glass rod to homogenize the contents in the water. The homogenous mixture is used as an extract for detection of the extra elements.
To our surprise no any other apparatus to conduct experiment for detection of extra elements in organic compounds is reported. However, the detection of these elements is important to find out functional groups helpful in identification of organic molecules. There are electronically controlled several dry lab methods to determine percentage of C (carbon), H (hydrogen), N (nitrogen), S (sulfur), and O (oxygen) in organic compounds on combustion of the compounds. But for extra elements with wet method no other device except Lassaigne is reported. Initially, an extra elements detection was initiated by Lassaigne in 1843 (Citation12) with subsequent modification by Jacobsen in 1879 on replacing sodium metal by potassium as an active reagent. However, no much change was noticed with potassium metal, though it was found simple but with poor results observed by students at bench (Citation13). Similarly, alternative methods of detecting elements have been developed and marked as Middleton test (Citation14) and a recent ignition method using oxygen developed by D. C. Ayres.
Results and discussion
The NOSIA is a simplified method for element detection as compared to traditional methods where many ignition tubes are smashed and resultant contents are filtered using funnel and filter paper. More than 50 lab sections of 40 undergraduate and postgraduate students have performed this laboratory experiment for element detection in their practical classes.
A comparison of resources with both methods is given in and (Citation6, Citation7). With NOSIA, use of a filter unit is unnecessary. In the traditional method about 40 mg of sodium and 50 mg of organic compound is used and the method is run with five tubes. Thus, the five tubes contain 40 mg × 5 = 0.2 g of sodium and 50 mg × 5 = 0.25 g organic compound. Additionally, very frequently, the sodium is lost when it jumps from the tube and the students take many sodium granules to compensate such loss. The average amount of sodium used per student is eight tubes requiring 40 mg × 8 = 0.32 g sodium and 50 mg × 8 = 0.40 g organic compound. If one laboratory section has 40 students then the sodium used is 0.32 g × 40 = 12.8 g. On an average, a laboratory may run four sections a day and amount of sodium used is 12.8 g × 4 = 51.2 g. The average weight of the glass tubes is about 2 g and thus the glass waste from eight tubes per student is 2 g × 8 = 16 g and for 40 students the glass used is 16 g × 40 = 640 g. For four sections, the glass waste is 640 g × 4 = 2560 g. With the traditional method, a filter assembly, filter paper, conical funnel, and flask to contain filtrate are required; however, the NOSIA method does not require such accessories. With NOSIA only 50 mg sodium and 50 mg organic compound is used per student and all of the glass waste is avoided.
Table 1. Comparative costs incurred with non-NOSIA and NOSIA for sodium extract preparation per student and per batch of 40 students for solid samples.
Table 2. Comparative costs incurred with non-NOSIA and NOSIA for sodium extract preparation per student and per batch of 40 students for volatile liquid samples.
Additionally, using the traditional method, extract preparation was obtained in 80 min against 20 min with NOSIA. Thus, for four laboratory sections, the laboratory was run for an additional four hours if the traditional method is used. Running laboratory for four additional hours also requires additional use of the exhaust fans, lights, water, etc. A single fan consumes 4 units of power per hour. In our lab, with four fans, and four lab sections per day, then 64 additional units of power were needed for the non-NOSIA method (4 units/hr × 4 hr × 4 fans). Likewise, lighting in our lab required 4 units of power per hour; consequently, the NOSIA method saves 16 units of power (4 units/hr × 4 hr) and the total reduction of electricity is 80 units. Our per unit cost for electricity is 4 Indian rupees, thus the savings from the NOSIA method is 320 rupees.
The water used per student for cleaning of the filter unit was 2 L (80 L for a laboratory section with 40 students). The biogas used for heating for fusion of eight breakable tubes per student is 0.1 kg m−3 (4.0 kg m−3 for a laboratory section with 40 students) and the cost per kg m−3 biogas was 30 rupees. Our total expenditure for biogas use, for the traditional method, is 120 rupees (30 rupees per lab section × 4 sections). Thus, the use of NOSIA makes a huge saving of resources with minimal discharge of pollutant effluents ().
The NOSIA method does not require any additional accessories, while reducing waste, reducing time and cost, and the method is safer, providing a green chemistry process.
Students, as well instructors, were so overwhelmed and excited by doing experiments with NOSIA that they wanted more and more laboratory experiments with a variety of organic compounds. The students and instructors appreciated avoiding the complication of filling the sodium pieces and the organic compound into the breakable ignition tubes, breaking the ignition tubes, and filtering off the extract. During filtration of the mixture in the traditional method, there also exists a possibility of injury by the pieces of the broken glass but with a use of NOSIA no such risk is involved.
The NOSIA is an asset for element detection experiment with volatile, toxic, and flammable organic compounds as it avoids the chances of fire or vapor emission. shows that volatile liquids such as nitrobenzene, pyridine, and others for element detection with the traditional method are taken in larger volumes as they quickly evaporate.
Additionally, the NOSIA could be used for identification of the elements in air samples by taking the air sample into the fusion zone of the NOSIA for a certain amount of time. Either the air sample can directly cover the sodium granules in the fusion zone or a solvent that dissolves the gases could be used in fusion zone.
Conclusion
Experiments for element detection conducted with the traditional method are wasteful and hazardous. The NOSIA technique is a safer and more economical method and it is especially of value for volatile liquids that have been a great difficulty due to several factors.
Use of NOSIA in place of the traditional method saves water, electricity, and other resources by 97%. The NOSIA method has been found to be a single-step process for element detection with no additional development of laboratory infrastructure with significant saving of consumable supplies. The NOSIA could be used for 10th and 12th standards students for teaching green chemistry concepts and for introducing the significance of extra elements in organic compounds.
Acknowledgment
Author is thankful to the Central University of Gujarat for infrastructural support.
References
- Singh, M. Anal. Lett., 2007, 40 (13), 2617–2623.
- Schmerber, K.; Borud, K.; Rothney, M.; Doane, A. Chem. Health. Safety, 2005, 12 (3), 30–35.
- Finar, I.L. Organic Chemistry, Vol. 1, 5th ed.; Thames Polytechnic, London: ELBS, 1975, 52–55.
- Robert, R.M.; Gilber, J.C.; Rodewald, L.B.; Wingrove A.S. Modern Experimental Organic Chemistry, 4th ed.; New York, NY: Holt-Saunders International, 1985.
- Singh, M. Surf. Interf. Anal., 2008, 40 (2), 76–80.
- Singh, M. J. Biochem. Biophys. Methods, 2006, 67 (2–3), 151–161.
- Singh, M. Surf. Interf. Anal., 2008, 40 (2), 15–20.
- Singh, M. Bulg. J. Chem. Edu., 2009, 18 (4), 82–88.
- Singh, M.; Matsuoka, H. Surf. Rev. Lett., 2009, 16, 599–608.
- Singh, M. Inter. J. Environ. Anal. Chem., 2011, 91 (3), 272–279.
- Singh, M. US Patent 987700, 2011.
- Lassaigne, J.L. Annalm, 1843, 48, 367.
- Gower, R.P.; Rhodes, I.P. J. Chem. Edu., 1969, 46, 606.
- Haynes, B. Qualitative Organic Analysis, 6th ed.; Macmillan: London, 1966.