Abstract
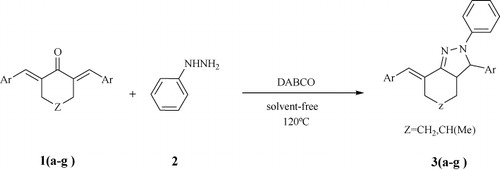
The aldol condensation of various aromatic aldehydes with cycloalkanones using ethanolic KOH afforded α,ά-bis (substituted-arylidene) cycloalkanones in excellent yields. The cyclization of the resulting cycloalkanones with phenyl hydrazine in the presence of catalytic amounts of DABCO (1,4-diazabicyclo[2.2.2]octane) under solvent-free conditions afforded 7-benzylidene-2,3-diphenyl-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives in good to excellent yields.
Introduction
In recent years, the use of organic chemistry as a primary tool for the synthesis of compounds with biological and medicinal properties has grown rapidly. Our interest in the amplification of practical processes for creating a variety of drug compounds led us to apply α,ά-bis (substituted-arylidene) cycloalkanones as a key intermediates for synthesis of compounds that possess various biological and medicinal scaffolds. Since these α,β-unsaturated ketones represent an important class of compounds not only for their biological activities such as cytotoxic analogs (Citation1), but also for their exceptional reactivities. That can be traced back to the presence of the carbonyl group conjugated with the double bond. This high regio selectivity of carbonyl and β-carbon reaction centers allows reaction with a wide range of nucleophiles (Citation2–Citation6).
The condensation of α,ά-bis (substituted-arylidene) cycloalkanones with nitrogen-containing binucleophilic agents is leading to an important class of heterocyclic compounds such as pyrimidine, pyrazoline, and indazoline. It is also worthy of mention that indazolines is a class of nitrogen-containing heterocyclic compounds which play a critical role in many biological processes (Citation7, Citation8) and exhibit crucial biological activities as antibacterial, antitumor, antimicrobial, antiviral, and antiprotozoal (Citation9–Citation11). As a matter of fact they are a focal point of an important amount of research interest.
In order to study their biological activities, several methods have been developed for their synthesis. Most of these methods provided the procedure of synthesizing 7-benzylidene-5-methyl-2,3-diphenyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine derivatives (Citation13–Citation15) and meanwhile only a limited number of 7-benzylidene-2,3-diphenyl-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives have been synthesized (Citation12, Citation13). However, for the most of these methods, the yield is low due to reverse and side reactions, and several and difficult purification steps are required.
The increasing attention for environmental protection has led us to develop chemical processes with maximum yield and minimum cost while using nontoxic reagents, solvents and catalysts. For this purpose, we create a method for the synthesis of some novel 7-benzylidene-2,3-diphenyl-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives, which is environmentally benign, simple and economical by introducing DABCO, a tertiary amine base with weak basicity and moderate hindrance (Citation14), as a nontoxic, nonvolatile, stable, inexpensive and easy to handle catalyst. Also this reaction requires neither solvents nor harmful reagents.
Results and discussion
In our initial experiments, synthesized compound 3a via the condensation reaction between 1a and phenyl hydrazine (), without using any catalyst in the EtOH/reflux condition at 2 hours. However, we found out that for the other derivatives 1b–1g reaction time under those conditions was much longer than that of 1a. For the derivatives of α,ά-bis (substituted-arylidene) cycloalkanones 1f and 1g where Z = CH(Me), the reaction time was especially long; the reaction did not reach completion even after 36 hours. Also the side reactions and ancillary products made it necessary to add difficult isolation and purification steps. Hence, our efforts focused on the search for a catalyst, which would help improving reaction time. For this purpose, the condensation reaction between compound 1f and phenyl hydrazine was selected as a model reaction in the presence of different catalytic systems and the results are summarized in . After extensive screening, we found that the optimized best yields and time profiles were obtained with 10 mol% of DABCO under solvent-free conditions at 120°C which furnished the corresponding indazole in 82% yield.
Table 1. Synthesis of 3f in the presence of various catalysts.
In order to determine the scope of this reaction, we attempted to synthesize a range of 7-benzylidene-2,3-diphenyl-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives under the same reaction conditions. The results are presented in .
Table 2. DABCO catalyzed synthesis of some new indazole derivatives.
Conclusion
In conclusion, we synthesized a number of novel 7-benzylidene-2,3-diphenyl-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives from phenylhydrazine and the corresponding α,ά-bis (substituted-arylidene) cycloalkanones via solventless synthesis catalyzed by DABCO in good to excellent yields.
Experimental
The α,ά-bis (substituted-arylidene) cycloalkanones 1a–g (Citation15, Citation16) were synthesized via aldol condensation as described previously (Citation16).
General procedure for the preparation of 7-benzylidene-2,3-diphenyl-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives(3a–g)
α,ά-bis (substituted-arylidene) cycloalkanone (1a-g), (1 mmol) was stirred at 120°C with phenyl hydrazine (2), (2 mmol) in presence of DABCO (10 mol%). After the completion of reaction, the reaction mixture was dissolved in ethanol (5 ml), and then poured onto crashed ice. The precipitate was separated by filtration, washed with water, and crystallized from ethanol to obtain the corresponding products.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/17518253.2014.976280
Supplemental_Material.pdf
Download PDF (111.4 KB)References
- Dimmock, J.R.A.; Padmanilayam, M.P.; Zello, G.A.; Nienaber, K.H.; Allen, T.M.; Santos, C.L.; Clercq, E.D.; Balzarini, J.; Manavathu, E.K.; Stables, J.P. Eur. J. Med. Chem. 2003, 38, 169.
- Deli, J.; Lorand, T.; Szabo, D.; Foldesi, A. Pharmazie. 1984, 39, 539.
- Kaushal, G. K. Polymer. 1995, 36, 1903.
- Ogawa, M.; Ishii, Y.; Nakano, T.; Irifune, S. Jpn. Kohai Tokkyo JP63192446 A2 (1988) (Chem. Abstr 1988, 63, 238034).
- Raj, A. A.; Raghunathan, R.; Sridevi Kumari, M.R.; Raman, N. Bioorg. Med. Chem. 2003, 11, 407.
- Xiaofang, L.; Xianyong, Y.; Yaqing, F. Chin. J. Chem. 2009, 27, 1531.
- Moreiraa, V.M.A.; Vasaitisb, T.S.; Njarb, V.C.O.; Salvadora, J.A.R.; Steroids. 2007, 2, 939.
- Narkevitch, V.B.; Anthony, N.J.; Cofre, V.; Jolly, S.M.; Murphy, K.L.; Ransom, R.W.; Reiss, D.R.; Tang, C.; Prueksaritanont, T.; Pettibone, D.J.; Bock, M.G.; Kuduk, S.D. Bioorg. Med. Chem. Lett. 2010, 20, 7011.
- Schmidt, A.; Beutler, A.; Snovydovych, B. Eur. J. Org. Chem. 2008, 4073.
- Cerecetto, H.; Gerpe, A.; Gonzalez, M.; Aran, V. J.; de Ocariz, C.O. Mini-Rev Med. Chem. 2005, 5, 869.
- Henderson, J.L.; Buchwald, S.L. Org. Lett. 2010, 12, 4442.
- El-Subbagh, H.I.; Abu-Zaid, S.M.; Mahran, M.A.; Badria, F.A.; Al-Obaid, A.M. J. Med. Chem. 2000, 43, 2915.
- Sviridenkova, N.V.; Vatsadze, S.Z.; Manaenkova, M.A.; Zyk, N.V. Russ. Chem. Bull. 2005, 54, 2590.
- Peng, J.H.; Hao, W.J.; Tu, S.J. J. Heterocycl. Chem. 2009, 46, 849.
- Mahdavinia, G.H.; Rostamizadeh, S.; Amani, A.M.; Mirzazadeh, M. Green Chem. Lett. and Rev.2012, 5, 255.
- Mahdavinia, G.H.; Mirzazadeh, M. E. J. Chem. 2012, 9, 49.