Abstract
The reaction of aldehydes with primary sulfonamides under solvent- and catalyst-free conditions is described. This method affords the N-sulfamoyl imines in short reaction times and high yields. Mild conditions, simplicity, inexpensive and easily available reagents, and absence of any auxiliary substances are some other advantages of this procedure.
Introduction
Imines, also called Schiff bases (Citation1) or azomethines, are versatile synthetic intermediates in organic transformations (Citation2–Citation3), they continue to be of significant interest (Citation4). Among them, N-sulfamoyl imines continue to attract the attention of chemists because the sulfonyl moiety has proven to be powerful activating group of the C=N bond in these compounds (Citation5). As a consequence, N-sulfonyl imines have been widely used in various transformations in organic synthesis for access at biologically active compounds (Citation6). They are excellent substrates in nucleophilic additions (Citation7), Aza Diels–Alder reaction (Citation8), reductions (Citation9), oxaziridine (Citation10) synthesis, asymmetric synthesis of β-amino acids derivatives (Citation11), and reactive alkenes in ene reactions (Citation12). In addition, they are excellent precursors for the preparation of optically active 2-imidazolines (Citation13).
There are several process available for the preparation of N-sulfonyl imines including the rearrangement of oximes o-sulfinates (Citation14), Lewis acid- or solid acid-catalyzed reactions of sulfonamides with aldehydes or acetals precursors (Citation15–Citation26), utilization of tellurium metal and chloramine T (Citation27), halogen-mediated conversion of N-(trimethylsilyl) imines in the presence of the corresponding sulfonyl chloride (Citation28), application of N-sulfinyl sulfonamides instead of sulfonamides to generate sulfonyl imines in situ (Citation29), catalyzed isomerization or rearrangement of sulfonyl aziridines (Citation30), solvent-free synthesis under microware irradiation (Citation31), or cyanuric chloride (Citation32) and iron-catalyzed under neutral conditions (Citation33). However, most of the reported methods suffer from drawbacks such as long reaction times, effluent pollution, hard reaction conditions, the use of toxic reagent, solvent, and unsatisfactory yields. Therefore, it seems desirable to develop a green procedure for the synthesis of N-sulfonyl imines. In the last decades solvent- and catalyst-free reactions have been demonstrated to new solutions to existing problems in organic synthesis. Solvent-free conditions often decrease reaction times, increased yields, and may enhance the regioselectivity and stereoselectivity of reaction (Citation34). More recently, Zolfigol et al. reported a green method for the synthesis of N-sulfonyl imines using WCl6 as novel, highly facile and reusable catalyst (Citation35). In continuation of our interest toward development of useful green synthetic methodology (Citation36–Citation40), we report the successful and efficient method for the synthesis of N-sulfonyl imines via the reaction of chloroethylsulfonamides with various aldehydes under solvent- and catalyst-free conditions.
Experimental
All commercial chemicals and solvents were without further purification. All reactions were carried out under inert argon atmosphere.
Melting points were determined in open capillary tubes on a Büchi apparatus and are uncorrected. 1H and 13C NMR spectra were recorded, respectively, in a 400 and 100 MHz Brücker spectrometer. Chemical shifts are reported in δ unit (ppm) with Trimethylsilane as reference. All coupling constants J are reported in Hertz. Multiplicity is indicated as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) and combination of these signals. All reactions were monitored by thin layer chromatography (TLC) on silica Merck 60 F254 pre-coated aluminum plates and were developed by spraying with Ninhydrin solution.
General procedure for the preparation of N-sulfamoyl imine
In a round-bottom flask 50 mL, the mixture of sulfamide (1 mmol) and aldehyde (1.1 mmol) was stirred at 100°C for the appropriate time ( and ). After completion of the reaction, as monitored by TLC, the reaction mixture was dissolved in dry ethyl acetate (5 mL) and was allowed to stand at room temperature for 1 hour. During this time, the powders of product formed which were collected by filtration.
Results and discussion
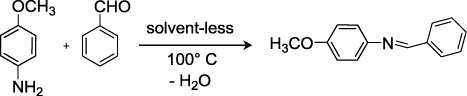
In order to investigate the optimum model reaction system, p-anisidine was chosen as a model substrate. We first examined the influence of the reaction temperature; the reaction of p-anisidine (1 mmol) with benzaldehyde (1.1 mmol) was studied under solvent-free at different temperatures. The results are summarized in . As shown in , the reaction was completed in 50 min; the imine was obtained in 94% yield.
Table 1. Synthesis of N-benzylidene-4-methoxyaniline under various temperatures.
In this case, the benzaldehyde plays the role of reagent and solvent. No reaction observed at temperatures lower than 50 °C.
When imine is formed from benzaldehyde and p-anisidine, one molecule of water is liberated per molecule of imine. Consequently, the formation of imines is facilitated when water is removed from the reaction mixture; a distillation system with inert gas (argon) was used to eliminate the water. To find the effect of the reaction system (solvent-free and temperature), the reaction was carried out at reflux in THF and CH2Cl2. No reaction occurs after 3 hours working time, this shows that the temperature plays the key role. Encouraged by the preliminary results and to increase the scope of this reaction, we extended this study to various aldehydes and synthetic N-chloroethylsulfamoyl amines. The choice of theses N-chloroethylsulfamoyl amines is dictated by the perspective of the mitotic inhibitor of the synthesized sulfamoyl imines.
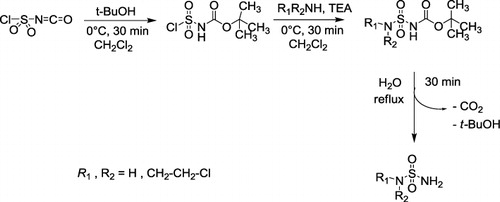
For an efficient process to the required sulfamoyl imines, we used the chemistry of chlorosulfonyl isocyanate (CSI) developed by our group (Citation41–Citation45) to access at the intermediate sulfamides. The preparation of N-Boc sulfamides is performed in dried dichloromethane with successive additions of tert-butanol and chloroethylamine or N,N-bis-chloroethylamine into CSI. The first one pot carbamylation sulfamoylation step is carried out in the presence of triethylamine as base, in 80–85% yield. The tert-butoxycarbonyl group removal is carried out in water, according to the method developed by Cheraiet et al. (Citation46) to afford the known sulfamides (Citation47–Citation48) in 92–97% yields ().
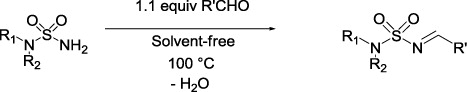
In the first time, we have examined the condensation of N-chloroethylsulfamide with various structurally aldehydes under solvent-free conditions (). The results are reported in . The reaction proceeded within 50 min in excellent yield. The results showed that all products (1a–7a) are not influenced by the electron withdrawing and electron-releasing substituent.
Table 2. Synthesis of N-(2-chloroethylsulfamoyl) imines under catalyst-free and solventless conditions.
The reaction of aldehyde with sulfonamide () gives the desired compound within 50–60 min in excellent yield (). The best result was obtained with the o-chlorobenzaldehyde. The structures of all products were established from their spectral properties (IR, 1H, and 13C NMR).
In order to extend our investigation and to increase the scope of this reaction, we extended this study to N,N-bis-chloroethylsulfamide. The results are summarized in . In all cases we obtained the N,N-bis-chloroethylsulfamoyl imines in good yield. The best result was obtained with benzaldehyde as substrate, affording sulfamoyl imine in 95% after 50 min.
Table 3. Synthesis of N,N-bis-(2-chloroethylsulfamoyl) imines under catalyst-free and solventless conditions.
The structures of all compounds were unambiguously confirmed by spectroscopic methods (IR, 1H, 13C NMR). For the resulting compounds, IR spectra showed bands at 1580–1640 cm–1(C=N) imine and exhibited characteristic absorption bands (SO2) at 1110–1170 cm–1 and 1340–1390 cm−1. 1H-NMR spectra showed the shift of proton corresponding to H-C=N from 8.1 to 8.8 ppm. 13C NMR data of all N-chloroethylsulfamoyl imines showed a signal between 165 and 175 ppm corresponding to imine carbon (H-C=N).
In the other hand, to compare the efficiency and the applicability of the solvent-free conditions with the other solvent system conditions, the catalyst-free reaction was performed in several solvents (3 mL) under reflux conditions. The corresponding results are depicted in .
Table 4. Effect of various solvents: ethanol, methanol, and toluene under refluxed conditions, and solvent-free conditions at 100°C.
As it can be seen from , no reaction occurred in MeOH after 24h reaction time. EtOH and Toluene gave much better results than MeOH, but still not satisfying, with a (<30%) yield. It was obvious to us that the solvent-free conditions are far better procedure than other solutions conditions.
Subsequently, N-(2-chloroethyl)sulfamide and N,N-bis-(2-chloroethyl)sulfamide were condensed with various aromatic aldehyde as well as croton aldehyde ((E)-but-2-enal), in short, to evaluate the efficiency of the reaction conditions; the respective results are summarized in and , all N-(2-chloroethyl)sulfamoyl imine and N,N-bis-(2-chloroethyl)sulfamoyl imine were obtained in good to excellent yields within 50–65 min at 100°C.
Encouraged by these results, we attempt this method with alkyl aldehyde (valeraldehyde), unfortunately no reaction occurred. We think that the electron-releasing of the alkyl can decrease the electrophilicity of aldehyde.
Conclusions
This letters describes a new, efficient, and green protocol for the synthesis of N-sulfamoyl imines by condensation of sulfamide and an arylaldehyde at fusion temperature. The required sulfamides can be readily synthesized from chloroethylamines and chlorosulfonyl isocyanate in two high-yielding steps. This new process has several advantages, such as high yield, short reaction time, low cost, simple experimental, easy isolation procedure, and solvent-free conditions. This method provides a green and much improved protocol over the existing methods.
The antimitotic evaluation of the resulting compounds and their incorporation into biomolecule analogs are currently underway and will be reported in due course.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/17518253.2015.1027287.
Supplemental_Material.pdf
Download PDF (1,019.2 KB)Acknowledgments
Fruitful discussions with Pr. Jacques Lebreton, Université de Nantes, France, Dr. Malika Ibrahim-Ouali, Université d’Aix Marseille II France are gratefully appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pataï, S. The Chemistry of Carbon-Nitrogen Double Bond; Wiley: London, 1970; Pataï, S. The Chemistry of Amino group; Wiley: London, 1968.
- Kobayashi, S.; Ishitani, H. Chem. Rev. 1999, 99, 1069; Boger, D.; Weinreb, S.N. Hetero Diels-Alders Methodology in Organic Synthesis; Academic Press: San Diego, CA, 1987.
- Bloch, R. Chem. Rev. 1988, 98, 1407.
- Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.-L.; Sanders, J.K.M.; Otto, S. Chem. Rev. 2006, 106, 3652.
- Weinreb, S.M. Top. Curr. Chem. 1992, 57, 4777.
- Jarrahpour, A.; Zarei, M. Molecules. 2006, 11, 49.
- Yi, M.; Wong, H.N.C. J. Org. Chem. 2004, 69, 2892.
- Boger, D.L.; Corbett, W.L.; Curran, T.T.; Kasper, A.M. J. Am. Chem. Soc. 1991, 113, 1713.
- Nishikori, H.; Yoshihara, R.; Hosomi, A. Synlett. 2003, 561.
- Zhou, X.-T.; Lin, Y.-R.; Dai, L.-X.; Sun, J.; Xia, L.-J.; Tang, M.-H. J. Org. Chem. 1999, 64, 1331.
- Colpaert, F.; Mangelinckx, S.; Kimpe, N.D. Org. Lett. 2010, 12, 1904.
- Melnick, M.J.; Weinreb, S.M.; Freyer, A. Tetrahedron Lett. 1988, 29, 3891. Tschaen, Turos, D.M.; Weinreb, S.M. J. Org. Chem. 1984, 49, 5058.
- Zhou, X.-T.; Lin, Y.-R.; Dai, L.-X.; Sun, J.; Xia, L.-J.; Tang, M.-H. J. Org. Chem. 1999, 64, 1331.
- Boger, D.L.; Corbett, W.L. J. Org. Chem. 1992, 57, 4777.
- Hasaninejad, A.; Sharghi, H. Phosphorus, Sulfur Silicon Relat. Elem. 2007, 182, 873.
- Wu, X.F.; Bray, C.V.L.; Bechki, L.; Darcel, C. Tetrahedron. 2009, 65, 7380.
- Deng, G.S.; Zou, J.Y.; Sun, T.F. Chin. Chem. Lett. 2011, 22, 511.
- Davis, F.A.; Kaminski, J.M.; Kluger, E.W.; Freilich, H.S. J. Am. Chem. Soc. 1975, 97, 7085.
- Davis, F.A.; Nadir, U.; Kluger, E.W.; Sedergran, T.C.; Panunto, T.W.; Billmers, R.; Jenkins, R.; Turchi, I.J.; Watson, W.H.; Chen, J.S.; Kimura, M. J. Am. Chem. Soc. 1980, 102, 2000.
- Sharghi, H.; Hosseini-Sarvari, M.; Ebrahimpourmoghaddam, S. ARKIVOC. 2007, xv, 255. Hasaninejad, A.; Zare, A. J. Sulfur Chem. 2007, 28, 357.
- Hasaninejad, A.; Zare, A.; Sharghi, H.; Shekouhy, M. ARKIVOC. 2008, xi, 64.
- Khalafi-Nezhad, A.; Parhami, A.; Zare, A.; NasrolahiShirazi, A.; Moosavi-Zare, A.R.; Hasaninejad, A. Can. J. Chem. 2008, 86, 456.
- Zare, A.; Hasaninejad, A.; Shekouhy, M.; Moosavi-Zare, A.R. Org. Prep. Proced. Int. 2008, 40, 457.
- Sharghi, H.; Khalafi-Nezhad, A. Phosphorus, Sulfur Silicon Relat. Elem. 2008, 183, 2769.
- Zare, A.; Moosavi-Zare, A.R.; Hasaninejad, A.; Parhami; Khalafi-Nezhad, A.; Beyzavi, M.H. Synth. Commun. 2009, 39, 3156.
- Zolfigol, M.A.; Khazaei, A.; Moosavi-Zare, A.R.; Zare, A. J. Iran. Chem. Soc. 2010, 7, 646.
- Trost, B.M.; Marrs, C. J. Org. Chem. 1991, 56, 6468.
- Georg, G.I.; Harriman, G.C.B.; Peterson, S.C. J. Org. Chem. 1995, 60, 7366.
- McFarlane, A.K.; Thomas, G.; Whiting, A. Tetrahedron Lett. 1993, 34, 2379.
- Wolfe, J.P.; Ney, J.E. Org. Lett. 2003, 5, 4607.
- Vass, A.; Dudas, J.; Varma, R.S. Tetrahedron Lett. 1999, 40, 4951.
- Wu, L.; Yang, X.; Waung, X.; Yan, F.J. Sulfur Chem. 2010, 31, 509.
- Wu, X.-F.; Vovard-Le Bray, C.; Bechki, L.; Darcel, C. Tetrahedron. 2009, 65, 7380–7384.
- Tanaka, K. Solvent-free Organic Synthesis; Wiley-VCH, GmbHand KGaA: Weinheim, 2004.
- Zolfigol, M.A.; Tavasoli, M.; Moosavi-Zare, A.R.; Arghavani-Hadi, P.; Darvishi, G.; Zare, A.; Khakyzadek, V. RSC Adv. 2013, 3, 7692.
- Amira, A.; K’tir, H.; Berredjem, M.; Aouf, N.-E. Monatch. Chem. 2014, 145, 509.
- Belghiche, R.; Cheraiet, Z.; Berredjem, M.; Abbessi, M.; Aouf, N.-E. Eur. J. Chem. 2012, 3, 305.
- Nadia, K.; Malika, B.; Nawel, K.; Med Yazid, B.; Regaïnia, Z.; Aouf, N.-E. J. Heterocycl. Chem. 2004, 41, 57.
- Lakrout, S.; K’tir, H.; Amira, A.; Berredjem, M.; Aouf, N.-E. RSC Adv. 2014, 4, 16027.
- K’tir, H.; Amira, A.; Berredjem, M.; Aouf, N.-E. Chem. Lett. 2014, 43, 851.
- Bendjeddou, A.; Djebbar, H.; Berredjem, M.; Hattab, Z.; Regaïnia Z.; Aouf, N.-E. Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181, 1351.
- Berredjem, M.; Bouasla, R.; Aouf, N.-E.; Barbey, C. X-ray Structure Analysis Online 2010, 26, 13.
- Bouchouk, D.; Colacino, G.E.; Toupet, L.; Dewynter, G.; Aouf, N.-E.; Martinez, J. Tetrahedron Lett. 2009, 50, 1100.
- Bendjeddou, A.; Djeribi, R.; Regaïnia, Z.; Aouf, N.-E. Molecules. 2005, 10, 1387.
- Berredjem, M.; Winum, J.‐Y.; Toupet, L.; Masmoudi, O.; Aouf, N.-E.; Montero, J.‐L. Synthetic Comm. 2004, 34, 1653.
- Cheraiet, Z.; Hessainia, S.; Ouarna, S.; Berredjem, M.; Aouf, N.-E. Green Chem. Lett. Rev. 2013, 6, 211.
- Abdaoui, M.; Dewynter, G.; Aouf, N.-E.; Favre, G.; Morère, A.; Montero, J.-L. Bioorg. Med. Chem. 1996, 4, 1235.
- Berredjem, M.; Djebbar, H.; Regaïnia, Z; Aouf, N.-E.; Dewnyter, G.; Winum, J.-Y.; Montero, J.-L. Phosphorus Sulfur Silicon. 2003, 178, 693.