Abstract
Benzanilide synthesis through amide bond formation was effectively carried out by palladium-doped clay catalyst using microwave irradiation under solvent-free conditions. Products were obtained with excellent yield in very short reaction time and only a small amount of the catalyst was used. The catalyst could be separated easily and recycled several times with insignificant loss in catalytic activity. No column purification was required and the products were purified by the crystallization method. The heterogeneous character of the catalyst in a ligand-free, solvent-free and base-free system supports for a practical and environmentally benign process.
1. Introduction
The formation of amide functionality is counted among the most attractive reactions in modern organic chemistry. Amides constitute one of the most important functional groups in contemporary chemistry and are essential for sustaining life ( Citation1). This important motif occurs prevalently in several pharmaceutical, biological, and agrochemical molecules as well as other natural products ( Citation2). Benzanilides are a special group of amides with such a wide range of biological activities that the benzanilide core has been called a privileged structure ( Citation3, Citation4). Currently the most exciting use of this core is in the field of kinase inhibitors. Imatinib (Gleevec), 1 () is an ATP-binding site kinase inhibitor specific for the c-Kit, BCR-ABL, and platelet-derived growth factor receptor kinases ( Citation5). Vascular endothelial growth factor receptor (VEGF-R) tyrosine kinase inhibitor 2 () is another class of benzanilide ( Citation6), which was postulated to bind to the ATP-binding site pocket in a mode similar to that of the anilinophthalazine kinase inhibitors. Benzanilides have also been reported to have activities as farnesyl transferase inhibitors 3 () ( Citation7) and acetyl-CoA carboxylase 4 () ( Citation8). Benzanilides can also serve as leads towards more conformationally constrained compounds such as quinazoline-2,4-diones, benzothiadiazin-4-ones, and benzodiazepine-2,5-diones ( Citation9–11).
The wide-range applicability of amides has encouraged comprehensive studies in the amide bond formation ( Citation2, Citation12). Synthesis of amides through coupling of activated carboxylic acids with amines is one of the long-established methods ( Citation13–15). Various alternative strategies have been formulated for this transformation ( Citation16–19). Preparations of amides by the condensation of acid chlorides with amines have been demonstrated in the presence of metal catalysts and bases using different solvent systems ( Citation20). Metal catalysed methods for preparing this important functionality have gained escalating interest ( Citation21–25). But some disadvantages such as harsh reaction conditions, low yields of aromatic amine substrates, low selectivity, tedious workup, requirement of stoichiometric amounts of various reagents, inert atmosphere, anhydrous organic solvents and long reaction time and formation toxic chemical waste seriously limited the application scope of such methods ( Citation26, Citation27). Hence, there are still plenty of significant challenges to develop facile, effective and more economical methods ( Citation28) for the formation of amides in both academic and industrial teams worldwide ( Citation29). Modern trend of amide synthesis is focused on the development of green methods. Gooßen et al. showed that a range of amides could be formed under solvent-free thermal conditions at a temperature of 160oC, in the presence of 3 Å molecular sieves ( Citation30). Hosseini-Sarvari and Sharghi synthesized amide bonds using ZnO as a catalyst under solvent-free conditions at 70oC, achieving some excellent yields in short reaction times ( Citation31). They also demonstrated the reusability of the ZnO catalyst, incurring only a small decrease in yield of amide after the third use. Mohamed Afzal Pasha et al. have recently demonstrated aluminium-catalysed efficient synthesis of anilides by the acylation of arylamines under ultrasonic conditions ( Citation32). Somnath Ghosh and Jhantu Das developed a procedure for benzoylation of aromatic and aliphatic amines in neat phase without the use of any solvent or alkali ( Citation33). Mandava V.B. Rao and Manojit Pal et al. demonstrated catalyst-free chemo-selective acylation of amines in water ( Citation34).
Palladium (Pd) strongly prefers the oxidation states 0 and +2, which are separated by a relatively narrow energy gap and responsible for its versatility in synthetic organic chemistry. Pd-catalysed C-N bond formation has emerged as a versatile tool for coupling aryl/alkyl/acyl halides with amines ( Citation35). Since homogeneous Pd catalysts are air sensitive, expensive and prone to contamination of the products, its heterogeneous counterpart, where the active metal can be incorporated into a solid support, becomes an imperative alternative.
Ion exchange capacity and the potential for increased interlayer spacing are important characteristics of clay minerals that strongly influence their physicochemical and technological properties. These characteristics, as well as their high surface area, have escorted clay minerals to be explored as catalyst supports ( Citation36, Citation37). In combination with microwave irradiation, solid-supported reagents can be exploited to accomplish an assortment of reactions in short times with high conversions and selectivity, exclusive of the requirement of solvents ( Citation38).
Herein, we report a simple, scalable, and highly efficient method of amide formation using a heterogeneous Pd-modified clay catalyst under solvent-free conditions with remarkable improvement over known procedures (Scheme 1). This heterogeneous catalyst is easy to prepare, low cost, environmentally benign, robust, easy to recover, recyclable and can effectively catalyse amide bond formation. The products are purified through crystallization without using any column chromatography. This avoids the use of large quantities of volatile organic solvents usually required for work-up and purification in many existing procedures. Moreover, solvent-free synthesis is probably the most efficient way to improve the ‘‘greenness’’ of complex organic molecule syntheses, as the solvent is generally the main source of waste ( Citation39).
2. Experimental design
2.1. Preparation of the catalyst
Pd-doped clay was prepared by suspending montmorillonite-KSF (10 g) with cation exchange capacity of 120 meq./100 g clay in 200 ml distilled water and the suspension was vigorously stirred at room temperature for 2 h. Palladium chloride dissolved in water (for 3.5 wt.% loading) was introduced to this suspension and the resulting mixture was stirred for 1 h at 80°C. After that sodium hydroxide solution was added dropwise (OH/Pd ratio=2) to this system and the resulting slurry was stirred at room temperature for 15 h. The solid products were filtered, washed several times with distilled water, dried first at room temperature, and then at 110o C for 12 h. The dried catalyst was calcined at 425o C for 3 h. It is characterized by powder X-ray diffraction using a D-8 ADVANCE (BRUKER AXS, GERMANY) X-ray diffractometer using Ni filter with Cu-Kα radiation Hitachi (H-7500) in the 2θ range 10–80o in the step scan mode (Step Size: 0.02o, Scan Speed: 2 s/step). The phases were identified by the search match procedure with the help of DIFFRACPLUS software using JCPDS databank. Temperature programmed reduction (TPR) and BET surface area were determined by CHEMBET-3000 TPR/TPD/TPO instrument. SEM of the catalyst was carried out using JEOL.JEM100CXII ELECTRON MICROSCOPE with ASID Accelerating Voltage 40.0 KV. IR spectra were recorded on Perkin-Elmer IR spectrophotometer. The specific surface area (m2g−1) of the catalyst was estimated with the N2 adsorption and desorption was determined at −196°C by means of an automated CHEMBET-3000 adsorption apparatus. XPS analysis was performed on a KRATOS-AXIS 165 instrument.
2.2. Procedure for the preparation of amides
A mixture of benzoyl chloride (1 mmol), aniline (1 mmol), and 5 mg catalyst were taken into a 5 ml microwave vial. The vial was capped with a Teflon pressure cap and subjected to microwave irradiation for 8 min at 150°C using a conventional kitchen microwave oven Electrolux EM30EC90SS operating at 260 watts. The reactions 1, 2 and 3 of were repeated using a Scientific Microwave Synthesizer CATA – R I operating at 300 watts, resulting in comparable yields. On completion of the reaction, followed by TLC examination, the mixture was cooled to room temperature, dissolved in ethyl acetate and filtered to separate the catalyst. The filtrate was concentrated under high vacuum. The crude products were purified by crystallization and the pure products were characterized by NMR and mass analysis and the characterization data of the synthesized compounds were compared with the literature reports.
Table 1. Reaction of 4-methoxy aniline with benzoyl chloride in the presence of Pd-clay catalyst under different reaction conditions.
3. Result and discussion
3.1. Morphological analysis of the catalyst
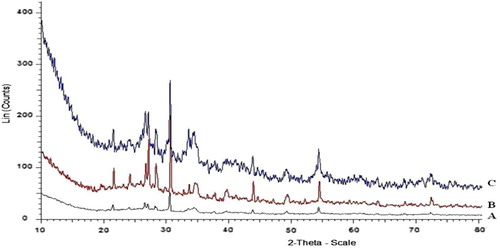
A comparative XRD pattern of montmorillonite-KSF (A), the fresh Pd-clay catalyst (B), and the used catalyst after 5th reaction cycle (C) are shown in . By comparison of XRD patterns it is clear that there is almost no variation and no additional peaks due to Pd are observed which may be due to the high dispersion of Pd nanoparticles.
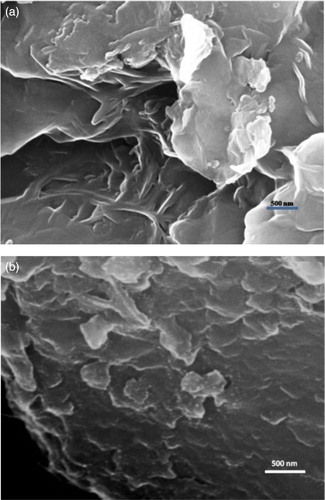
The SEM images of the montmorillonite-KSF and Pd-clay with 20000 magnifications are displayed in . We were overjoyed with the amazing ultrafine size and narrow distribution obtained for the supported Pd nanoparticles. It is clear that after loading copper on the montmorillonite-KSF the smooth surface of the clay turns patchy so it is confirmed that the Pd particles are highly dispersed over the support surface in the form of nanoaggregates. The nano-sized PdO distributed in mosaic form on the surface of the clay. From the SEM (EDAX) elemental analysis it was found that the catalyst prepared contains 3.09% of copper loaded.
3.2. Catalytic application of Pd-clay
In the present study, clay-encapsulated Pd nanoparticles are used as catalysts in the benzanilide synthesis through amide bond formation (Scheme 1). The layered structure of montmorillonite-KSF, acting as a polar support, is expected to provide closer proximity between the reagents ( Citation40). Based on adsorption isotherm and XRD studies H. Kostelníkova et al. reported that aniline is adsorbed readily into the montmorillonite interlayer being perpendicularly oriented to silicate sheet (with an increase in d001 spacing) ( Citation41). This increase in basal spacing will not only facilitate addition of benzoyl chloride, but also ensure closer proximity between the two layers.
The reaction between 4-methoxy aniline (1 mmol) and benzoyl chloride (1 mmol) in the presence of Pd-doped clay catalyst was selected as a model reaction in order to optimize various reaction parameters (Scheme 1). Upon investigating the effect of temperature we observed that reaction does not move under conventional stirring at room temperature and only traces of the desired product were formed at 100°C both with solvent and without solvent (, entries 1–9). Under ultrasonic irradiation for 2 h, traces of product formation were observed at room temperature under solvent-free conditions as well as with solvent (, entries 10 and 11). Microwaves were found to have a profound effect on the conversion and the reaction yield under solvent-free conditions. The optimized power range for the amide transformation was established as 260 watts for 8 min (, entries 12–14). We observed that 5 mg catalyst is an optimum amount for the model reaction. Decreasing the catalyst amount to 3 mg leads to a decrease in the product yield and no increase in the product yield or reaction time was observed upon increasing the amount of catalyst to 15 mg (, entries 15–17).
When as such clays like bentonite, KSF, kaolin and K10 were attempted for the model reaction, very low conversions with very less selectivity were obtained (, entries 1–4). While other prepared catalysts like clay doped with Ni, Co, Cu, Sn and Zn furnished very less conversion for the model reaction under optimized conditions and formation of some decomposition products was observed with these catalysts (, entries 6–10). The catalytic activity and selectivity of Pd-doped montmorillonite-KSF catalyst was excellent without the formation of any undesired product (, entry 5). With PdO and PdCl2 as catalysts, traces of the desired product were formed along with several by-products (, entries 11 and 12). Therefore, we can conclude that the Pd-clay catalyst system is much superior to commercially available alternatives with respect to catalyst loading, reaction time and conversion.
Table 2. Reaction of aniline and benzoyl chloride in the presence of various catalysts.
With optimized reaction conditions in hand, we further attempted to understand the generality of the procedure. To study the scope and the limitations of this novel method, we investigated the reaction using several electron-donating and electron-withdrawing substituted anilines as well as benzoyl chlorides. The reaction was found to be compatible with various functional groups such as Cl, Br, F, OMe, OEt and NO2. showing excellent chemo-selectivity. Various anilines-containing electron-donating or electron-withdrawing functional groups were treated with various benzoyl chlorides and no remarkable difference in the yield of the desired products was observed. All the reactions performed under microwave conditions proceeded cleanly to give the corresponding amides. It was observed that anilines-bearing electron-donating groups require little lesser time than the amine substituted with electron-withdrawing group. Similar observation is noticed in the case of benzoyl chloride derivatives when they are attached with electron-withdrawing and electron-donating groups.
3.3. Recyclability test
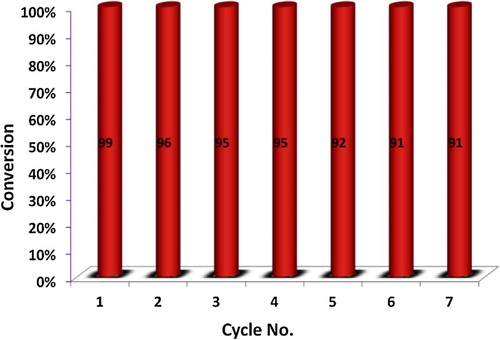
The catalyst was removed from the reaction mixture by filtration after dissolving the reaction mixture in ethanol, washed with ethanol, dried and reused seven times for the model reaction. The reusability test of the catalyst is shown in . It clearly reveals that the catalyst can be reused several times without any significant loss in the catalytic activity.
4. Conclusions
In conclusion we report a microwave-assisted, solvent-free synthesis of benzanilides using Pd-clay as a heterogeneous recyclable and robust catalyst. This method is efficient and convenient for the synthesis of amides with excellent yields and high selectivity in short reaction time. Pd-doped clay is active, selective, stable and easy to prepare. The product purification through crystallization without using any column chromatography avoids large quantities of volatile organic solvents usually required for work-up and purification in many existing procedures and very less time is required for this operation. The simple and straightforward experimental manipulation, solvent-free reaction, zero production of undesired by-products and easier work-up make the process environmental- and nature-friendly. This proves that the formation of amides can be achieved by catalytic quantity of Pd-doped clay avoiding the use of homogeneous reagents, such as NaOH, KOH and pyridine ().
Table 3. Pd-clay-mediated synthesis of benzanilides.
Supplemental data and research materials
Supplemental data for this article can be accessed at the Taylor & Francis website, doi: 10.1080/17518253.2015.1065349.
Supplementary_Material.docx
Download MS Word (2.8 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allen, C.L.; Williams, J.M. Chem. Soc. Rev. 2011, 40, 3405–3415. doi: 10.1039/c0cs00196a
- Li, H.; Fronczek, F.R.; Vicente, M.G. Tetrahedron Lett. 2012, 52, 6675–6678. doi: 10.1016/j.tetlet.2011.10.002
- Saeed, A.; Shahid, H.; Ulrich, F.; Turkish J. Chem. 2008, 32, 481–486.
- El-Araby, M.; Guo, H.; Pottorf, R.S.; Player, M.R. J. Comb. Chem. 2004, 6, 789–795. doi: 10.1021/cc0499284
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Nat. Rev. Drug Discov. 2002, 1, 493–502. doi: 10.1038/nrd839
- Manley, P.W.; Furet, P.; Bold, G.; Bruggen, J.; Mestan, J.; Meyer, T.; Schnell, C.R.; Wood, J.; Haberey, M.; Huth, A.; Kruger, M.; Menrad, A.; Ottow, E.; Seidelmann, D.; Siemeister, G.; Thierauch, K.H. J. Med. Chem. 2002, 45, 5687–5693. doi: 10.1021/jm020899q
- Russo, P.; Ottoboni, C.; Falugi, C.; Reinhold, W.; Riou, J.F.; Parodi, S.; O'Connor, P.M. Annal. N. Y. Acad. Sci. 1999, 886, 252–256. doi: 10.1111/j.1749-6632.1999.tb09429.x
- Hay, B.A.; Abrams, B.; Zumbrunn, A.Y.; Valentine, J.J.; Warren, L.C.; Petras, S.F.; Shelly, L.D.; Xia, A.; Varghese, A.H.; Hawkins, J.L.; Van Camp, J.A.; Robbins, M.D.; Landschulz, K.; Harwood, Jr., H.J. Bioorg. Med. Chem. Lett. 2007, 17, 4411–4414. doi: 10.1016/j.bmcl.2007.06.031
- Yoshikawa, M.; Sasakawa, C.; Makino, S.; Okada, N.; Lett, M.C.; Sakai, T.; Yamada, M.; Komatsu, K.; Kamata, K.; Kurata, T.; Lindberg, A.A.; Pál, T.; Makino, S.; Okada, N.; Lett, M.C.; Sakai, T.; Yamada, M.; Komatsu, K.; Kamata, K.; Kurata, T. Microbiol. Sci. 1988, 5, 333–334, 339.
- Mohamed, L.W., El-Yamany, M.F. Arch. Pharm. Res. 2012, 35, 1369–1377. doi: 10.1007/s12272-012-0806-3
- Moradei, O.; Vaisburg, A.; Martell, R.E. Curr. Top. Med. Chem. 2008, 8, 841–858. doi: 10.2174/156802608784911581
- Allen, J.V.; Bardelle, C.; Blades, K.; Buttar, D.; Chapman, L.; Colclough, N.; Dossetter, A.G.; Garner, A.P.; Girdwood, A.; Lambert, C.; Leach, A.G.; Law, B.; Major, J.; Plant, H.; Slater, A.M. Bioorg. Med. Chem. Lett. 2011, 21, 5224–5229. doi: 10.1016/j.bmcl.2011.07.047
- Al-Zoubi, R.M., Marion, O.; Hall, D.G. Angew. Chem. Int. Ed. Engl. 2008, 47, 2876–2879. doi: 10.1002/anie.200705468
- Valeur, E., Bradley, M. Chem. Soc. Rev. 2009, 38, 606–631. doi: 10.1039/B701677H
- Dunetz, J.R., Xiang, Y.; Baldwin, A.; Ringling, J. Org. Lett. 2011, 13, 5048–5051. doi: 10.1021/ol201875q
- Lin, Y.S.; Alper, H. Angew. Chem. Int. Ed. Engl. 2001, 40, 779–781. doi: 10.1002/1521-3773(20010216)40:4<779::AID-ANIE7790>3.0.CO;2-1
- Ju, J.; Jeong, M.; Moon, J.; Jung, H.M.; Lee, S. Org. Lett. 2007, 9, 4615–4618. doi: 10.1021/ol702058e
- Pawan, Y.P.P.; Tambade, J.; Bhanushali, Mayur J.; Bhanage, Bhalchandra M. Tetrahedron Lett. 2008, 49, 2221–2224. doi: 10.1016/j.tetlet.2008.02.049
- Wu, X.F.; Neumann, H.; Beller, M. Chem. Asian J. 2010, 5, 2168–2172. doi: 10.1002/asia.201000418
- Bo Wang, F.Y.; Shan, Yi-Fan; Qiu, Wen-Wei; Tang, Jie. Tetrahedron. 2009, 65, 5409–5412. doi: 10.1016/j.tet.2009.04.046
- Gunanathan, C.; Ben-David, Y., Milstein, D. Science. 2007, 317, 790–792. doi: 10.1126/science.1145295
- Soule, J.F., Miyamura, H.; Kobayashi, S. J. Am. Chem. Soc. 2011, 133, 18550–18553. doi: 10.1021/ja2080086
- Zeng, H.; Guan, Z. J. Am. Chem. Soc. 2011, 133, 1159–1161. doi: 10.1021/ja106958s
- Wang, Y.; Zhu, D.; Tang, L.; Wang, S.; Wang, Z. Angew. Chem. Int. Ed. Engl. 2011, 50, 8917–8921. doi: 10.1002/anie.201102374
- Yamaguchi, K.; Kobayashi, H.; Oishi, T.; Mizuno, N. Angew. Chem. Int. Ed. Engl. 2012, 51, 544–547. doi: 10.1002/anie.201107110
- Allen, C.L.; Chhatwal, A.R.; Williams, J.M. Chem. Commun. (Camb.). 2012, 48, 666–668. doi: 10.1039/C1CC15210F
- Xie, J., Jiang, H., Cheng, Y., Zhu, C. Chem. Commun. (Camb.). 2012, 48, 979–981. doi: 10.1039/C2CC15813B
- Jun Liang, J.L.; Shang, Zhi-cai. Tetrahedron. 2011, 67, 8532–8535. doi: 10.1016/j.tet.2011.08.091
- Huamin Li, J.X.; Xue, Qicai; Cheng, Yixiang; Zhu, Chengjian. Tetrahedron Lett. 2012, 53, 6479–6482. doi: 10.1016/j.tetlet.2012.09.039
- Gooßen, L.J.; Ohlmann, D.M.; Lange, P.P. Synthesis. 2009, 1, 160–164. doi: 10.1055/s-0028-1083277
- Hosseini-Sarvari, M.; Sharghi, H. J. Org. Chem. 2006, 71, 6652–6654. doi: 10.1021/jo060847z
- Reddy, M. B. M.; Jayashankara, V. P.; Pasha, M. A. Green Chem. Lett. Rev. 2013, 6, 1, 107–112. doi: 10.1080/17518253.2012.710650
- Ghosh, S.; Das, J. Org. Chem. Int. 2010, 1–3. doi: 10.1155/2010/743186
- Dulla, Balakrishna; Vijayavardhini, Suryadevara; Rambaua, Dandela; Anuradha, Vegendla; Rao, Mandava V.B.; Pal, Manojit. Curr. Green Chem. 2014, 1, 73–79. doi: 10.2174/22133461114019990002
- Beccalli, E.M.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107, 5318–5365. doi: 10.1021/cr068006f
- Sahoo, A.K.; Dar, Bashir A.; Patidar, P.; Sharma, P.R.; Mupparapu, N.; Vyas, D. S.; Sharma, Maity, M.; Singh, B. J. Ind. Eng. Chem. 3013, 19, 407–412.
- Chakraborty, A.; Dar, B.A.; Sharma, P.R.; Shrivastava, V.; Bhowmik, A.; Vyas, D.; Bhatti, P.; Sharma, M.; Singh, B. J. Ind. Eng. Chem. 2013, 19, 732–738. doi: 10.1016/j.jiec.2012.10.018
- Steven, V. Ley, Baxendale, Ian R.; Bream, Robert N.; Jackson, Philip S.; Leach, Andrew G.; Longbottom, Deborah A.; Nesi Marcella, Scott, James S.; Storer, Ian R.; Taylor, Stephen J. J. Chem. Soc. Perkin Trans. 1 2000, 23, 3815–4195.
- Thomas-Xavier Métro, J.B.; Reidon, T.; Sarpoulet, J.; Martineza, J.; Lamaty, F. Chem. Commun. 2012, 48, 11781–11783. doi: 10.1039/c2cc36352f
- Amarajothi Dhakshinamoorthy, P.V.; Tharmaraj, Vairaperumal; Pitchumani, Kasi. Catal. Commun. 2011, 16, 15–19. doi: 10.1016/j.catcom.2011.08.026
- Kostelníková, H.; Praus, P.; Turicová, M. Acta Geodynamica et Geomaterialia. 2008, 5, 83–88.