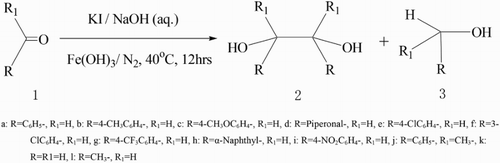
ABSTRACT
A small amount of ferric hydroxide was found to catalyze KI-activated coupling of aromatic aldehydes and acetophenone to give the corresponding pinacols in high yields. The ratio of meso to dl isomer was approximately 1:1. Most aliphatic aldehydes and ketones were unreactive under the same conditions.
1. Introduction
Pinacol coupling reaction is one of the most important reactions for the formation of the carbon–carbon bond, and, although discovered in 1859, it can still find many applications to the diastereoselective synthesis of vicinal diols, which can be used as intermediates for the construction of biologically important natural product skeletons. Numerous reagents for pinacol reactions are known, which include Al–Hg ( Citation1), Rieke Mg ( Citation2), active Mn ( Citation3), SmI2 ( Citation4), SmI2–TMSCl (TMSCl = trimethylsilyl chloride) ( Citation5), Cr(II) complexes ( Citation6), Zn–TMSCl–Ti(III) ( Citation7), and TiCl3 ( Citation8) in organic solvent. There were also reports of pinacolization in absolute methanol using Al–KOH ( Citation9) and Sm–I2–Ti(OPr)4 ( Citation10), however, these methods required absolutely anhydrous conditions in an inert atmosphere. Moreover, because of economic and environmental concerns, the use of water as a solvent for pinacol coupling reactions has generated considerable interest ( Citation11).
Recently, all pinacol coupling reactions in aqueous media required metal reduction of carbonyl compounds, by usage of Ti ( Citation12), Cu–Zn ( Citation13, Citation14), Al ( Citation15, Citation16), Mn ( Citation17), In ( Citation18), Mg ( Citation19), Ga ( Citation20), or Sm ( Citation21, Citation22), affording inter- or intramolecular coupling products of carbonyl compounds. These reactions produced metal ion and even heavy metal ion with the potential to harm the environment.
To date, anion used for coupling reaction of carbonyl compounds in aqueous media has just been reported ( Citation23, Citation24). Herein, we wish to report the study of using KI for coupling reactions of carbonyl compounds in water. This method only changes ion valences and does not increase ionic concentration.
2. Results and discussion
In our experiment, it was found that KI, together with a very small amount of Fe(OH)3, was highly effective for pinacol coupling reactions of carbonyl compounds in aqueous media to afford pinacol 2, as shown in (). The corresponding alcohol 3 was also formed as a by-product ().
To begin our study, the reaction of benzaldehyde with KI was examined under various conditions in order to optimize the coupling condition ().
Table 1. Pinacol coupling reaction of benzaldehyde (1.0 mmol) with KI (3.0 mmol) under various conditions.
No pinacol product was observed in the absence of additives (entries 1 and 2, ). Little pinacol product (<10%) was observed when only KI/Fe3+ was employed in water or in acidic solution (entries 3–6, ), this may be attributed to the fact that Fe3+/Fe2+ (generated from KI/Fe3+) has a higher standard electrode potential [E0 (Fe3+/Fe2+) = 0.771 eV). An interesting recent discovery is that a small amount of ferric hydroxide (Fe(OH)3) is able to catalyze KI-mediated coupling reaction of benzaldehyde in alkaline solution (entries 7–9, ).
It was proposed that [Fe(OH)2] produced by KI/Fe(OH)3 in alkaline solution activated pinacol coupling of benzaldehyde due to lower standard electrode potential E0{[Fe(OH)2]/Fe(OH)3] = −0.56 eV}, a high yield of pinacol was obtained as a mixture of meso to dl isomers with the ratio of meso to dl isomer approximately 1:1. The yield of pinacol was significantly improved if the reaction were run under protection of nitrogen (entries 10–12, ), because ferrous hydroxide is easily oxidized into ferric hydroxide with oxygen. Raising the temperature helped to enhance the yield of pinacol (entries 10–12, ). However, increasing the concentration of ferric hydroxide (Fe(OH)3) hardly increased the yield of pinacol (entries 9, ). After a survey of the reaction conditions, it was found that coupling of benzaldehyde (1 mmol) with KI (3 mmol) in sodium hydroxide (5%, 5 ml) at 40°C could provide the highest yield of pinacol in the presence of Fe(OH)3 (0.1 mmol) under N2.
Subsequently, Fe(OH)3 was used as additive to investigate the coupling reaction of other substrates under the same conditions. The results are listed in ().
Table 2. KI/Fe(OH)3 promoted pinacol coupling of carbonyl compounds in sodium hydroxide at 40°C under N2.
From () it is seen that pinacol coupling of carbonyl compounds promoted by KI/ Fe(OH)3 in alkaline solution usually has a high yield (70–95%). In most cases, high yields of pinacols were obtained as mixtures of dl and meso isomers under such reaction conditions. It was noteworthy that the electrostatic effect on the aromatic ring and steric hindrance around the carbonyl group could affect the yields of diols. Aromatic aldehydes substituted by electron-withdrawing groups gave higher yields than aldehydes substituted by an electron-donating group at the same position, and for example, benzaldehyde bearing trifluoromethyl, chloro or bromo group proceeded similarly to yield pinacol 2 as the major products (entries 5–7, ). Interestingly, nitrobenzaldehyde can also be coupled; in comparison, it is usually hard to form pinacol coupling by using a metal promoter (entry 9, ). An increase in the steric hindrance around the carbonyl group affected the ratio of meso to dl isomer, the preponderant product was compound of lesser steric hindrance, such as diols from piperonal or from 1-naphthaldehyde (entries 4, 8, ). Furthermore, as the substituent group substituted was big enough, no desired pinacol was observed with benzophenone (entry 11, ). Unlike aromatic carbonyl compounds, most aliphatic aldehydes and ketones provided none of the desired diols, while the main products were complex mixtures.
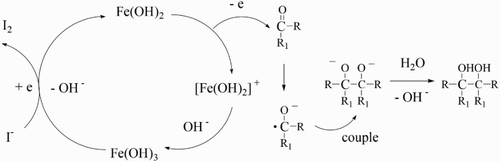
Compared to the previous work, we proposed that likely mechanism for the reaction to be as follows (). First, Fe(OH)3 is reduced to Fe(OH)2 by KI in alkaline aqueous solution. Second, the intermediate of a ketyl radical anion is created by electron transfer from Fe(OH)2 to the carbonyl substrate. Third, ketyl radical anions couple to form pinacols.
3. Experimental
IR (Perkin-Elmer, 2000 FTIR), 1H NMR (CDCl3, 400 MHz), 13C NMR (CDCl3, 100 MHz) and MS-GC (HP5890 (II)/HP 5972, EI) spectra were obtained at the Center of Analytical Configuration of Anhui Jianzhu University. Flash chromatographic sheet employed was purchased from Anhui Liangchen Silicon Material Co., Ltd and all material from Aldrich and used directly as received.
General procedure for pinacol coupling of aldehydes and ketones, carbonyl compounds (1 mmol), Fe(OH)3 (0.1 mmol) and NaOH (5%, 5 ml), were sequentially placed in a round-bottomed flask, then KI (3 mmol) was added to the mixture. The reaction mixture was stirred for 12 h at 40°C under N2. The reaction mixture was then extracted with ethyl ether. Organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate and concentrated in vacuo. The crude product mixture contained pinacol dl-2, pinacol meso-2 and alcohol 3, with the ratio found by 1H NMR analysis. Pure pinacols were obtained by recrystallization from EtOAc/hexane (1:6, V:V) or by flash chromatography on silica gel eluting with petroleum ether/acetic ether (4:1, V:V). All compounds obtained were consistent with authentic ones in literatures ( Citation13–16, Citation20).
1,2-Bi(p-nitro-phenyl)-1,2-ethanediol (2i): IR (KBr), 3325, 2895, 1652, 1553, 1524, 1381, 1045 cm−1; 1H NMR (CDCl3, 400 MHz) δ: 6.87–7.16 (m, 10H, Ph), 2.03–2.15 (br s, 2H, OH), 4.87 (meso,), 4.71 (dl) (s, 2H, CH-OH), 7.38–8.24 (m, 8H, Ph); 13C NMR (CDCl3, 100 MHz) δ: 79.56, 79.87, 123.38, 123.42, 127.51, 128.01, 138.61, 138.86, 139.25, 139.36; HRMS: calcd for C14H12N2O6: 304.2807, found: 304.2852.
4. Conclusion
In conclusion, this study demonstrated a novel pinacol coupling of carbonyl compounds mediated by KI/Fe(OH)3 in aqueous media. It is noteworthy that anion offers electron to carbonyl substrate to achieve pinacol coupling, therein, Fe(OH)3 is performed as catalyst. This reaction is very efficient and applicable to some aldehydes and ketones. This procedure provided a convenient and practical method for the formation of the carbon–carbon bond.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
We appreciate financial support from the Natural Science Fund of Anhui Province Education Office (KJ2013A073).
References
- Hulce, M.; Lavaute, T. Tetrahedron Lett. 1988, 29, 525–528.
- Furstner, A.; Csuk, R.; Rohrer, C. J. Chem. Soc. Perkin Trans. 1988, 1, 1729–1734.
- Rieke, K.D.; Kim, S.H. J. Org. Chem. 1998, 63, 5235–5239.
- Namy, J.L.; Souppe, J.; Kagan, H. B. Tetrahedron Lett. 1983, 24, 765–766.
- Honda, T.; Katoh, M. Chem. Commum. 1997, 4, 369–370.
- Svatõs, A.; Bloland, W. Synlett. 1998, 5, 549–551.
- Lipski, T.A.; Hilfiker, M.A.; Nelson, S.G. J. Org. Chem. 1997, 62, 7397–7412.
- Clerici, A.; Porta, O. Tetrahedron Lett. 1982, 23, 3517–3520.
- Khurane, J. M.; Sehgal, A. J. Chem. Soc. Chem. Commun. 1994, 5, 571.
- Yanada, R.; Negoro, N.; Yanada, K.; Fujita, T. Tetrahedron Lett. 1997, 38, 3271–3274.
- Li, C. J. Chem. Rev. 1993, 93, 2023–2035.
- Clerici, A.; Porta, O. J. Org. Chem. 1982, 47, 2852–2856.
- Yuan, S.Z.; Liu, J.; Jiang, G.S. Chin. J. Org. Chem. 2009, 29, 1158–1160.
- Yuan, S.Z.; Jiang, G.S. Chin. J. Appl. Chem. 2007, 24, 1455–1457.
- Yuan, S.Z.; Wang, Z.Y.; LI, Z. Chin. J. Chem. 2006, 24, 141–145.
- Hazarika, B.K.; Dutta, D.K. Synthetic Commun. 2011, 41, 1088–1093.
- Li, C. J.; Meng, Y.; Yi, X. H. J. Org. Chem. 1998, 63, 7498–7504.
- Lim, H.J.; Keum, G.; Kang, S.B.; Chung, B.Y. Tetrahedron Lett. 1998, 39, 4367–4368.
- Zhang, W.C.; Li, C.J. J. Org. Chem. 1999, 64, 3230–3236.
- Wang, Z.Y.; Yuan, S.Z.; Zha, Z.G.; Zhang, Z.D. Chin. J. Chem. 2003, 21, 1231–1235.
- Talukdar, S.; Fang, J.M. J. Org. Chem. 2001, 66, 330–333.
- Matsukawa, S.; Hinakubo, Y. Org. Lett. 2003, 5, 1221–1223.
- Sun, L.; Sahloul, K.; Mohamed, M. ACS Catal. 2013, 3, 2568–2573.
- Szostak, M.; Fazakerley, N.J.; Parmar, D.; Procter, D.J. Chem. Rev. 2014, 114, 5959–6039.