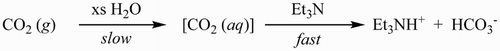ABSTRACT
The widely used chromatographic eluent, aqueous triethylammonium bicarbonate, can be efficiently prepared as 2 M stock solution by carbonation of a mixture of triethylamine and water in a commercially available pressure reactor (20–25 psi). This improved process reduces carbon dioxide waste emissions by ca. 90% compared to traditional gas bubbling at atmospheric pressure.
1. Introduction
An aqueous solution of triethylammonium bicarbonate (TEAB) ( Citation1, Citation2) is widely used as a volatile buffer mobile phase and ion-pairing agent ( Citation3, Citation4) for chromatographic purification of nucleic acids ( Citation5–13) and other biomolecules ( Citation14, Citation15). TEAB can be isolated as a crystalline trihydrate (mp. 17°C, dec) ( Citation1) but it is more conveniently handled as a concentrated aqueous solution. TEAB is commercially available as a 1 M solution, albeit at a very high price compared to its components: water, triethylamine, and carbon dioxide ( Citation16). Literature preparations of aqueous 1–3 M TEAB involve, with minor variations ( Citation2–7, Citation12, Citation14), slowly bubbling carbon dioxide gas into an ice-cold mixture of triethylamine and water until a homogeneous solution and pH 7.5–8.0 are obtained (). These sources are sufficient for most laboratory-scale uses.
Our high-volume preparative synthesis laboratory uses >40 L per week of 0.1 M TEAB for high-performance liquid chromatography purification of chemically modified ssDNA aptamers, termed SOMAmer® Reagents ( Citation17, Citation18). Use of the commercial 1 M TEAB product has been precluded by its cost and we have found that multi-liter scale-up of the carbon dioxide bubbling procedure has several drawbacks:
The rate of absorption of carbon dioxide gas into an ice-cold triethylamine–water mixture (mass transfer) is remarkably slow at atmospheric pressure. In our hands, preparation of a 2 L batch of 2 M TEAB requires 3–4 h of vigorous carbon dioxide bubbling with an extra-fine gas dispersion tube. While this may partly result from the local high altitude, it is consistent with the time scale reported by others for carbonations performed closer to sea level (Citation7, Citation12).
The efficiency of carbon dioxide absorption is low: a 39 lb cylinder of carbon dioxide typically affords ∼20 L of 2 M TEAB (10% yield). Most of the carbon dioxide bubbled into the mixture is immediately wasted as greenhouse gas.
Vigorous ebullition of the reaction mixture with carbon dioxide causes evaporation of variable amounts of triethylamine, leading to a strong amine odor in the fume hood and a variable titer in the final TEAB solution.
2. Materials and methods
2.1. Chemicals
Triethylamine (>99.5%), catalog BB359-1, was purchased from Honeywell/Burdick & Jackson, Muskegon, MI, USA. Deionized water was prepared with a Milli-Q Integral 10 water system, EMD Millipore, Billerica, MA, USA. Carbon dioxide (research grade 99.998%) was purchased from Specialty Gases of America, Inc., Toledo, OH, USA.
2.2. Equipment
Carbon dioxide was dispensed using a low-pressure gas regulator from Victor Specialty Products, Model SGT500/40, from Thermadyne, St. Louis, MO, USA. The pH measurements were performed with a SympHony Model SB80PC pH meter from VWR International, Radnor, PA, USA. A detailed parts list for the Ace Glass™ pressure reactor system is provided in the Supplementary Material.
3. Results and discussion
3.1. Pressurized carbonation process
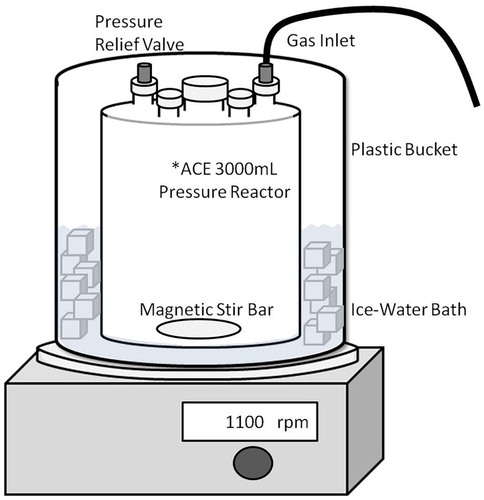
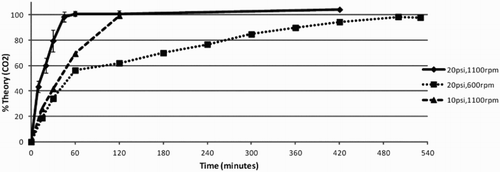
A more efficient and economical preparation of 2 M TEAB can be accomplished by introducing the carbon dioxide gas under moderate pressure (20–25 psi) to a triethylamine/water mixture in a closed system. We have used a relatively inexpensive, commercially available, 3 L glass pressure reactor ( Citation19) () to prepare 2 L batches of 2 M TEAB. With vigorous magnetic stirring (>1000 rpm) to turbulently mix the gas and liquid phases, the reaction time is ≤1 h () and the yield from carbon dioxide is essentially quantitative.
3.2. Experimental: 2 M TEAB (aqueous)
A 3 L cylindrical glass pressure reactor was visually inspected for the absence of cracks and then charged with triethylamine (556 mL, 4 mol), deionized water (1480 mL), and a large (2–3′′) magnetic stir bar. The reactor was sealed and cooled in ice-water in a plastic bucket (containment) with rapid (>1000 rpm) magnetic stirring. The reactor headspace was briefly flushed with carbon dioxide. The reactor was sealed and then charged to a pressure of 20–25 psi with carbon dioxide. After 45–60 min, the audible flow of gas slowed and a clear to slightly turbid solution of 2 M TEAB was obtained (internal temperature 20 ± 5°C). After venting excess gas pressure, the pH was confirmed to be 7.6 ± 0.2 (range 7.47–7.73, n = 6) and the mass of the solution was found to have increased by 172–178 g (98–101% theory as carbon dioxide, n = 6). The 2 M TEAB stock solution can be stored in the refrigerator (4°C) in a tightly closed serum bottle for up to two days and diluted with deionized water and organic solvents, as needed, to prepare the desired TEAB mobile phases for chromatography.
3.3. Safety
DO NOT attempt to substitute a serum bottle, round-bottom flask, or any other container, for the pressure reactor, unless the substitute has been specifically designed and rated for pressurized applications. Safe procedures for working with pressurized glassware have been described ( Citation20). Preliminary experiments indicate that good results can also be obtained in a well-agitated, pressure-rated 316 stainless steel mixing tank.
4. Conclusions
The widely used volatile buffer mobile phase component, TEAB (2 M aqueous), can be prepared in a commercially available pressure reactor (20–25 psi) by carbonation of a triethylamine/water mixture. This new process significantly reduces carbon dioxide waste emissions compared to previously reported procedures which involved gas bubbling at atmospheric pressure.
Disclosure statement
All three authors are full-time employees and shareholders of SomaLogic Inc. and performed this research as part of their regular paid employment.
Supplemental data
Supplemental data for this article can be accessed at the Taylor & Francis website, doi:10.1080/17518253.2015.1091039.
Supplemental_Material.docx
Download MS Word (115.9 KB)References
- Bachelet, M.; Guibe, M. Bull. Soc. Chim. Fr. 1951, 18 (7–8)554.
- Fournier, J.; Gouju, D.; Champetier, G. Comptes Rendus Acad. Sci. Paris. 1967, 264 (6), 502–503.
- Petritis, K.; Dessans, H.; Elfakir, C.; Dreux, M. LC GC Europe. 2002, 15 (2), 98–102.
- Wilson, A.D.; Stewart, F.F. RSC Adv. 2014, 4, 11039–11049.
- Leder, P. Methods Enzymol. 1968, 12 (Pt. B), 837–839.
- Mahoney, C.W.; Yount, R.G. Anal. Biochem. 1984, 138 (1), 246–251.
- Ip, C.Y.; Ha, D.; Morris, P.W.; Puttemans, M.L.; Venton, D.L. Anal. Biochem. 1985, 147, 180–185.
- Shailubhai, K.; Singh, R.K.; Schmuke, J.J.; Jacob, G.S. Anal. Biochem. 1996, 243, 165–170.
- Xiong, W.; Glick, J.; Yiqing, L.; Vouros, P. Anal. Chem. 2007, 79 (14), 5312–5321.
- Zlatev, I.; Lavergne, T.; Debart, F.; Vasseur, J.J.; Manoharan, M.; Morvan, F. Org. Lett. 2010, 12 (10), 2190–2193.
- Ye, G.; Beverly, M. Rapid Commun. Mass Spectrosc. 2011, 25 (21), 3207–3215.
- Ravalico, F.; Messina, I.; Berberian, M.V.; James, S.L.; Migaud, M.E.; Vyle, J.S. Org. Biomol. Chem. 2011, 9, 6496–6497.
- Sharma, V.K.; Glick, J.; Vouros, P. J. Chromatogr. A. 2012, 1245, 65–74.
- Sakai, T.T.; Riordan, J.M. J. Chromatogr. 1979, 178 (1), 302–306.
- Schmidt, C.; Hesse, D.; Raabe, M.; Urlaub, H.; Jahn, O. Proteomics. 2013, 13, 1417–1422.
- Sigma–Aldrich Corporation, St Louis, MO, USA. Sigma Product 90360, 500 mL = $308.50 (February 2015).
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; Flather, D.; Forbes, A.; Foreman, T.; Fowler, C.; Gawande, B.; Goss, M.; Gunn, M.; Gupta, S.; Halladay, D.; Heil, H.; Heilig, J.; Hicke, B.; Husar, G.; Janjic, N.; Jarvis, T.; Jennings, S.; Katilius, E.; Keeney, T.R.; Kim, N.; Koch, T.H.; Kraemer, S.; Kroiss, L.; Le, N.; Levine, W.; Lollo, B.; Mayfield, W.; Mehan, M.; Mehler, R.; Nelson, S.K.; Nelson, M.; Nieuwlandt, D.; Nikrad, M.; Ochsner, U.; Ostroff, R.M.; Otis, M.; Parker, T.; Pietrasiewicz, S.; Resnicow, D.I.; Rohloff, J.; Sanders, G.; Sattin, S.; Schneider, D.; Singer, B.; Stanton, M.; Sterkel, A.; Stewart, A.; Stratford, S.; Vaught, J.D.; Vrkljan, M.; Walker, J.J.; Watrobka, M.; Waugh, S.; Weiss, A.; Wilcox, S.K.; Wolfson, A.; Wolk, S.K.; Zhang, C.; Zichi, D.; PLoS ONE. 2012, 5 (12), 5004. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0015004. (accessed June 11, 2015)
- Rohloff, J.C.; Gelinas, A.D.; Jarvis, T.C.; Ochsner, U.A.; Schneider, D.J.; Gold, L.; Janjic, N. Mol. Therapy Nucleic Acids. 2014, 3, e201. http://www.nature.com/mtna/journal/v3/n10/full/mtna201449a.html. (accessed June 11, 2015)
- Ace Glass, Vineland, NJ, USA, Part 6436-31. This One-Piece 3000 mL Heavy-duty Glass Reactor Is Rated to 45 psi at 100°C. The Complete Pressure Reactor System Costs <$2000 and Thus Pays for Itself with the Second 2 L Batch of 2M TEAB, Compared to Price of the Commercial Product (Reference 16). A Complete Parts List for the Reactor Is Provided in the Supporting Information.
- Reactors Catalog, Bench & Kilo Class. Ace Glass Incorporated. 2011. http://www.aceglass.com/html/3dissues/Reactors/offline/download.pdf (accessed Aug 25, 2015).