ABSTRACT
An efficient and green approach for synthesizing chromeno[2,3-b]pyridine derivatives through one-pot three-component reactions of salicylaldehydes, thiols, and malononitrile has been developed by Fe3O4@SiO2–NH2 nanocatalyst in aqueous ethanol media under reflux conditions. The present procedure provides several advantages such as environmentally benign, straightforward, excellent yields, short reaction times, cost-effective, good recyclability, little catalyst loading, and facile catalyst separation for the preparation of chromeno[2,3-b]pyridines as important privileged medicinal scaffold. In addition, aminopropyl-coated Fe3O4@SiO2 nanoparticles were fully characterized by scanning electron microscopy, X-ray diffraction, energy dispersive analysis of X-ray, vibrating sample magnetometer, and FT-IR analysis.
Introduction
Recently, functionalized magnetite nanoparticles have been more interested in the various fields such as diagnostics, biotechnology ( Citation1), cell biology, and pharmaceutics frequently due to their superparamagnetic properties present at the small particle sizes ( Citation2,Citation3). Metal organic solids are hybrid materials which have produced by the community of the metal ion and organic ligands, which have already shown a vast range of promising attributes in gas sorption, ion exchange, catalysis, sensing, optics, magnetism, etc. ( Citation4–6). Because of the vast range of the properties, one of the main challenges is the miniaturization of this system to design and construct the novel metal organic nanoparticles. Magnetite nanoparticles have been immobilized on different catalyst supports, including polymers, carbons, and silicas because of easily retrievable and reusable heterogeneous catalyst they are still in high demand, and a striking typicality of supported iron oxide nanoparticles catalyst is that they can be readily separated using an external magnet, which achieves a simple separation of catalyst without filtration ( Citation7–11). Furthermore, they do not only show high catalytic activities but also a high degree of chemical consistency and they do not swell in organic solvents. Recently, extensive research has been performed on a magnetic catalyst which has led to substantial progressions in nanomaterial synthesis. In recent years, functionalized magnetite nanoparticles have used as efficient catalytic systems in many chemical transformations including oxidation of alcohols ( Citation12), synthesis of α-amino nitriles ( Citation13), 1,1-diacetates from aldehydes ( Citation14), diazepine derivatives ( Citation15), indazolo[2,1-b]phthalazine-triones and pyrazolo[1,2-b]phthalazine-diones ( Citation16), 3,4-dihydropyrimidin-2(1H)-ones ( Citation17), 2-amino-4H-chromen-4-yl phosphonates ( Citation18), 1,4-dihydropyridines ( Citation19), and pyrrole synthesis ( Citation20). In addition, various organic reactions such as Knoevenagel condensation and Michael addition ( Citation21), Suzuki and Heck cross-coupling ( Citation22), asymmetric aldol reaction ( Citation23), Suzuki coupling ( Citation24), asymmetric hydrogenation of aromatic ketones ( Citation25), acetalization reaction ( Citation26), reduction of nitro aromatic compounds ( Citation27), cyanosilylation of carbonyl compounds ( Citation28), Henry reaction ( Citation29), and enantioselective direct addition of terminal alkynes to imines ( Citation30) have been done using functionalized magnetite nanoparticles.
Development of novel synthetic methodologies in order to the preparation of compound libraries based on privileged structures is the significant area of the research. One of the approaches to address this challenge involves the development of multi-component reactions (MCRs) in which three or more reactants are combined together in a single reaction flask to generate a product incorporating most of the atoms contained in the starting materials. In addition to the intrinsic atom economy and selectivity underlying such reactions, simpler procedures and equipments, time, and energy savings as well as environmental friendliness have all led to a sizable effort to design and implement MCRs in both academia and industry. The chromene ring system is considered as one of the most imperative heterocyclic compounds in nature as it has the distinction of being the parent ring in countless derivatives of biological relevance.
Chromeno[2,3-b]pyridine scaffolds have also been successfully utilized for the generation of libraries for diverse medical applications. In addition, chromenopyridines have been reported to have antiproliferative ( Citation31), cancer chemopreventive ( Citation32), antibacterial (including antitubercular) ( Citation33), antimyopic ( Citation34), antihistaminic ( Citation35), hypotensive ( Citation36), antirheumatic ( Citation37), and antiasthmatic activities ( Citation38). The synthetic routes for the preparation of substituted chromenopyridines mainly involve: the reaction of 4-chlorocoumarin-3-carbaldehyde with malononitrile ( Citation39), 2-amino-3-formylchromone with cyclic active methylene compounds, 2-amino-4-(phenylsulfanyl)-4H-chromene-3-carbonitrile and malononitrile ( Citation40), 2,2-disubstituted chroman-4-one with aromatic aldehydes and 2-cyanoacetamide ( Citation41), 4-chloro-2H-3-chromene carbaldehydes on reaction with ethyl-3-aminocrotonate ( Citation42) and the reaction of 2,2-disubstituted chroman-4-one with aromatic aldehydes and 2-cyanoacetamide ( Citation43).
One of the most attractive routes to the synthesis of chromeno[2,3-b]pyridines involves the cyclocondensation of aldehydes, malononitrile, and thiols. There are a few methods such as using K2CO3 ( Citation44), Et3N ( Citation45), ZrP2O7 nanoparticles ( Citation46), and SnO nanoparticles ( Citation47) as catalysts which have been reported to the synthesis of chromeno[2,3-b]pyridine derivatives. In the context of our interest on sustainable protocols in the synthesis of heterocyclic compounds using MCRs and nanocatalysts ( Citation48–53), herein we report a novel, green, and mild method for the synthesis of chromeno[2,3-b]pyridines via the multi-component coupling reaction in the presence of Fe3O4@SiO2–NH2 NPs as a green, available, economic, and environmentally benign nanocatalyst.
Results and discussion
In the preliminary experiments Fe3O4@SiO2–NH2 nanostructures were prepared and characterized by energy dispersive analysis of X-ray (EDX), scanning electron microscopy (SEM), X-ray diffraction (XRD), FT-IR, and vibrating sample magnetometer (VSM) analysis.
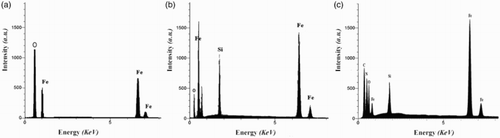
The chemical purity of the samples as well as their stoichiometry was tested by EDX study. The EDX spectrum which is given in (a) shows the presence of Fe and O as the only elementary components of Fe3O4 NPs. EDX spectrum of Fe3O4@SiO2 ((b)) shows that the elemental compositions are (Fe, O, and Si) of core-shell nanoparticles. EDX spectrum of Fe3O4@SiO2–NH2 ((c)) shows that the elemental compositions are (Fe, Si, O, C, and N) of coated-magnetic nanoparticles.
The morphology and structure of the as-prepared samples were characterized by SEM. (a) shows that Fe3O4 nanoparticles are spherical in shape with an average size about 20 nm. (b) indicates that Fe3O4@SiO2 nanoparticles still keep the morphological properties of Fe3O4 except a slightly larger particle size and smoother surface. Fe3O4@SiO2–NH2 nanoparticles with a diameter of 45 nm are shown in (c). This indicates that amino groups were successfully coated on the Fe3O4@SiO2 nanoparticles.
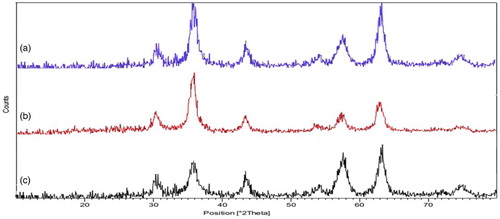
The structure of Fe3O4 (a), Fe3O4@SiO2 (b), and Fe3O4@SiO2–NH2 (c) was analyzed by XRD spectroscopy (). XRD diagram of the bare Fe3O4 NPs shows patterns which included spinel ferrites pattern. All the strong peaks appeared at 2θ = 30.2°, 36.4°, 43.7°, 53.5°, 56.3°, 62.3°, and 73.8° are indexed to the highly crystalline cubic spinel structure of Fe3O4 nanoparticles ( Citation54,Citation55). The same sets of characteristic peaks were also observed for Fe3O4@SiO2 and Fe3O4@SiO2–NH2, indicating the stability of the crystalline phase of Fe3O4 nanoparticles during silica coating and surface amino-functionalization. The XRD data further suggest that the effect of the modifiers on the crystal structure of core-shell samples is negligible.
The average metal nanoparticles (MNPs) core diameter of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2–NH2 was calculated to be about 18, 25, and 40 nm, respectively from the XRD results by Scherrer's equation, (D = Kλ/βcosθ), where β FWHM (full-width at half-maximum or half-width) is in radian and θ is the position of the maximum of diffraction peak, K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the X-ray wavelength (1.5406 Å for Cu Kα).
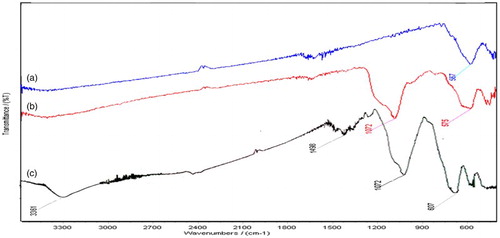
The FT-IR spectra of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2–NH2 nanoparticles are shown in . For the bare magnetic nanoparticles ((a)), the vibration band at 567 cm–1 is the typical IR absorbance induced by structure Fe–O vibration. In the case of Fe3O4@SiO2 nanoparticles ((b)), the band at 1072 cm–1 is corresponding to Si–O–Si antisymmetric stretching vibrations, being indicative of the existence of SiO2 on the nanoparticles. The absorption bands at 1072 cm–1 observed on Fe3O4@SiO2 and Fe3O4@SiO2–NH2 can be ascribed to the stretching and deformation vibrations of SiO2, reflecting the coating of silica on the magnetite surface. Successful aminopropyl functionalization of the silica layer on Fe3O4@SiO2 was also evidenced by the absorption at 3361, 1498, and 692 cm–1 attributed to the stretching and bending vibrations of amino groups. The absorption peaks in the region 2800–3025 cm–1 were associated with the stretching vibration of methylene groups of Fe3O4@SiO2–NH2 ((c)). The results verified the formation of a silica shell on the Fe3O4 surface and the amino-functionalization of the silica shell ( Citation56,Citation57).
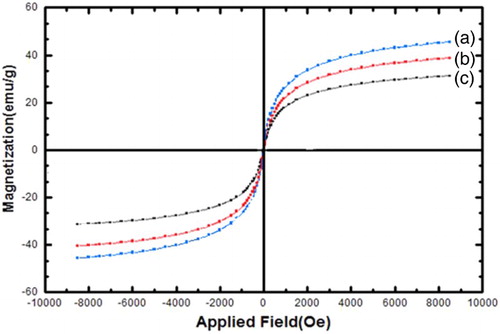
The magnetic properties of the uncoated magnetic iron oxide (Fe3O4), Fe3O4@SiO2, and Fe3O4@SiO2–NH2 were measured by VSM at room temperature (). In , the hysteresis loops which are related to superparamagnetic behavior can be clearly observed for all the nanoparticles. Superparamagnetism is the responsiveness to an applied magnetic field without retaining any magnetism after the removal of the applied magnetic field. From M versus H curves, the saturation magnetization value (Ms) of uncoated Fe3O4 NPs was found to be 47.12 emu g–1. For Fe3O4@SiO2 and Fe3O4@SiO2–NH2, the magnetization obtained at the same field were 41.23 and 32.42 emu g–1, respectively, lower than that of uncoated Fe3O4. These results indicated that the magnetization of Fe3O4 decreased considerably with the increase of SiO2 and aminopropyl groups. This is mainly attributed to the existence of nonmagnetic materials on the surface of the nanoparticles.
Generally, the magnetization of Fe3O4 nanoparticles does not change by itself even coated by other materials but less magnetic properties was observed bacuase of functionalization of nanopartiles with nonmagnetic materials.
In the continuation of this research, in order to optimize reaction conditions, the reaction of salicylaldehyde (1a), malononitrile (2), and thiophenol (3a) was carried out as model reaction in the presence of various catalytic system including homogenous and heterogeneous catalysts (Scheme 1). As can be seen from , among the various catalysts, Fe3O4@SiO2–NH2 nanoparticles were founded to be the most effective catalysts to the synthesis of 2,4-diamino-5-phenylsulfanyl-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4a) under reflux conditions.
Table 1. Preparation of 2,4-diamino-5-phenylsulfanyl-5H-chromeno [2,3-b]pyridine-3-carbonitrile (4a) using various catalysts.a
It seems that high surface area and better dispersion of nanoparticles in the reaction mixture are reasons for the better activities of Fe3O4@SiO2–NH2 NPs. The presence of many hydroxyl groups on the surface of the MNPs leads to reaction with 3-aminopropyltriethyloxysilane and formation of Si–O bonds which support terminal NH2 functional groups on the shell of the Fe3O4 core.
Next, we investigated the effect of quantity of Fe3O4@SiO2–NH2 nanoparticles on the synthesis of 2,4-diamino-5-phenylsulfanyl-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4a). The reaction was performed in the absence and also in the presence of 0.002, 0.005, 0.01, 0.02, and 0.03 g of Fe3O4@SiO2–NH2 NPs. The best result was obtained using 0.01 g of Fe3O4@SiO2–NH2 nanoparticles under reflux conditions (, Entry 4).
Table 2. Effect of quantity of Fe3O4@SiO2–NH2 NPs on the model reaction.a
shows that the solvent has a great effect in acceleration of the reaction. The best results (94% Yield) were obtained in 45 min in 50% aqueous ethanol under reflux conditions for the MCR of salicylaldehyde (1a), malononitrile (2), and thiophenol (3a) (, entry 6).
Table 3. The synthesis of chromeno[2,3-b]pyridine (4a) using different solvents.
To research the scope of this process, we used a variety of salicylaldehydes and thiophenols to study these three-component reactions under the optimal conditions (Scheme 2, ). We found that various salicylaldehyde derivatives cyclized smoothly to afford the products. shows that salicylaldehydes with electron-withdrawing groups such as NO2 and Cl reacted slower and in lower yields in comparison with electron-releasing group such as OH. As shown in , these condensation reactions can be performed in the presence of aromatic and aliphatic thiols, but the rate of the reactions involving aliphatic thiols is slightly decreased in comparison with aromatic ones. However, the yields of products formation were satisfactorily observed when we used aliphatic thiols. In addition, aromatic thiols with electron-donating groups reacted faster than those with electron-withdrawing groups as expected. Also sterically hindered aromatic thiols required longer reaction times.
Table 4. Synthesis of chromeno[2,3-b] pyridine derivatives using Fe3O4@SiO2–NH2 nanoparticles.a
Proposed mechanism
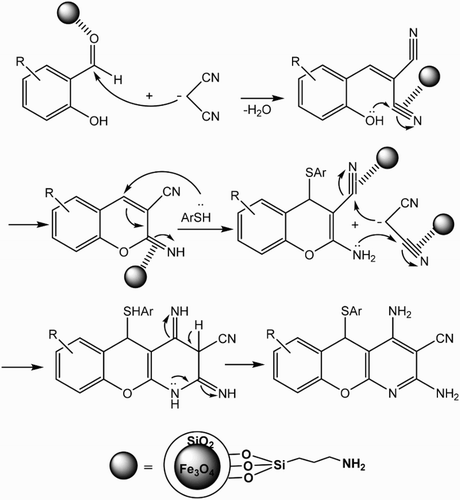
A possible mechanism for the synthesis of chromeno[2,3-b]pyridine derivatives using Fe3O4@SiO2–NH2 NPs is shown in Scheme 3. We suppose that nanoparticles show dual role in this MCR. The catalytic active site in the Fe3O4@SiO2 NPs is Fe+3 which behaves as a Lewis acid and attaches to carbonyl groups of salicylaldehyde derivatives and nitrile to accelerate the conjugate and direct additions of nucleophiles to corresponding substrates ( Citation58,Citation59). Another rule of the catalyst is related to Brønsted basic (–NH2) properties of functionalized Fe3O4 which plays a crucial catalytic role in the described transformation. The NH2 groups on the surfaces of nanoparticles separate the acidic hydrogen of substrates for more efficiency in the nucleophilic attacks.
Experimental
Chemicals were purchased from the Sigma-Aldrich and Merck in high purity. All of the materials were of commercial reagent grade and were used without further purification. All melting points are uncorrected and were determined in capillary tube on Boetius melting point microscope.1H NMR and 13C NMR spectra were obtained on a Bruker 400 MHz spectrometer with DMSO-d6 as a solvent using tetramethylsilane as an internal standard. FT-IR spectrum was recorded on Magna-IR, spectrometer 550. The elemental analyses (C, H, N) were obtained from a Carlo ERBA Model EA 1108 analyzer. Powder X-ray diffraction (XRD) was carried out on a Philips diffractometer of X'pert Company with mono chromatized Cu Kα radiation (λ = 1.5406 Å). Microscopic morphology of products was visualized by SEM (LEO 1455VP). The mass spectra were recorded on a Joel D-30 instrument at an ionization potential of 70 eV. Magnetic properties were obtained on a BHV-55 VSM. The compositional analysis was done by EDX (Kevex, Delta Class I).
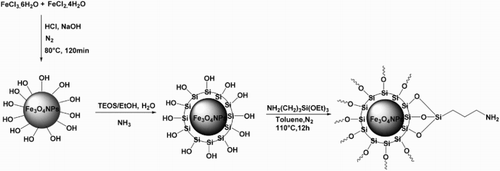
Preparation of Fe3O4 nanoparticles
Fe3O4 MNPs were prepared according to a previously reported procedure by Hu et al. ( Citation60) using the chemical co-precipitation method. Typically, FeCl3·6H2O (2.7 g) and FeCl2·4H2O (1 g) were dissolved in 100 ml of 1.2 mmol l–1 aqueous HCl followed by ultrasonic bath for 30 min. Then, 1.25 mol l–1 aqueous NaOH (150 ml) was added under vigorous stirring and a black precipitate was immediately formed. The resulting transparent solution was heated at 80°C with rapid mechanical stirring under N2 atmosphere. After vigorous stirring for 2 h, the black products were centrifuged, filtered out, and washed with deionized water and alcohol for several times, and finally dried at 60°C for12 h.
Preparation of Fe3O4@SiO2 nanoparticles
Fe3O4@SiO2 core-shell particles were prepared via modified Stöber sol-gel process ( Citation61). Thirty milligram as-prepared Fe3O4 submicrospheres were ultrasonically dispersed in a solution containing 160 mL ethanol, 40 mL water, and 10 mL concentrated ammonia (28 wt%). Then, 0.4 mL TEOS was added dropwise to the solution under sonication, followed by mechanically stirring for 3 h at room temperature. Subsequently, the resulting particles were separated using a magnet and washed with deionized water and ethanol. This step was repeated several times before drying at 60°C for 12 h.
Preparation of Fe3O4@SiO2–NH2 nanoparticles
Fe3O4@SiO2–NH2 MNPs were prepared according to a previously reported procedure by Jiahong Wang et al. ( Citation62). Amino-functionalized Fe3O4@SiO2 nanoparticles were prepared by surface functionalization of Fe3O4@SiO2 nanoparticles using (3-aminopropyl)triethoxysilane (APTES). Two gram of Fe3O4@SiO2 nanoparticles and 50 ml of toluene were added to a 250-ml three-necked flask and then ultrasonically dispersed for 15 min. Four milliliter of APTES (Sigma) was then added to the flask, and the mixture was refluxed at 110°C with continuous stirring for 12 h under a nitrogen flow (40 ml/min). The resulting functionalized Fe3O4@SiO2 was gathered by filtration followed by washing with ethanol and acetone several times and drying at 50°C under vacuum for 12 h. The materials obtained are referred to as Fe3O4@SiO2–NH2 nanoparticles (Scheme 4).
General procedure for the synthesis of chromeno[2,3-b]pyridine derivatives (4a-l)
A mixture of salicylaldehyde derivatives (1.5 mmol), thiol (1.5 mmol), malononitrile (3 mmol), and Fe3O4@SiO2–NH2 NPs (0.01 g) was refluxed in 50% aqueous ethanol (8 mL). After completion of the reaction (monitored on thin layer chromatography), the reaction mixture was cooled and then the catalyst was separated by an external magnet. The solid obtained was filtered and washed well with water. The residue was dissolved in DMF (2 ml), the undissolved part in DMF was filtered off, and water (3 ml) was added to the filtrate. The resulting precipitate was filtered and dried well under vacuum pump, and the crude solid product was crystallized from hot MeOH to afford the pure product in high yield.
Spectral data of new products
2,4-Diamino-5-(2-bromophenylsulfanyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4b)
Yellow solid, m.p 244–246°C, 1H NMR (DMSO-d6, 400 MHz) δ: 5.88 (s, 1H), 6.57 (s, 2H), 6.85–6.89 (m, 3H), 6.98 (s, 2H), 7.04–7.10 (m, 4H), 7.12–7.27 (m, 2H), 7.49–7.52 (d, 2H); 13C NMR (DMSO-d6, 100 MHz) δ : 160.5, 158.2, 152.6, 147.2, 145.4, 131.5, 131.0, 125.5, 124.1, 119.4, 118.8, 116.5, 112.5, 110.7, 84.8, 55.4, 38.4: IR (KBr) v: 3427, 3357, 2205, 1406–1625, cm–1; MS (EI) (m/z): 425 (M+); Anal. calcd for C19H13BrN4OS: C 53.66, H 3.08, N 13.17. Found C 53.42, H 3.17, N 13.25.
2,4-Diamino-5-(4-bromophenylsulfanyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4c)
Yellow solid, m.p. 259–261°C, 1H NMR (DMSO-d6, 400 MHz) δ: 5.80 (s, 1H), 6.57 (s, 2H), 6.66–6.86 (d, 2H), 6.83–6.86 (d, 2H), 7.00 (s, 1H), 7.10–7.15 (m, 1H), 7.23–7.27(d, 2H), 7.31–7.34(d, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 160.9, 158.2, 152.1, 144.8, 132.0, 131.6, 125.7,125.3, 124.4, 122.6, 122.3, 118.9, 116.2, 112.5, 110.00, 84.9, 55.3, 38.1: IR (KBr) v: 3427, 3352, 2211, 1410–1625, cm–1; MS (EI) (m/z): 425 (M+); Anal. calcd for C19H13BrN4OS: C 53.66, H 3.08, N 13.17. Found C 53. 61, H 3.15, N 13.13.
2,4-Diamino-5-(4-methylthiophenylsulfanyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4d)
Yellow solid, m.p 265–267°C, 1H NMR (DMSO-d6, 400 MHz) δ: 2.27 (s, 3H), 5.68 (s, 1H), 6.46 (s, 2H), 6.65 (d, 2H), 6.80 (d, 1H), 6.94 (s, 4H), 7.15 (d, 2H), 7.19 (d, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 159.6, 159.5, 155.3, 150.2, 137.3, 135.9, 128.8, 128.5, 128.2, 126.0, 123.6, 121.8, 116.4, 115.7, 86.3, 70.3, 45.6, 23.6; IR (KBr) v: 3459, 3352, 2320, 1403–1624, cm–1; MS (EI) (m/z): 392 (M+); Anal. calcd for C20H16N4OS2: C 61.20, H 4.11, N 14.27. Found C 61.28, H 4.06, N 14.18.
2,4-Diamino-6-hydroxy-5-(4-bromophenylsulfanyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4l)
Yellow solid, m.p 238–240°C, 1H NMR (DMSO-d6, 400 MHz) δ: 5.45 (s, 1H), 6.23–6.28 (m, 2H), 6.35 (s, 2H), 6.84–6.89 (m, 3H), 7.43 (d, 2H), 7.76 (d, 2H), 9.37 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 160.3, 156.8, 149.5, 137.9, 137.0, 130.9, 130.4, 129.6, 129.2, 129.1, 127.4, 127.1, 125.1, 122.5, 117.2, 113.7, 87.0, 70.9, 41.9; IR (KBr) v: 3489, 3373, 3156, 2214, 1652, 1408–1622, cm–1; MS (EI) (m/z): 441 (M+); Anal. calcd for C19H13BrN4O2S: C 51.71, H 2.97, N 12.70. Found C 51.78, H 2.91, N 12.73.
Catalyst recovery
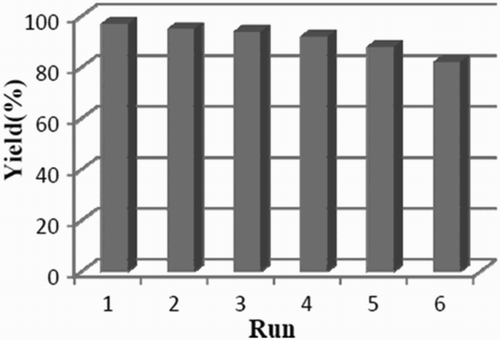
After the reaction was complete, the recovered nanoparticles were washed several times with methanol and ethylacetate and then dried at 50°C for 10 h. To investigate lifetime and level of recoverability of the Fe3O4@SiO2@NH2 NPs, the model study was carried out several times by use of recycled magnetite nanoparticles. We observed that the recovered magnetite nanoparticles could be used for six successive runs with a slight decrease in activity as indicated in .
Conclusions
We have described a rapid, green, and highly efficient method for the green synthesis of chromeno[2,3-b]pyridine derivatives using Fe3O4@SiO2–NH2 nanoparticles in aqueous ethanol media under reflux conditions. The products were obtained in excellent yields and the reaction times were significantly low. The present approach demonstrates a simple and appropriate method for the three-component coupling of salicylaldehydes, thiols, and malononitrile to synthesize some chromeno[2,3-b]pyridine derivatives in the presence of Fe3O4@SiO2–NH2 NPs. The use of these nanoparticles leads to the principles of green chemistry. This methodology offers significant improvements such as simplicity in operation, and green aspects with avoiding expensive or corrosive catalysts and solvents. In addition, Fe3O4@SiO2–NH2 nanoparticles as an environmental friendly, non-toxic, non-volatile and reusable catalyst could also be successfully recovered and recycled at least for six runs without any significant loss in activity.
Disclosure statement
No potential conflict of interest was reported by the author.
Supplemental data and research materials
Supplemental data for this article can be accessed at the Taylor & Francis website, doi: 10.1080/17518253.2015.1107139
Supplemental_Material.docx
Download MS Word (17.1 KB)Additional information
Funding
References
- Hu, W.; Wilson, R.J.; Koh, A.; Fu, A.; Faranesh, A.Z.; Earhart, C.M.; Osterfeld, S.J.; Han, S.; Xu, L.; Guccione, S.; Sinclair, R.; Wang, S.X. Adv. Mat. 2008, 20, 1479–1483.
- Scherer, C.; Figueiredo Neto, A.M. Braz. J. Phys. 2005, 35, 718–727.
- Raming, T.P.; Winnubst, A.J.A.; Van Kats, C.M.; Philipse, A.P. J. Colloid Interface Sci. 2002, 249, 346–350.
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. J. Mater. Chem. 2004, 14, 2161–2175.
- Chorny, M.; Polyak, B.; Alferiev, I.S.; Walsh, K.; Friedman, G.; Levy, R.J. Faseb J. 2007, 21, 2510–2519.
- Gu, H.; Xu, K.; Xu, C.; Xu, B. Chem. Commun. 2006, (9), 941–949.
- Teunissen, W.; Bol, A.A.; Geus, J.W. Catal. Today. 1999, 48, 329–336.
- Nemati, F.; Golmohammadi Afkham, M.; Elhampour, A. Green. Chem. Lett. Rev. 2014, 7, 79–84.
- Yoon, H.; Ko, S.; Jang, J. Chem. Commun. 2007, (14), 1468–1470.
- Yang, H.H.; Zhang, S.Q.; Chen, X.L.; Zhuang, Z.X.; Xu, J.G.; Wang, X.R. Anal. Chem. 2004, 76, 1316–1321.
- Stevens, P.D.; Li, G.; Fan, J.; Yen, M.; Gao, Y. Chem. Commun. 2005, (35), 4435–4437.
- Sadri, F.; Ramazani, A.; Massoudi, A.; Khoobi, M.; Tarasi, R.; M. Shafiee, A.; Azizkhani, V.; Dolatyari, L.; Joo, S.V. Green. Chem. Lett. Rev. 2014, 7, 257–264.
- Kassaee, M.Z.; Masrouri, H.; Movahedi, F. Appl. Catal. A-Gen. 2011, 395, 28–33.
- Esmaeilpour, M.; Sardarian, A.R.; Javidi, J. Appl. Catal. A-Gen. 2012, 445–446, 359–367.
- Maleki, A. Tetrahedron Lett. 2013, 54, 2055–2059.
- Kiasat, A.R.; Davarpanah, J. J. Mol. Catal. A-Chem. 2013, 373, 46–54.
- Zamani, F.; Izadi, E. Catal. Commun. 2013, 42, 104–108.
- Mohammadi, R.; Kassaee, M.Z. J. Mol. Catal. A-Chem. 2013, 380, 152–158.
- Gawande, M.B.; Bonifácio, V.D.B.; Varma, R.S.; Nogueira, I.D.; Bundaleski, N.; Amjad, C.; Ghumman, A.; Teodoro, M.N.D.; Branco, P.S. Green Chem. 2013, 15, 1226–1231.
- Mahmoudi, H.; Jafari, A.A. ChemCatChem. 2013, 5, 3743–3749.
- Nemati, F.; Heravi, M.M.; Saeedi Rad, R. Chin. J. Catal. 2012, 33, 1825–1831.
- Du, Q.; Zhang, W.; Ma, H.; Zheng, J.; Zhou, B.; Li, Y. Tetrahedron. 2012, 68, 3577–3584.
- Yang, H.; Li, S.; Wang, X.; Zhang, F.; Zhong, X.; Dong, Z.; Ma, J. J. Mol. Catal. A-Chem. 2012, 363–364, 404–410.
- Li, W.; Zhang, B.; Li, X.; Zhang, H.; Zhang, Q. Appl. Catal. A-Gen. 2013, 459, 65–72.
- Hu, A.; Yee, G.T.; Lin, W. J. Am. Chem. Soc. 2005, 127, 12486–12487.
- Wang, P.; Kong, A.; Wang, W.; Zhu, H.Y.; Shan, Y.K. Catal. Lett. 2010, 135, 159–164.
- Zamani, F.; Kianpour, S. Catal. Commun. 2014, 45, 1–6.
- Atashkar, B.; Rostami, A.; Tahmasbi, B. Catal. Sci. Technol. 2013, 3, 2140–2146.
- Alizadeh, A.; Khodaei, M.M.; Beygzadeh, M.; Kordestani, D.; Feyzi, M. Bull. Korean Chem. Soc. 2012, 33, 2546–2552.
- Zeng, T.; Yang, L.; Hudson, R.; Song, G.; Moores, A.R.; Li, C. Org. Lett. 2011, 13, 442–445.
- Kolokythas, G.; Pouli, N.; Marakos, P.; Pratsinis, H.; Kletsas, D. Eur. J. Med. Chem. 2006, 41, 71–79.
- Azuine, M.A.; Tokuda, H.; Takayasu, J.; Enjyo, F.; Mukainaka, T.; Konoshima, T.; Nishino, H.; Kapadia, G. J. Pharmacol. Res. 2004, 49, 161–169.
- Srivastava, S.K.; Tripathi, R.P.; Ramachandran, R. J. Biol. Chem. 2005, 280, 30273–30281.
- Toshiro, S.; Noriko, W. Eur. Pat. Appl. EP 647445 A1 19950412, 1995.
- Ito, Y.; Kato, H.; Yasuda, S.; Kato, N.; Iwasaki, N.; Nishino, H.; Takeshita, M. Jpn. Kokai Tokkyo Koho JP 06107664 A2 19940419, 1994.
- Goto, K.; Yaoka, O.; Oe, T. PCT Int. Appl. WO 8401711 A1 19840510, 1984.
- Maruyama, Y.; Goto, K.; Terasawa, M. Ger. Offen. DE 3010751 19810806, 1981.
- Ukawa, K.; Ishiguro, T.; Kuriki, H.; Nohara, A. Chem. Pharm. Bull. 1985, 33, 4432–4437.
- Violina, T.; Angelova, I.T.; Toma, G. J. Heterocycl. Chem. 2014, 51, 1031–1035.
- Zeba, N.S. Tetrahedron Lett. 2012, 37(53), 4974–4978.
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kor- nienko, A. J. Org. Chem. 2007, 72, 3443–3453.
- Ramadasa, S.; Krupadanama, G.L. Synth. Commun. 2000, 30, 1103–1114.
- El-Essawy Farag, A.; El-Etrawy Abd-Allah, S. J. Heterocycl. Chem. 2014, 51, 191–195.
- Mishra, S.; Ghosh, R. Synth. Commun. 2012, 42, 2214–2229.
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. Tetrahedron Lett. 2006, 47, 9309–9312.
- Safaei-Ghomi, J.; Kiani, M.; Ziarati, A.; Shahbazi-Alavi, H. J. Sulfur. Chem. 2014, 35, 450–457.
- Safaei-Ghomi, J.; Shahbazi-Alavi, H.; Heidari-Baghbahadorani, E.J. RSC Adv. 2014, 4, 50668–50677.
- Ghasemzadeh, M.A.; Safaei-Ghomi, J. Acta. Chim. Slov. 2014, 62, 103–110.
- Hedayati, V.R.; Ghasemzadeh, M.A. J. Chem. Res. 2015, 39, 56–61.
- Ghasemzadeh, M.A.; Safaei-Ghomi, J.; Zahedi, S. J. Serb. Chem. Soc. 2013, 78, 769–779.
- Ghasemzadeh, M.A.; Safaei-Ghomi, J.; Molaei, H. C. R. Chimie. 2012, 15, 969–974.
- Ghasemzadeh, M.A.; Safaei-Ghomi, J. J. Chem. Res. 2014, 5, 313–316.
- Mirhosseini-Eshkevari, B.; Ghasemzadeh, M.A.; Safaei-Ghomi, J. Res. Chem. Intermed. 2015, 41, 7703–7714.
- Yang, T.; Shen, C.M.; Gao, H.J. J. Phys. Chem. B. 2005, 109, 23233–23236.
- Pecharroman, C.; Gonzalez-Carreno, T.; Iglesias, J.E. Phys. Chem. Miner. 1995, 22, 21–29.
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamurad, M.; Tomad, H.E. J. Magn. Magn. Mater. 2004, 279, 210–217.
- Shen, X.C.; Fang, X.Z.; Zhou, Y.H.; Liang, H. Chem. Lett. 2004, 33, 1468–1469.
- Joseph, Y.; Kuhrs, C.; Ranke, W.; Ritter, M.; Weiss, W. Chem. Phys. Lett. 1999, 314, 195–202.
- Martnez, R.; Ramon, D.J.; Yus, M. Adv. Synth. Catal. 2008, 350, 1235–1240.
- Hu, Y.; Zhang, Z.; Zhang, H.; Luo, L.; Yao, S. J. Solid State Electro.Chem. 2012, 16, 857–867.
- Deng, Y.H.; Qi, D.W.; Deng, C.H.; Zhang, X.M.; Zhao, D.Y. J. Am. Chem. Soc. 2008, 130, 28–29.
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. J. Colloid Interface Sci. 2010, 349, 293–299.


![Scheme 1. The model reaction for the synthesis of chromeno[2,3-b]pyridine (4a).](/cms/asset/b85ba49c-b4f7-495c-9519-910e9ef0c7ca/tgcl_a_1107139_f0007_b.gif)
![Scheme 2. One-pot synthesis of chromeno[2,3-b]pyridines catalyzed by Fe3O4@SiO2–NH2 NPs.](/cms/asset/b2096ca6-6ef4-4a2a-9f97-9a0bd0966759/tgcl_a_1107139_f0008_b.gif)