ABSTRACT
Polyhydroxyalkanoates (PHAs) are intracellular aliphatic polyesters synthesized as energy reserves, in the form of water-insoluble, nano-sized discrete and optically dense granules in cytoplasm by a diverse bacteria and some archae under conditions of limiting nutrients in the presence of excess carbon source. Bacteria synthesize different PHAs from coenzyme A thioesters of respective hydroxyalkanoic acid, and degrade intracellularly for reuse and extracellularly in natural environments by other microorganisms. In vivo, PHAs exist as amorphous mobile liquid and water-insoluble inclusions but in vitro, exhibit material and mechanical properties, ranging from stiff and brittle crystalline to elastomeric and molding, similar to petrochemical thermoplastics. Further, they are hydrophobic, isotactic, biocompatible and exhibit piezoelectric properties. But as commodity plastics their applications are limited by high production cost, low yield, in vivo degradation, complexity of technology and difficulty of extraction. Therefore, to replace the conventional plastic with PHAs, it is prerequisite to standardize the PHA production systems.
Introduction
Expansion of conventional petrochemical plastics production and consumption are having a significant impact both visibly and invisibly on the environment and society. Improper disposal of plastics has threatened natural environment worldwide since long time ago ( Citation1). Conventional petrochemical plastics are recalcitrant to microbial degradation and accumulate in environment at a rate of 28 million tons per year ( Citation2) In 2011, nearly 280 million tons of petrochemicals-based polymers were produced with expected increased in 4% per annum to 2016. Production of synthetic polymers is expected to increase to around 810 million tons by 2050 ( Citation3). The challenging problems associated with petroleum-based plastic accumulation in the environment and rapid depletion of natural resources being used in their production are motivating factors for research into sources and tools for alternatives to petroleum-based polymers. Considering current advances in biopolymers research, bacterial polyhydroxyalkanoates (PHAs) which are biodegradable thermoplastics have shown great potential as a replacement for petroleum-based plastics. Therefore, to overcome these problems, the production and applications of eco-friendly PHAs become inevitable.
PHAs are a class of polyesters of various hydroxycarboxylic acids, which are produced and accumulated by a large number of bacteria under unbalanced growth conditions as intracellular hydrophobic inclusions of carbon and energy storage compounds in the cytoplasm to levels as high as 90–97% of the cell dry weight ( Citation4–9) or as an electron sink mechanism for redundant reducing power under the condition of limiting nutritional elements such as N, P, S, O or Mg in the presence of excess carbon source ( Citation10–13). They are synthesized as byproducts not the major ones when there is no sufficient nutrient to promote their growth and do not play an essential role in development of the producing organisms, but convey survival in the biological community and environment, therefore, microbial PHAs are secondary metabolites ( Citation14). The accumulated PHAs are degraded by intracellular depolymerases and metabolized as carbon and energy source as soon as the supply of the limiting nutrient is restored ( Citation15, Citation16). The presence of sudanophilic, lipid-like inclusions which were soluble in chloroform was initially observed in Azotobacter chroococcum in early last century ( Citation17). The chemical composition of similar inclusions in Bacillus megaterium was later identified as poly(3-hydroxybutyric acid) (P(3HB) or PHB) by Lemoigne ( Citation18, Citation19) and coworkers ( Citation20) During the following 30 years, interest in this material was negligible. The first report on functions of PHB appeared in 1958 by Macrae and Wilkinson ( Citation21). They reported the rapid biodegradability of PHB produced by B. megaterium, by Bacillus cereus and B. megaterium itself. From here on, the interest in PHB increased dramatically. Once PHAs are extracted from bacterial cells, these polymers show crystalline, flexible, elastic and thermoplastic properties ( Citation22–25). Further, they are synthesized from renewable carbon resources, based on agriculture or even on industrial wastes or fermentation feedstocks ( Citation25–27) so do not lead to the depletion of finite resources ( Citation28). Bacterial PHAs gained particular interest since they are completely biodegradable, non-toxic, biocompatible and also sources for commercially useful pool of chiral monomers ( Citation29–33). Growing concerns about environmental pollution have renewed interest in the development of PHAs which are totally degraded by microorganisms present in most environments and ecologically useful alternatives to synthetic conventional plastics which are non-degradable, and release harmful chemicals like hydrogen chloride and hydrogen cyanide during incineration ( Citation31, Citation34, Citation35). Besides being biodegradable, PHAs are recyclable like the petrochemical thermoplastics ( Citation7). Since their discovery, all these properties have made these microbial polyesters very attractive as a source of alternative biodegradable materials to conventional petrochemical-based plastics ( Citation23, Citation24, Citation36). Therefore, they are considered for several applications in the packaging industry, biomedical, medicine, agriculture and food industry, or as raw materials for the synthesis of enantiomerically pure chemicals and the production of paints ( Citation28, Citation37–39). Due to wide industrial application, PHB are considered as green plastics. Unfortunately, the current production costs are much higher than for petroleum-based synthetic plastics, which make PHAs substantially more expensive and hamper the widespread usage of this high-quality material ( Citation30, Citation40, Citation41). However, environmental-friendly features such as biodegradability and biocompatibility, which are lacking in conventional plastics, have continued as the commercial interest in PHAs ( Citation42). In the early 1990s, despite higher costs of production, a German company Wella started using PHAs as packaging material for some of its hair-care products ( Citation43). Recently, PHAs have attracted considerable attention due to their potential use as biodegradable thermoplastics and sources of chiral monomers ( Citation34, Citation44, Citation45). Thus, research is currently being performed to improve productivity, to reduce production costs and, more importantly, to produce specific functionalized PHAs ( Citation46–48). Therefore, the aim of the present study is to review the current advances and progress in biosynthesis, accumulation, extraction, chemical and physical properties, biocompatibility, degradation and potential applications of bacterial thermoplastic PHAs.
Chemical structure
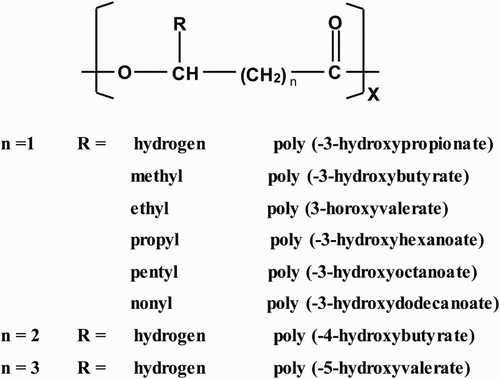
The PHAs that have been identified to date are rather complex class of primarily aliphatic/linear, head-to-tail and optically active biopolyoxoesters and composed of (R)-3-hydroxy fatty acid monomers () ( Citation7, Citation9, Citation43, Citation45). In these polymers, the carboxyl group of one monomer forms an ester bond with the hydroxyl group of the neighboring monomer () ( Citation18, Citation30). In all PHAs that have been characterized so far, the hydroxyl-substituted carbon atom is of the R-configuration due to the stereo specificity of PHA biosynthetic enzymes, except in some special cases where there is no chirality () ( Citation26, Citation43, Citation49, Citation50). Because of the chiral (R) center in the backbone, the chemical synthesis of PHA is difficult ( Citation49). At the same C-3 or β position, an alkyl group which can vary from methyl to tridecyl is positioned (). However, this alkyl side chain is not necessarily saturated: aromatic, unsaturated, halogenated, epoxidized and branched monomers have been reported as well ( Citation51–55). The majority of PHAs are composed of R(-)-3-hydroxyalkanoic acid monomers ranging from C3 to C14 carbon atoms with a variety of saturated or unsaturated and straight or branched chain containing aliphatic or aromatic side groups ( Citation27, Citation56). Approximately 150 different hydroxyalkanoic acids are now known to occur as constituents of PHAs ( Citation57) and this number continues to increase with the introduction of new types of PHA through the chemical or physical modification of naturally occurring PHA, or through the creation of genetically modified organisms to produce PHA with specialized functional groups ( Citation58). The molecular weight of the PHA is in the range of 2 × 105 to 3 × 106 Da, depending on the number of carbon atoms constituting monomer units, the type of microorganism and growth conditions ( Citation16, Citation43). Based on the number of carbon atoms in the monomer units, PHAs can be divided into four groups. The short chain length polyhydroxyalkanoates (scl-PHAs), which consist of C3–C5 atoms, medium chain length polyhydroxyalkanoates (mcl-PHAs) consisting of C6–C14 atoms and long chain length polyhydroxyalkanoates (lcl-PHAs) containing >C14 monomers, and PHAs containing both scl- and mcl-monomer units have been identified in several bacteria ( Citation59–64). In addition, the random copolymer poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P(3HB-3HH) or P(HB-HH) was found to be accumulated in some Aeromonas strains ( Citation64). This grouping is due to the substrate specificity of the PHA synthesis that only accepts 3-hydroxyalkanoates (3HAs or HAs) of a certain range of carbon length ( Citation26). The PHA synthases of Alcaligenes eutrophus can only polymerize 3HAs (scl) while that of Pseudomonas oleovorans only polymerize 3HAs (mcl). For scl-PHAs, the monomer units are oxidized at positions other than the third carbons while for mcl-PHAs, all the monomers units are oxidized at the third position except in few cases ( Citation28). A lot of PHAs (mcl) containing various functional groups such as olefins, branched alkyls, halogens, aromatic and cyano have been reported ( Citation28, Citation53, Citation55). This flexibility of PHA biosynthesis makes it possible to design and produce related biopolymers having useful physical properties ranging from stiff and brittle plastic to rubbery polymers ( Citation26).
Screening of bacteria for PHA production
To date two major methods have described for screening of PHA-producing natural bacteria from environments, namely, phenotypic-based screening and genotypic-based screening. There are many phenotypic detection methods for detecting intracellular PHA granules, which are applied to the screening of PHA producers, including Sudan black B staining ( Citation65–67), Nile blue A staining ( Citation68–70) and Nile red ( Citation71), which result in dark blue or fluorescent granules. Alternative staining methods have been developed for directly staining colonies ( Citation72) or growing bacteria on plates containing Nile blue A or Nile red ( Citation68, Citation73), resulting in fluorescent colonies that can be visualized by UV illumination. PHA-producing colonies on plates containing Sudan black B appear as black-bluish ( Citation74). It was reported that PHAs stained by Nile red show a similar fluorescence behavior, maximum at an excitation wavelength between 540 and 560 nm and an emission wave length between 570 and 605 nm detected by fluorescence spectroscopy or flow cytometry ( Citation75, Citation76). This is suitable for the analysis of granule size distributions in biotechnology ( Citation77). The staining of cell suspensions during cultivation experiments revealed that Nile red has a high potential for the quantitative determination of hydrophobic bacterial polyhydroxyalkanoic acids. Such optical methods offer the advantages of online monitoring in real time with high specificity ( Citation71). Although these staining methods are quite sensitive, but it is rather time-consuming and labor-intensive work to screen a large number of environmental isolates. Moreover, prior to identification and isolation of PHA-producing bacteria by phenotypic methods, it is necessary to provide appropriate carbon sources, nutrient limitation conditions and a long culture time is required for PHA granule accumulation to the bacterial cells ( Citation78, Citation79). Sudan black B is non-specific to PHA as it also stains other lipid bodies. Nile blue A and Nile red are reported to be more specific than Sudan black B for detection ( Citation79). In addition, these methods cannot distinguish between bacteria that accumulate PHA granules and those that accumulate lipid compounds ( Citation78). Hong et al. developed a rapid noninvasive technique using Fourier Transformed Infrared Spectroscopy (FT-IR) which allows detecting intracellular PHA accumulation in intact cells within a few seconds. If the PHA content is high in the cells, the FTIR spectra of PHA can also differentiate the various structures of PHB (scl), mcl-PHAs and PHA consisting of HB and mcl-3HA monomers ( Citation80). Genotypic-based screening method is specific, reliable and quick which circumvents the major drawbacks inherent in phenotypic detection methods ( Citation78, Citation81–84). For genotypic-based screening, the degenerate or and specific oligo nucleotide primers are designed based on multiple sequence alignment results and are used as PCR primers to detect PHA synthase genes ( Citation78, Citation79, Citation81, Citation83–85). For rapid detection, colony PCR technique is employed for screening PHA producers from the environment ( Citation78, Citation80). The PHA accumulation ability of well-separated colonies isolated from environmental samples can be directly validated by PCR with no further culturing or chromosomal DNA extraction procedures. In addition to its application to the screening of wild-type isolates, the individual PCR-amplified product is also suitable as a specific probe for PHA operon detection in producers and cloning ( Citation78).
Diversity among PHA-producing bacteria
PHA producers have been reported to reside at various ecological niches which are naturally or accidently exposed to high organic matter or growth-limited conditions such as dairy wastes, hydrocarbon-contaminated sites, pulp and paper mill wastes, agricultural wastes, activated sludges of treatment plants, rhizosphere and industrial effluents ( Citation86). A wide range of taxonomically and physiologically different natural bacteria and some archae accumulate PHAs as storage reserve materials and deposit them as insoluble granules in the cytoplasm ( Citation10, Citation11, Citation69, Citation70, Citation83, Citation84). After the discovery of PHB from the bacterium B. megaterium ( Citation18), over 300 different bacteria, including Gram-negative and -positive species, have been reported to accumulate various PHAs ( Citation11, Citation28, Citation83, Citation86, Citation87). To date, most PHA-producing bacteria were found to be Gram-negative. Comparatively, a limited number of Gram-positive bacteria has been reported in genera Bacillus, Caryophanon, Clostridium, Corynebacterium, Micrococcus, Microlunatus, Microcystis, Nocardia, Rhodococcus, Staphylococcus and Streptomyces ( Citation58). As for archaea is concerned, PHA production to date, however, has been limited to haloarchaeal species, specifically the genera Haloferax, Halalkalicoccus, Haloarcula, Halobacterium, Halobiforma, Halococcus, Halopiger, Haloquadratum, Halorhabdus, Halorubrum, Halostagnicola, Haloterrigena, Natrialba, Natrinema, Natronobacterium, Natronococcus, Natronomonas and Natronorubrum ( Citation88).
Bacteria used for the production of PHAs can be divided to two major groups based on the culture conditions required for PHA synthesis ( Citation35). First group of bacteria requires the limitation of an essential nutrient such as nitrogen, phosphorus, magnesium or sulfur for the synthesis of PHA from an excess carbon source. The bacteria included in this group are Ralstonia eutropha, Protomonas extorquens and P. oleovorans. The second group of bacteria does not require nutrient limitation for PHA synthesis and the polymer is accumulated during growth phase. It includes Alcaligenes latus, a mutant strain of Azotobacter vinelandii, and recombinant Escherichia coli. These characteristics are important to be considered while the production of PHA.
In another way, PHA-producing bacteria can also broadly be divided into four groups according to the number of carbon atoms in the monomeric units of the PHAs produced ( Citation39). One group of bacteria, including Ralstonia eutropha (formerly A. eutrophus), produces scl-PHAs, while the other group, including P. oleovorans and most Pseudomonads belonging to the rRNA homology group I, can accumulate mcl-PHAs using 3-hydroxyacyl-CoA intermediates of β-oxidation pathway when cultured on various alkanes, alkanols or fatty acids ( Citation11, Citation26, Citation83, Citation84). Although the majority of bacteria accumulate either scl- or mcl-PHA, several bacteria (third group) have also been found to synthesize PHAs (copolymers) containing both scl- and mcl-HA. This largely depends on the substrates provided and polyester synthases with different substrate specificities ( Citation45, Citation89). In the presence of propionic or valeric acid, A. eutrophus (among other bacteria) also produces copolymers of 3-hydroxybutyric acid and 3-hydroxyvaleric acid (HV) P(HB–HV) ( Citation90). Copolymers of 3-hydroxybutyric acid with 4-hydroxybutyric acid can also be produced in A. eutrophus ( Citation91). The bacteria Rhodospirillum rubrum ( Citation59), Rhodocyclus gelatinosus ( Citation60) and Rhodococcus sp ( Citation59) produced terpolyesters consisting of 3HA units of C4, C5 and C6 from hexanoate. Aeromonas caviae produced a random copolymer of HB and 3-hydroxyhexanoate (HH) ( Citation92–94). Pseudomonas strain GP4BH1 produced PHA containing HB and 3-hydroxyoctanoate (HO) from octanoate and PHA containing HB, HO, and 3-hydroxydecanoate (HD) from gluconate ( Citation95). Pseudomonas sp. strain 61–3 isolated from soil was reported to produce a blend of a PHB homopolymer and a random copolymer (P(HB–HA)) consisting of HA units of C4 to C12 from sugars and alkanoic acids ( Citation62, Citation63, Citation96). Boyandin et al. reported the luminescent bacteria Photobacterium leiognathi and Vibrio harveyi as new producers of two- and three-component heteropolymeric PHA containing hydroxybutyric acid as the main monomer, and hydroxyvaleric and hydroxyhexanoic acids monomers as minor components ( Citation97).
In addition, two different types of polyester granules were formed in the same cell ( Citation98). A recombinant strain of P. oleovorans expressing R. eutropha PHB biosynthesis genes has been shown to synthesize a blend of a PHB homopolymer and a copolymer of HHx and HO units when grown on octanoate ( Citation99). Both polyesters were stored as separated granules within the cells ( Citation100). Fourth group of bacteria including Fluorescent pseudomonads is capable of synthesizing lcl-PHAs ( Citation101). In addition, Pseudomonas fluorescens and several other Pseudomonas strains were found to produce a P(HB-–HA) copolymer consisting of HA units of C4 to C12 from HB and 1,3-butanediol ( Citation102). Although Thiocapsa pfennigii accumulated only a PHB homopolymer from various carbon sources, a recombinant P. putida strain harboring the PHA synthesis genes of T. pfennigii produced a P(HB–HHx–HO) terpolymer from octanoate ( Citation103). Interestingly, the occurrence of PHA is not limited to the intracellular collection in granules. PHB with lower molecular weight (cPHB; Mw < 14,000 Da) was also found in B. subtilis, A. vinelandii and Streptomyces lividans ( Citation104–106) in association with polyphosphate and calcium ions. In addition, non-storage PHAs that are of low molecular weight, PHBs have also been detected in the cytoplasmic membrane and cytoplasm of E. coli ( Citation31).
Biosynthesis
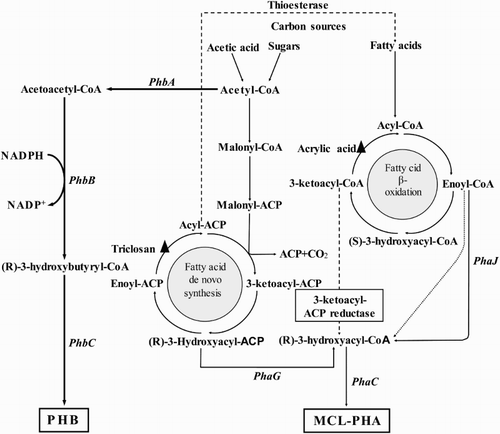
PHAs are synthesized by several enzymatic reactions from acetyl-CoAs catalyzed by substrate-specific PHA synthases located in the cytosol of the cell where PHA accumulation takes place ( Citation12, Citation13, Citation26, Citation28, Citation107, Citation108). The majority of bacteria synthesize scl-PHA, PHB and the second major class of PHA is composed of mcl-(R)-3-hydroxyfatty acids ( Citation45). The biosynthetic pathway of PHB consists of three enzymatic reactions catalyzed by three different enzymes (). The first reaction consists of the condensation of two acetyl coenzyme A (acetyl-CoA) molecules into acetoacetyl-CoA by β-ketoacylCoA thiolase (phbA). The second reaction is the reduction of acetoacetyl-CoA to (R)-3-hydroxybutyryl-CoA by an NADPH-dependent, (R)-specific acetoacetyl-CoA dehydrogenase/reductase (phbB). Lastly, the (R)-3-hydroxybutyryl-CoA monomers the direct precursor of PHB are polymerized into PHB by P(3HB) polymerase (phbC) () ( Citation28, Citation45, Citation109). PHB can also be synthesized through de novo fatty acid biosynthesis and β-oxidation pathways from sugars and fatty acids ( Citation110). In contrast, PHAs composed of mcl-(R)-3-hydroxyfatty acids are synthesized converting intermediates of fatty acid metabolism to (R)-3-hydroxyacyl-CoA (). If the carbon source is oxidized to acetyl-CoA excluding the fatty acid β-oxidation pathway, then fatty acid de novo biosynthesis intermediates are diverted towards PHA biosynthesis catalyzed by the transacylase (PhaG). This specific transacylase catalyses the transfer of the (R)-3-hydroxyacyl moiety of the respective acyl carrier protein (ACP) thioester to CoA () ( Citation111, Citation112). If the carbon source is oxidized through the fatty acid β-oxidation pathway, then the (R)-specific enoyl-CoA hydratase (PhaJ) catalyses the oxidation of enoyl-CoA to (R)-3-hydroxyacyl-CoA ( Citation98). (R)-3-hydroxyacyl-CoA is a substrate for the PHA synthase (PhaC) and the direct precursor of PHA biosynthesis ( Citation45) ().
Figure 2. Anabolic pathways towards PHA (scl and mcl) synthesis in bacteria. Dashed lines represent engineered biosynthesis pathways. Triangles depict targets for inhibitors enabling PHA synthesis. PhaA/PhbA (β-Ketothiolase), PhaB/PhbB (NADPH-dependent acetoacetyl-CoA reductase), PhaC/PhbC (PHA synthase), PhaG (Transacylase) and PhaJ (R-specific enoyl-CoA hydratase).
In vitro formation of PHA granules
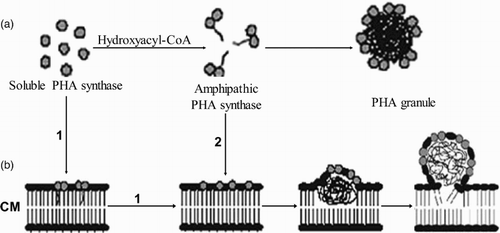
Gerngross and Martin were the first to demonstrate in vitro synthesis of PHB and self-assembly of spherical granules by only using purified polyester synthase and substrate () ( Citation113). This study clearly defined that the PHA synthases possessed all the features required for self-organization into spherical particles. This was further supported by establishment of in vitro PHA synthesis using purified PHA synthases from other microorganisms, such as for example from Cupriavidus necator, Allochromatioum vinosum, Pseudomonas aeruginosa (PhaC1 and PhaC2) and P. oleovorans (PhaC1) ( Citation114–116). The class II PHA synthases were only recently purified and provision of 3-hydroxydecanoyl-CoA as a substrate was sufficient for in vitro synthesis of poly(3-hydroxydecanoate) ( Citation115, Citation116).
In vivo formation of PHA granules
Two models of PHA granule formation have been described: (i) the micelle model and (ii) the budding model (). Both models consider the defined location of the polyester synthase and to some extent the phasin protein on the surface of the granule. The micelle model is certainly supported by PHA granule formation in vitro and in the absence of membranes () ( Citation45, Citation117). However, electron microscopy studies showing membrane-like material surrounding PHA granules in intact cells ( Citation118) or isolated granules ( Citation119) provided evidence for the budding model. Tian et al. showed that early stage granules are not randomly distributed in the cytoplasm and close to the inner cell membrane ( Citation120), as would be anticipated from the two models of granule formation ( Citation121).They found that emerging granules arose from only the center of the cell at unknown mediation elements suggesting a new model for PHA granule formation () ( Citation120). Dennis et al. observed large structures (35 nm) on the surface of PHB-containing granules from C. necator cells using atomic force microscopy which might function as synthesis–degradation centers ( Citation121). Recent fluorescence microscopic studies employing green fluorescent protein (GFP)-labeled PHA synthase, that is, GFP, were fused to the N-terminus of class I and class II PHA synthases, without affecting PHA particle formation, enabled in vivo monitoring of PHA granule formation as well as subcellular localization ( Citation122). In this study, early stage granules were found to be localized at the cell poles suggesting that granule formation starts at the cell poles according to the budding model. Localization of granule formation was found to be dependent on nucleoid structure suggesting that nucleoid occlusion occurred ( Citation122). It remains unclear whether PHA chain synthesis is required for subcellular localization of granule formation. Interestingly, this study led to the observation that small emerging granules are rapidly oscillating between the cell poles. This might play a role in equal distribution of storage materials between the daughter cells ( Citation122). Overall, these in vivo studies using GFP-labeled polyester synthase supported the budding model by localizing granule formation close to the cytoplasmic membrane sat the cell poles ().
Structure of PHA granules
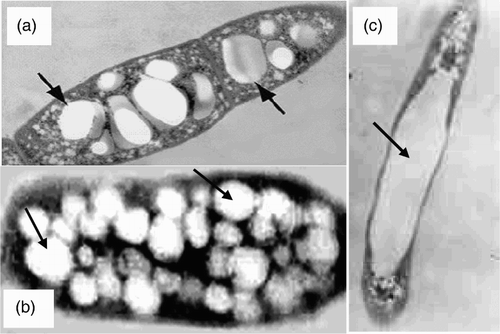
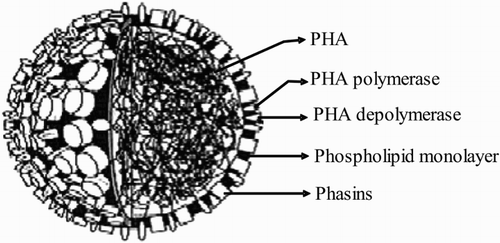
Generally PHAs are accumulated as light-refracting discrete granules inside the cell ( Citation123, Citation124). These PHA granules can be stained specifically with Sudan black B or light fluorescent stains Nile blue A and Nile red () ( Citation65, Citation68, Citation71, Citation125, Citation126). This accumulation in granules separates the polymers from the cell lumen and, consequently, the osmotic pressure of the cell is not changed extensively () ( Citation26). The size and number per cell varies depending on the different species (). About 8–13 granules per cell having a diameter range of 0.2–0.5 µm were observed in A. eutrophus ( Citation16, Citation127). Whereas P. oleovorans is estimated to have about one or two large granules ( Citation128). The PHA granules are mostly spherical and surrounded by a phospholipids membrane ( Citation129) separating two crystalline protein layers ( Citation130, Citation131) composed of the PHA polymerases ( Citation132), the intracellular PHA depolymerase ( Citation133), amphipathic phasing proteins ( Citation117, Citation134), PHA-specific regulator proteins ( Citation135–137) and additional proteins with unknown function () ( Citation128). Granules membrane coat is about 2 nm thick, containing 0.5% lipid and 2% protein, of the granule weight ( Citation138). PHA polymerases are present in the cytosol; however, they are only active when localized on the surface of granules ( Citation103, Citation132, Citation134). The intracellular depolymerases are required for mobilization of the reserve polyester and are attached to the granule surface ( Citation139). Among the proteins associated to PHA granules, phasins constitute a very diverse group are the most dominant proteins of relatively small molecular size ( Citation124, Citation140, Citation141). A few that have been studied in detail revealed that they are associated with a structural role of PHA granules and may have several functions such as coating, stabilizing granules as structural proteins by non-covalently attached to the polyester core of granules () ( Citation30, Citation135, Citation142, Citation143, Citation144) or activating genes and/or enzymes involved in PHA synthesis ( Citation124). Phasins promote PHA biosynthesis and their copy number has impact on PHA granule size ( Citation143, Citation144). Phasins are inhibiting individual granules from coalescing and agglutinating with other granules and acting as a barrier between the polymer and other cellular components ( Citation123, Citation124, Citation145). In phasins mutants of R. eutropha, the cells accumulate less PHB, and they possess only one single large granule because separate granules coagule due to the “nacked” surface if the hydrophobic PHA molecules get in contact () ( Citation140, Citation142, Citation146). It was also speculated that phasins might have a protective function to reduce the passive attachment of cytosolic proteins. Simulation of the self-assembly process showed that phasins might impact on the kinetics of granule formation by reducing the lag phase ( Citation147). Various PHA-specific regulators such as PhbR from C. necator ( Citation137, Citation142). PhaF from Pseudomonads ( Citation135, Citation148, Citation149) and PhaR from Paracoccus denitrificans ( Citation136, Citation150) were found to bind non-covalently to the PHA granules. The cytosolic levels of these repressor proteins are supposed to be low when PHA granules are formed. Additional proteins (PhaI, PhaD and PhaS) were found to be granule-associated and co-regulated in Pseudomonads, whose function has not been clarified yet ( Citation128, Citation135, Citation148, Citation149).
Methods for extraction
Efficient recovery and purification of PHA from cells is required for its cost-efficient industrial production but the extraction of PHA from cells poses yet another challenge. Several protocols have been described and patented over the past 35 years. In all methods, it is essential to concentrate cells by centrifugation, cross-flow filtration and flocculation which are the methods of choice. Subsequently, either water-based separation or solvent-based extraction can be chosen as isolation methods. For solvent-based extraction of PHA, the concentrated biomass is dried either by freeze- or spray-drying. PHA is extracted directly from ground biomass by dissolving it in an organic solvent, usually chloroform, chlorolimited methylene chloride, propylene carbonate and dichloroethane ( Citation70, Citation151, Citation152, Citation153). After removing cellular components (residuals) by filtration and centrifugation, the PHA is precipitated in a non-PHA-dissolving solvent such as cold ethanol or methanol ( Citation154–158). In contrast to mcl-PHAs, scl-PHAs are not soluble in acetone, thus, enabling a separation of both PHA classes ( Citation159). Recovery of PHA from bacterial cells using organic solvents is often applied in industrial processes and in laboratory scale PHA extractions due to the recovery efficiency of the process, simplicity, rapidity, polymer purity obtained and the possible removal of endotoxins from the recovered polymer, which is important for medical applications ( Citation160–162). Solvents extract the polymer without degrading it by improving the cellular membrane permeability and subsequent solubilization of the PHA ( Citation162). But a large amount of hazardous solvent is needed to repeat the same process. Thus, this method is not environmentally friendly and unsuitable for mass production of bioplastic ( Citation22). Excellent recovery was reported for the extraction in supercritical CO2, which may be especially applicable to reduce the endotoxins contamination from genetically engineered E. coli strains ( Citation29). The second protocol is digestion-based extraction strategies that utilize enzymatic treatment of cellular components to release PHA and design to avoid the use of organic solvents. In this method, the cells need to be broken up and various chemical additives added to digest non-PHA material. Bacterial cells debris is treated with a cocktail of enzymes (including proteases, nucleases and lysozymes) and detergents (5% sodium dodecylsulfate) to remove proteins, nucleic acids and cell walls, leaving the PHA intact. Finally, the PHA is concentrated through cross-flow filtration and obtained as white latex of 95% purity, ready to be used as coating material ( Citation22, Citation25, Citation158, Citation163). Compared to solvent-extracted PHA, the molecular weight may be lower following enzymatic recovery methods despite the mild reaction conditions ( Citation164). One critical consideration of these chemical-based methods is that harsh chemical treatment to achieve high purities may lead to a reduction of the molecular weight of the polymer ( Citation165). Yang et al. developed an strategy for poly(hydroxybutyrate-co-hydroxyvalerate) (P(HB-co-HV)) recovery using linear alkylbenzene sulfonic acid LAS-99 as an alternative to the commonly used sodium dodecyl sulfate. In this method, only 21% of the surfactant is required ( Citation166), compared to previous SDS-based methods. The main disadvantages of chemical-based strategies are the large amount of salt produced as a by-product and the amount of surfactant-containing wastewater generated from the process, potentially resulting in high costs for wastewater treatment. Singh et al. reviewed a novel self-disruption cell system for recovery PHA developed in B. megaterium ( Citation167). In this system, a gene cassette carrying the cell lysis system (holin and endolysin of B. amyloliquefaciens phage) ( Citation168) was inserted into the E. coli–B. subtilis shuttle vector pX. In this expression system, xylR-xylA' target genes are induced by xylose but inhibited by glucose, which acts as an anti-inducer ( Citation169). It synchronizes the processes of spontaneous cell lysis and substrate exhaustion, which results in the release of accumulated PHAs. The efficiency of this regulatory process can be enhanced by manipulating the YoeB, a cell wall-associated protein, which gets induced in response to antibiotics stress. The expression of yoeB in B. subtilis is under a xylose-inducible promoter. yoeB mutants display an increased rate of autolysis in response to nutrient depletion and various cell envelope stress conditions. The process of autolysis in B. subtilis 168 can be aided by mutating yoeB gene and in the process making it independent of xylose regulation ( Citation168).
Biodegradability of PHA
The biodegradation of PHAs in natural environments (river and sea waters, soil, sludge and compost) is one of the commercially attractive features which distinguishe the PHAs from petroleum-based plastics ( Citation170–175). The main advantage of PHAs over other types of biodegradable plastic is that they do not require special environmental conditions and can degrade in either aerobic or anaerobic environments through thermal degradation or enzymatic hydrolysis ( Citation176, Citation177). Biodegradation of PHAs is performed by microorganisms, which inhabit a specific natural environment ( Citation176). But in the absence of biological agents (bacteria, algae and fungi) PHAs are practically not subject to mass lost under normal conditions ( Citation178) and they are degraded in biological media to form products innocuous to the environment. PHA biodegradation is performed by microorganisms that secrete intra- or extracellular PHA depolymerases, which differ in their molecular organization and substrate specificity ( Citation179). While intracellular PHA depolymerases are synthesized by PHA-producing bacteria and are used by them to hydrolyze their own PHA storages, extracellular enzymes are produced by other microorganisms to utilize PHAs usually released into environment after death and cell lysis of PHA-accumulating cells ( Citation180). The first microorganisms degrading poly-3-HB were isolated over 50 years ago ( Citation181). Six hundred PHA depolymerases from various microorganisms have been identified by now; comparison of their amino acid sequences provided a basis for uniting them in 8 superfamilies including 38 families ( Citation179). The same strain can contain several genes encoding PHA depolymerases with different specificities. The ability to degrade extracellular PHAs is determined by the activity and type of PHA depolymerases, which hydrolyze the polymer by surface erosion to water-soluble monomers and/or oligomers a substrate for microorganisms. All PHA-degrading bacteria, algae and fungi use extracellular enzymes to depolymerize, or break down, PHAs and, through a process known as mineralization, absorb the remaining fragments for use as minerals ( Citation182). The end products of PHA degradation in both aerobic and anaerobic environments are carbon dioxide and water, while in anaerobic conditions methane is also produced ( Citation28, Citation175). If the limiting growth factor is supplied again, the accumulated PHAs can be also intracellularly depolymerized by degradation enzymes via hydroxycarboxylic acids to acetyl-CoA and reused in metabolism ( Citation16, Citation28). All microorganisms that synthesize PHAs contain intracellular depolymerase system for this purpose. Studies using R. eutropha showed that the intracellular degradation of PHB inclusions is a very slow process ( Citation183). The rate of PHB degradation was calculated to be about 10 times slower than the rate of its synthesis ( Citation184, Citation185). It may be because that PHA inclusions are protected by granule-associated proteins on the surface from attack by the depolymerase ( Citation186). But, Merrick et al. ( Citation187) proposed that an activator acts to modify an inhibitor present on native PHB inclusions, thereby allowing the intracellular depolymerase to access its substrate ( Citation188). Based on the finding that the number of polymer chains was almost constant during the PHB degradation process, it was suggested that the intracellular depolymerase is an exotype hydrolase acting at the carbonyl terminus of the polymer chain ( Citation184). Low concentrations of diisopropyl fluorophosphate inhibit the depolymerase activity leading to the suggestion that this enzyme is a serine esterase ( Citation188). In comparison to the extracellular depolymerase which attacks crystalline PHA, the mechanism for intracellular depolymerase is presumed to be different because of the amorphous nature of the intracellular PHA inclusions. The end products of PHA degradation in aerobic environments are carbon dioxide and water, while methane is also produced in anaerobic conditions ( Citation28, Citation175). In vivo and in vitro PHA degradation is estimated in terms of weight loss (surface erosion), molecular weight decrease, increase in the degree of crystallinity and loss of mechanical properties ( Citation171). PHA degradability is influenced by many factors, including the specificity and activity of extracellular depolymerases, physical and chemical properties of the polymer (stereo-configuration of its molecules, composition, shape of specimen, crystallinity and molecular weight, polydispersity), surface area, microbial activity of the disposal environment, the pressure of other nutrient materials, physicochemical conditions (pH, temperature, oxygen availability, moisture, salinity, acidity of the environment, etc.), the shape and size of PHA-based devices, and weather and climate in different regions ( Citation28, Citation176, Citation180, Citation189). It has been reported that poly(3-hydroxybutyrate-co-4-hydroxybutyrate) had a much faster degradation rate than homopolymer PHB (178). While according to the current investigation of Boyandin et al., the homopolymer of 3-hydroxybutyric acid is degraded at higher rates than the P(HB-HV) ( Citation176). Similarly, the average rates of mass loss were 0.04–0.33% per day for films and 0.02–0.18% for compact pellets, suggesting preferential degradation of the amorphous phase over pellet. The differences in degree of degradation are due to differences in the kinetics and mechanism of PHA biodegradation is caused by qualitative and quantitative dissimilarities between microbial communities of different habitats and other environmental and specimen-related factors ( Citation176).
In microbially active environments, PHAs are initially viewed by microorganisms as energy sources and then start to degrade ( Citation107, Citation190). Microorganisms colonize on the surface of the polymer and secrete exodepolymerases which degrade P(HB–HV) into HB and HV units. These units are then used up by the cell as a carbon source for biomass growth ( Citation15). P(HB–HV) is water insoluble and is not affected by moisture, does not degrade under normal conditions of storage and is stable indefinitely in air ( Citation107, Citation171). The effect of different environments on the degradation rate of PHB and P(HB–HV) has been studied by several workers ( Citation171, Citation184). Degradation occurs most rapidly in anaerobic sewage and slowest in seawater. Lee showed that P(HB–HV) completely degraded after 6, 75 and 350 weeks in anaerobic sewage, soil and sea water, respectively ( Citation107, Citation191). Effective PHA destructors include various bacteria from widespread soil and water genera (Pseudomonas, Alcaligenes, Comamonas, Streptomyces, Ilyobacter) ( Citation192), as well as fungi (Ascomycetes, Basidiomycetes, Deuteromycetes, Mastigiomycetes and Myxomycetes) ( Citation180). Potential microbial PHA destructors are generally isolated by inoculation of microbiological samples on agar plates or latex medium based on PHA particles or granules as the only carbon and energy source. Microbial exodepolymerases hydrolyze PHA to soluble products forming lysis zones on surface of the plate ( Citation175). The ability to degrade extracellular PHA depends on the secretion of specific PHA depolymerases which hydrolyze the polymer to water-soluble products ( Citation181, Citation192) and is widely distributed among bacteria ( Citation171, Citation193). Aerobic and anaerobic PHA-degrading bacteria of many taxa were isolated from various ecosystems, and several PHA depolymerases were isolated and characterized ( Citation185, Citation194, Citation195). All purified depolymerases were specific for PHB and/or other scl-PHAs, such as the PHB depolymerases of A. faecalis, Comamonas sp., or P. lemoignei ( Citation196–198) or for mcl-PHAs, such as the poly(3-hydroxyoctanoate) (P(HO)) depolymerase of P. fluorescens GK13 ( Citation194). Whereas most PHA-degrading bacteria analyzed so far apparently produced only one PHA depolymerase, Pseudomonas lemoignei was found to have at least five PHA depolymerases ( Citation199). The structural genes of several PHA depolymerases have been cloned and sequenced ( Citation197, Citation200–202). Biochemical analysis of the purified depolymerase proteins and analysis of the DNA-deduced amino acid sequences revealed that PHA depolymerases apparently possess a catalytic triad consisting of serine, histidine and aspartate residues which have been demonstrated to form the active site in bacterial lipases ( Citation203). In addition, the hydrolysis of several scl-PHAs by lipases has also been reported ( Citation204). Therefore, the commercial production of PHA as a biological source will bring down the use of expensive chemicals and also reduce the load on pollution.
In vivo biocompatibility
PHAs such as PHB and their breakdown products 3-hydroxyacids have been found in a wide range of organisms, from bacteria to higher mammals. A number of studies have shown that PHB take part in formation of transmembrane ion channel complexes in eukaryotic and prokaryotic cell membranes ( Citation31, Citation205–207). Interestingly, degradation of PHB polymer results ultimately in the production of, R-3-hydroxybutyric acid which also is a normal constituent of blood at concentrations between 0.3 and 1.3 mM and known to be associated with ketone body formation. There is a strong likelihood that surgical implants, sutures, etc. produced from PHA will not result in an immune response in the host organism. Furthermore, sterilization of PHA-based materials does not appear to affect the average molecular weight (Mw), tensile strength or other properties ( Citation208, Citation209). Surface properties of PHA films have been shown to be favorable for proliferation and attachment of tissue culture cells ( Citation210, Citation211), suggesting that PHA is suitable for scaffolding material in tissue engineering. The P(HBco-HV-co-HHx) polymer exhibits the greatest surface roughness, as well as the highest water contact angle characteristics which are important for adherence and proliferation of cells on PHA surfaces. For example, mesenchymal stem cells were shown to adhere and proliferate on several PHA substrates, with a terpolymer poly(hydroxybutyrate-cohydroxyvalerate-co-hydroxyhexanoate) (P(HB-co-HVco-HHx)) yielding the optimum results ( Citation212, Citation213). Further, Sevastianov et al. have also tested the PHA matrices for hemocompatibility by inspecting the response of mammalian blood when incubated with polymer films ( Citation161). It was shown that PHB or P(HB-co-HV), when in contact with blood, did not affect platelet responses, nor did the polymer activate complement system ( Citation214). However, more involved polymer purification procedures had to be followed to significantly reduce the amount of bacterial cell wall material associated with the purified PHA ( Citation161).
Biocompatibility of PHB has been demonstrated in vivo under subcutaneous implantation of PHB films ( Citation161). Tissue reaction to films from PHB of different Mw (300, 450, 1000 and 1500 kDa) implanted subcutaneously was relatively low and did not change from tissue reaction to control glass plate. The low tissue reaction to implanted PHB films indicates the high biocompatibility of PHB in vivo ( Citation161, Citation214). This high biocompatibility of PHB is due to the presence of natural PHB oligomers and HB, the intermediate product of PHB degradation, in animal tissues at normal conditions in comparison with chemically synthesized biodegradable polymers, for example, polylactides and polyglycolides. Therefore, Toxicological certificate of Institute of Medical Technique (Ministry of Health, Russia) approves of PHB for application in medicine as non-toxic and biocompatible mate ( Citation214). Given these findings, it is highly understandable that this family of polymers is biocompatible and ideal material for biomedical applications.
Physical properties
Within the cell, PHAs exist as mobile liquid, amorphous ( Citation26, Citation83, Citation87, Citation89, Citation91) and water-insoluble inclusions ( Citation43, Citation22). However, after extraction from the cell with organic solvents, PHAs become highly crystalline and show material properties that are similar to conventional commodity plastics such as polypropylene, polyethylene and polystyrene () ( Citation22, Citation215–217). Because, in vivo, non-covalently attached phasins as structural proteins stabilizing the PHA granules ( Citation30, Citation141) and inhibiting individual granules from coalescing and agglutinating with other granules ( Citation123, Citation145). In addition, water is a minor component of PHA inclusions and therefore it was suggested that water could act as a plasticizer ( Citation218, Citation219). About 5–10% of water was estimated to be present in the nascent PHB inclusions which upon removal allows for the polymer chains to rearrange into lamellar crystals ( Citation220). Based on this finding, it was suggested that water molecules could form hydrogen bonds with the carbonyl groups of the polyester backbone to form “pseudo cross-links” between adjacent polymer chains. This type of molecular arrangement may also explain the mobile amorphous nature of PHA inclusions as well as the plastic deformation phenomenon ( Citation94, Citation118, Citation220) observed in freeze-fracture experiments ( Citation221). But for extraction, when PHA inclusions are subjected to physical treatments such as centrifugation, they readily coalesce into larger masses and this can lead to the apparent acceleration of crystallization ( Citation222). Furthermore, any damage to the surface coating of the inclusion will allow heterogeneous nucleation, that is, crystallization induced by external molecules other than PHA, further accelerating crystallization ( Citation223). The definition of their thermal and mechanical properties is normally expressed in terms of the glass-to-rubber transition temperature (Tg) of the amorphous phase and the melting temperature (Tm) of the crystalline phase () ( Citation43, Citation217). The mechanical properties of PHA range from brittle to flexible and elastic, depending on the branched length of the HAs or from the distance between the ester linkages in the polymer backbones. Typically, PHAs with short pendant groups are hard crystalline materials, whereas PHAs with longer pendant groups are elastomeric () ( Citation29). The scl-PHA such as PHB is stiffer and more brittle than polypropylene phase ( Citation217, Citation224). Because of its brittleness, PHB is not very stress-resistant. Also, the relatively high Tm of PHB (around 170°C) is close to the temperature where this polymer decomposes thermally and thus limits the ability to process the homopolymer. However, copolymers consisting of HB and HV P(HB–HV) have better mechanical properties than homopolymer PHB () ( Citation10, Citation11, Citation216). Copolymers consisting of HB and HH are less stiff and brittle than PHB, thus improve the mechanical properties of P(HB–HV) even further ( Citation225), making them comparable with conventional plastics such as polypropylene, polystyrene, polyethylene terephthalate and high-density polyethylene (). Actually, when copolymer formation occurs with HB and HV monomer units, the properties of the material (Biopol; ICI) alter as a consequence of decreased crystallinity and Tm. These result, in mechanical terms, in a decrease in stiffness (Young's modulus) and an increase in toughness, producing more desirable properties for commercial application. Consequently, a range of properties is feasible from the hard and brittle homopolymer via a balance of stiffness and toughness to soft and tough for copolymers with a high incorporation of HV ( Citation26). Besides the mechanical properties, various HA monomer structures affect the PHA degradation rates. It has been reported that poly(3-hydroxybutyrate-co-4-hydroxybutyrate) had a much faster degradation rate than homopolymer PHB ( Citation178). mcl-PHAs are rubbery and flexible materials with low crystallinity and can be used in a wide range of applications which cannot be fulfilled by PHB and other scl-PHAs ( Citation223) but these physical properties of mcl-PHAs are considered unsuitable for applications due to their low Tm and Tg ( Citation226, Citation227). In contrast to PHB and P(HB–HV), mcl-PHAs have a much lower level of crystallinity and are more elastic ( Citation226, Citation228) but not strong enough for applications. These mcl-PHAs potentially have a different range of applications than the scl-PHAs. PHA with monomer chain lengths to 12 carbon atoms or more occur as sticky, more elastic and liquid ( Citation178, Citation229). However, in general, all the bacterial PHAs are (i) biodegradable thermoplastic and/or elastomeric compounds which can be processed with apparatus used by the plastic manufacturing industry without losing biodegradability. In addition, they are (ii) insoluble in water and resistance to hydrolytic degradation, (iii) highly crystalline, (iv) exhibit a rather high degree of polymerization ranging from 105 to almost 107 Da, (v) they are optically active and (vi) isotactic (enantiomerically pure chemicals consisting, in general, only of the R-stereoisomers). They are (vii) non-toxic, (viii) biocompatible and (ix) exhibit piezoelectric properties as revealed (at least) for poly(3HB) and poly(3HB-co-3HV) ( Citation7, Citation30, Citation50). These features make them highly competitive with polypropylene, the petrochemical-derived plastic ( Citation31, Citation216, Citation217, Citation230).
Table 1. Comparison of physical properties of various polyhydroxyalkanoates with conventional plastics.
Tunable hydrophilicity, hydrolytic stability and controlled degradation
All PHAs are susceptible to degrade by hydrolysis to some extent and under normal conditions they are water stable ( Citation231). But the high degree of polymerization, high crystallinity, isotacticity (only the enantiomer of absolute configuration R is present in these polymers) and hydrophobicity are among the factors limiting the bioavailability of PHAs which can affect the rate of biodegradation in natural environment ( Citation231, Citation232). PHA biodegradation corresponds to hydrolysis involving endo- or exo-enzymatic systems in the breaking cleavage of esters bonds. This type of degradation is needed for environmental applications. In the case of therapeutic and biomedical uses, a simple hydrolysis is required. Hydrolytic degradation of PHAs is not evident and is depending on the structure particularly on the nature of the side chains and materials crystallinity ( Citation231). Therefore, PHAs need to have tunable hydrophilicity, chemical functionalities and appropriate hydrolytic stability to expand their therapeutic applications towards more advanced areas. To overcome this issue, three methods have been reported so far. The first way to obtain more hydrophilic PHAs is synthetic strategies which consist in the preparation of unsaturated PHAs with polar functional groups such as carboxylic, hydroxyl or epoxy groups. The functionalization turns the reactive pendant double bonds into hydrophilic functions ( Citation231, Citation232). Functional PHAs with hydrophilic groups such as carboxylic, amine or hydroxyl on the side chains were prepared by biotechnological syntheses but the fermentation conditions are difficult and generally polymers are obtained with very low yields ( Citation231, Citation87). The most suitable products in regard to hydrolysis concern are PHAs containing carboxylic groups in side chains. Because, carboxylic groups promote water penetration into the polymer and participate in hydrolysis ester groups through better water penetration and catalysis ( Citation231). The second method is preparation of PHA-based water-soluble polymers by the block/graft copolymerization of PHAs with hydrophilic components in various polymeric architectures ( Citation232). In this case, these hydrophilic moieties/reactive functions are used for grafting oligomers of hydrolysable polylactic acid (PLA) or hydrophilic polyethylene glycol (PEG). Otherwise block copolymers with more crystalline polycaprolactone (PCL) are prepared, aiming at nanoparticles formation in the view of drug release. Therefore, the hydrophilic/hydrophobic balance of these materials is controlled by chemical modification and their stability/hydrolysis. The hydrophilic PEG could increase the particles hydrolysis whereas the hydrophobic PLA could increase their stability by internalization inside the core of the particles. Third method has been investigated to increase degradation by blending with other polymers or plasticizers which disrupt the polymer crystallinity, improve processability by lowering the processing temperature and consequently accelerated hydrolysis ( Citation231, Citation233). According to the presence of PEG or PLA in blend of PHB/HV with a hydrophilic polymer, PEG or a hydrolysable polyester, racemic poly(-lactic acid) PLA50 could increase the hydrolytic rate ( Citation233). From these reports, it is concluded that the combination of bioconversion and organic chemistry allows modulating more precisely the physical properties of these bacterial polymers such as solubility, hydrophilic/hydrophobic balance and bioavailability.
Lifespan of PHA compared to conventional plastics
It is widely accepted that the use of long-lasting polymers for short-lived applications (engineering, packaging, catering, surgery, hygiene) is not entirely adequate. Therefore, selection of type of plastic for certain manufacturing should be made considering the lifespan of plastic commodity on the environmental conditions. So that increasing environmental problems associated with discarded plastics can be controlled. These petroleum-based conventional plastics are very stable in harsh conditions especially against the attack of chemical degradation and microbial decomposition, thus rendering them durable, highly resistant and blessed with a very long lifespan in the environment ( Citation234), since the synthetic plastics are designed in a way to suit the constant performance and trustable qualities that are used for long life span, therefore causing them to be inert to natural and chemical breakdown ( Citation235). Further, the first development of plastics at the beginning of this century has been the goal of industry and science to improve the resistance of these materials to microbial attack. Therefore, conventional plastic materials have, in contrast to microbial PHAs, almost unlimited lifespan ( Citation236). Generally, natural degradation of conventional plastics begins with photodegradation, which leads to thermooxidative degradation ( Citation237). Ultraviolet light from the sun provides activation energy required to initiate incorporation of oxygen atoms into the polymer. This causes the plastic to become brittle and to break into smaller and smaller pieces, until the polymer chains reach sufficiently low molecular weight to be metabolized by microorganisms ( Citation237). The microbes either convert the carbon in the polymer chains to CO2 or incorporate it into biomolecules. However, this entire process is very slow, and it can take 50 or more years for plastic to fully degrade ( Citation238). For these different reasons, reaching the conditions of conventional plastic replacements by degradable polymers, particularly for short-term applications (packaging, agriculture … ), is of major interest to the society as a whole, from the plastic industries to the citizens.
Although, microbial PHAs like conventional plastics are also thermoplastics, moldable, naturally UV-resistant and could be tailor-made for numerous applications ranging from stiff packaging goods to highly elastic materials for coatings. But their complete biodegradability in the environment and naturalness make them quite beneficial in certain short-lifespan applications for single-use packaging, catering, surgery, hygiene, drug delivery and agriculture ( Citation239). But according to Beucker and Marscheider-Weidemann bioplastic can have a short or a long lifespan depending on the material or the compound used to produce them ( Citation240). Degradation of commercially available shampoo bottles and film made from PHA (Biopol@ bottles; molecular composition: 92% 3-HB, 8% 3-HV, the inner and outer surface areas of the bottles = 336 and 356 cm2, respectively, the average mass = 31.90 g (n = 13) in depth of 85 m of an aquatic ecosystem (Lake Lugano, Switzerland) under natural conditions for 250 days has measured the degradation rates of 10–20 mg/d giving an average lifespan of 5–10 years for the specific bottle type ( Citation241). The degradation rates vary with the water depth, that is, with the spatial heterogeneity of the ecosystem. Bottles made from polyethylene–starch (PE + S; 9% and 13% starch, respectively) blends showed no degradation at all. In order to reduce the challenging burdens of currently being used plastic on health and environment, it is needed to determine the lifespan of plastic products in the real usage conditions.
Applications of PHA
Bacterial PHA first received commercial attention way back in the early 1960s from an American company, W.R. Grace, but apparently was discontinued due to some technical problems ( Citation242). Although patents were originally filed in the USA by Baptist J.N. in 1962, but first industrial production of PHB and PHA did not occur until 1982. The major petroleum crisis of the 1970s then motivated a British company, ICI Bioproducts (now known as Zeneca Bio Products) to get involved with this bacterial polymer and marketed them under the trade name Biopol ( Citation26). Besides, currently a number of companies are developing PHAs for use in plastics worldwide including Kaneka in Japan, and P&G Chemical, and BP and Metabolix in the USA. Kaneka and P&G Chemical have teamed up to commercialize a product “Nodax” (also known as Nodak™, which is a specialized PHA). Nodak™ has already been made into a variety of different prototype objects such as plastic fiber or twine and molded plasticware such as plates and cups. Metabolix is already producing preliminary PHA materials, but is teaming up with BP for two years to produce bioplastics ( Citation243). In response to an increased awareness of global environmental problems, PHA is gaining serious attention as a potential substitute for non-biodegradable polymers. Now, the advances in PHA production have opened up the potential opportunities to a number of applications ( Citation29). The production of PHA is intended to replace synthetic non-degradable polymers for applications especially in packaging, agriculture, leisure, fast-food, hygiene as well as medicine and biomedical since PHA is biocompatible and degradable.
Packaging, molding and coating
PHAs are natural and biodegradable thermoplastic polyesters, and hence the majority of their applications are as replacements for petrochemical polymers currently in use for packaging and coating applications ( Citation26, Citation31, Citation230, Citation244). They can be used in a wide variety of products including bags, containers, paper coatings, pens, golf tees, razors, feminine hygiene products, diaper backsheets, utensils, cosmetic containers, bottles, cups and materials for food packaging ( Citation26, Citation28, Citation31, Citation38, Citation190). The latex of PHAs can be used to produce a water-resistant layer for paper, film or cardboard ( Citation190, Citation245). Manufacturers in the USA used PHB and copolymer P(HB–HV) as water-proof films on the back of diaper sheets ( Citation31, Citation246). This copolymer P(HB–HV), with flexibility and impact resistance, was marketed under the trade name Biopol™ by ICI/Zeneca and later by Monsanto till 1995. PHAs can also be used to replace petrochemical polymers in toner and developer compositions or as ion-conducting polymers. PHAs can be used as a latex, for instance for paper-coating applications ( Citation26). New plastic properties can be achieved by blending PHA with other polymers and there is much activity in this field ( Citation28, Citation30, Citation247, Citation248). Often the mixture changes the crystallinity of the plastic, and crystallization rate and finally also the mechanical properties of the material. Thus, a mixture of 40–60% of PCL in PHB improved the mechanical properties over P(HB–HV). A 40% PCL and 60% PHB mixture had a decreased oxygen permeability that was only 5% of that of polyethylene ( Citation249). Maekawa and coworkers reported that blends of PHB and cellulose propionate were completely miscible since they had a single glass transition, a depression in the equilibrium melting temperature of PHB and a decrease in spherulitic growth rate of the PHB component. Also tensile strength was better for the blend of PHB/cellulose propionate than for PHB only ( Citation250). However, not all blends are compatible.
(2) Medical and pharmaceutical applications
In addition to their biodegradability, many PHAs are also biocompatible. Their breakdown products are 3-hydroxyacids, which are naturally found in animals. Such as PHB is biocompatible, which is not surprising when considering the fact that R-3-hydroxybutyric acid is a normal constituent of blood at concentrations between 0.3 and 1.3 mM ( Citation31, Citation205, Citation206) and is also found in the cell membrane of eukaryotes ( Citation207). These PHAs have the potential to become important and very useful compounds for medical applications such in wound management used as surgical suture, skin substitutes, nerve cuffs, surgical meshes, implants, gauzes, staples, gauzes, swab, lubricating powders ( Citation29, Citation30, Citation38), blood vessels, tissue scaffolds and bone fracture fixation plates ( Citation3, Citation251, Citation252). On account of their varied and diverse properties, the PHA biopolymers provide a three-dimensional scaffold means to support the cell growth and then degrade away leaving viable tissue, with potential product applications for the cardiovascular system, cornea, pancreas, gastrointestinal system, kidney and genitourinary system, musculoskeletal system, nervous system, teeth and oral cavity, skin and so forth ( Citation29, Citation32). In orthopaedy, PHAs can also be used as scaffolds for cartilage engineering, bone graft substitutes and spinal cages. PHAs can be used to engineer the heart valves, cardiovascular fabrics, pericardial patches and vascular grafts ( Citation3, Citation30, Citation32, Citation253). PHAs are also useful as stereoregular compounds which can serve as chiral precursors for the chemical synthesis of optically active compounds ( Citation32, Citation254). Such compounds are particularly used to synthesize the micro- and nanosphere of PHAs as biodegradable carriers or drug delivery systems for controlled release of therapeutics such as medicines, hormones, insecticides and herbicides into systemic circulation are receiving attention ( Citation30, Citation31, Citation253, Citation255, Citation256). According to Sudesh et al., PHAs have the oil absorption and retention capacities which offer commercial application value in the cosmetics and skin care industries ( Citation257). Voinova et al. have investigated the possibility of use of PHAs as a biodegradable carrier for pesticides (α-hexachlorocyclohexane and lindane) for targeted and controlled delivery of these compounds to soil. According to their investigation, pesticides embedded in a PHA carrier are released gradually and slowly, without surges, as the polymer is degraded by the soil microflora ( Citation258).
They are also used as osteosynthetic materials in the stimulation of bone growth owing to their piezoelectric properties, in bone plates and blood vessel replacements ( Citation31). PHA can be easily depolymerized to a rich source of optically active, pure, bi-functional hydroxy acids. PHB for instance is readily hydrolyzed to R-3-hydroxybutyric acid and used in the synthesis of Merck's anti-glaucoma drug “Truspot”. In tandem with R-1, 3-butanediol, it is also used in the synthesis of β-lactams ( Citation31). However, currently, the medical and pharmaceutical applications of PHAs are limited due to the slow biodegradation and high hydraulic stability in sterile tissues ( Citation259). The presence of 150 different monomers identified yet and new types of PHA through the chemical or physical modification of naturally occurring PHA gave rise to diverse properties. With these features PHA is also considered as pharmaceutically active compound and currently investigated as potential anti-HIV drugs, anti-cancer drugs, antibiotics, etc. ( Citation58).
PHAs appear to be potentially useful in controlling bacterial pathogens in certain aquaculture applications ( Citation260). For example, administration in the feed of 1000 mg/l of PHB particles of an average diameter of 30 μm, or addition of inactivated cells (107 cells ml−1) of PHB-containing Brachymonas bacteria (equivalent to 10 mg L−1 PHB) to the culture water of brine shrimp (Artemia nauplii) larvae, conferred a complete protection from a virulent strain of the intestinal pathogen Vibrio campbellii ( Citation261). Other similar reports have claimed an inhibitory effect of PHB on certain gut microflora of the giant freshwater prawn (Macrobrachium rosenbergii) larvae ( Citation260). Administration of PHB in the feed significantly increased the survival of the prawn larvae and improved their development. The total bacterial counts and Vibrio spp. counts were significantly reduced in PHB-fed larvae compared to the control larvae ( Citation3).
(3) Denitrification in water and wastewater treatment
A promising application of PHAs is as the solid substrate for denitrification of water and wastewater. This type of denitrification, termed here “solid-phase denitrification”, has several advantages over the conventional system supplemented with liquid organic substrate. PHAs serve not only as constant sources of reducing power for denitrification but also as solid matrices favorable for development of microbial films ( Citation189). In addition, in contrast to conventional processes, the use of PHAs has no potential risk of release of dissolved organic carbon with the resultant deterioration of effluent water quality. Denitrification processes using PHAs actually give high rates of nitrogen removal ( Citation262). Due to its oil absorption capacities, PHB has been successfully tested for removing lipid-soluble organic pollutants from water ( Citation257, Citation263).
(4) Miscellaneous applications
PHAs with long side chain hydroxyacids have been used in pressure-sensitive adhesive formulations. PHAs can be used to produce dairy cream substitutes or flavor delivery agents in foods. Moreover, PHAs are also used to produce fiber materials, such as non-woven fabrics ( Citation7). The PHAs are considered as a source for the synthesis of chiral compounds (enantiometrically pure chemicals) and are raw materials for the production of paints ( Citation31, Citation32, Citation264). In addition to its range of material properties and resulting applications, PHAs promise to be a new source of small molecules. PHA can be hydrolyzed chemically, and the monomers can be converted to commercially attractive molecules such as β-hydroxy acids, 2-alkenoic acids, β-hydroxyalkanols, β-acyllactones, β-amino acids and β-hydroxyacid esters ( Citation265). The last class of chemicals is currently receiving attention because of potential applications as biodegradable solvents ( Citation7). Besides helping to replace the existing solvents, β-hydroxy acid esters and derivatives are likely to find growing use as “green solvents” similar to lactic acid esters. The conversion of hydroxy acids into crotonic acids such as 1,3-butanediol, lactones, etc. would help improve the market value as they have an existing market demand in thousands of tones ( Citation31).
Economical aspects
One of the problems preventing the pilot-scale production, commercial application and wide use of PHA as commodity plastics is its high production cost. High production cost has hindered the use of PHAs, since their final price is considerably much higher than that of petrochemical-based synthetic plastic materials ( Citation30, Citation40, Citation41, Citation158, Citation266, Citation267). Factors involved include the product yield, complexity of the technology and hence the capital cost of the plant, and the ease or difficulty of product separation. Consequently, selection of the organism and substrate can critically influence costs ( Citation26, Citation267). For example, PHB from Gram-negative organisms are widely used in a variety of products but due to endotoxin in the outer membrane lipopolysaccharides (LPS), Bacillus species which lack LPS are extensively used in biopharmaceutical applications ( Citation70) but compared to Gram-negative bacteria, Gram-positive bacteria were mostly found to produce scl-PHA and at lower PHA contents between about 2% and 50% CDM, that is why Gram-positive bacteria have yet to be adopted for commercial PHA production ( Citation58). Further, Koller et al. have suggested to link ecology with economy, because bacteria residing at ecological niches like estuarine sediments, marine microbial mats, rhizosphere, groundwater sediments and engineered ecosystems with fluctuating nutrient contents support the population actively involved in PHA accumulation to meet the metabolic energy requirements during starvation period and this concept can be implemented industrially to reduce the cost of biopolymers commercially with sustainable production processes ( Citation268). Therefore, it is economical to use naturally producing microorganism due to safety and constant productivity rate, and there is great potential for producing PHAs in low-cost substrates, which can reduce PHAs production costs, because the cost of substrate is the most important factor for PHAs production ( Citation269–271). Moreover, novel-constituent polymers will reduce the unfavorable characteristics of PHA. The cost of raw material itself accounts for 40–50% of the total production cost. Recovery of polymer from the biomass is a vital stage of the process. Large-scale solvent extraction, although giving high recovery, is an expensive technique and involves large volumes of solvent and high capital investment in the solvent recovery plant ( Citation26). From an economical point of view, the price of the product ultimately depends on the substrate (mainly carbon source) cost, PHA yield on the substrate and the efficiency of product formulation in the downstream processing ( Citation31, Citation107). It is a prerequisite to standardize all the fermentation conditions, and physical and chemical parameters for the successful implementation of commercial PHA production systems ( Citation269–273). PHAs production costs can be reduced by several means, including the use of cheap substrates, such as whey, favored in countries with important dairy industries, or the enhancement of product yield, for example by using recombinant E. coli ( Citation7, Citation35, Citation267, Citation274). Molasses, from either sugarcane or beet, is one potential cheap carbon source for PHA production. B. cereus grown in 1% (w/v) beet molasses produced P(3HB) concentration of 0.1 g/l and a polymer content of 73.8%, whereas in 3% molasses P(3HB) was increased by 70% but the polymer content was halved ( Citation275). Another sucrose-rich inexpensive substrate is sugarcane liquor, which was used by Jiang et al. to produce P(3HB) using P. fluorescens ( Citation276). In a 5-l batch bioreactor, Castilho et al. obtained 22 g/l of P(3HB), at a 70% polymer content ( Citation277). Santimano et al. reported a comparative use of low-cost agro-industrial wastes (rice chaff, citrus pulp, coconut oil cake, cotton seed cake, cane molasses, bakery waste) for the production of PHA by Bacillus sp. strain COL1/A6. The highest PHA content was obtained from hydrolyzed wafer residues (62.41 ± 1.04% of dry cell wt.), followed by cane molasses (54.68 ± 1.36% of dry cell wt.) and hydrolyzed citrus pulp 47.5 ± 1.01% of dry cell wt.) ( Citation278). Using organic matter from wastes and wastewaters can be used as a substrate for the production of PHB, with combined advantages of reducing disposal cost and production of value-added products. A hydrolysis step is commonly needed before inoculation with pure cultures ( Citation279, Citation280). However, so far, only low PHB content and productivity were achieved from waste products ( Citation264). Since, the harsh conditions like nutrient limitation and stress are necessary to trigger PHA production and intracellular accumulation, therefore, environmental parameters such as pH and temperature have significant effects on microbial growth and PHA production ( Citation281). Further, there is diversity among bacteria from different ecological habitats in their PHA production, modes of growth, type of microorganisms and media ingredients. But, the most of standardization studies on PHA-producing bacteria, including Gram-negative and Gram-positive species, have reported the optimum pH 7 and incubation at 30–37°C for maximum yield ( Citation282–287).
Additionally, the biochemical and metabolic activities including the ability to utilize different carbon and nitrogen sources, and hydrolytic enzyme (amylase, lipase and protease) activities of bacterial candidates were also reported to play an important role in PHA synthesis. Therefore, it is economical to use microbes which produce a variety of enzymes to solubilize waste composites and cheap substrates. According to Sangkharak and Prasertsan, bacteria belonging to genera Bacillus, Aeromonas sp. and Alcaligenes sp. had ability to produce high amounts (2–6.58 g/l) of PHAs and high levels of hydrolytic enzyme activities which may result in efficient utilization of municipal wastewater, palm oil mill effluent, glycerol and molasses, and its conversion into PHAs with a variety of PHA monomers ( Citation270).
To reduce the substrate cost, recombinant strains utilizing a cheap carbon source and corresponding fermentation strategies have also been developed ( Citation177, Citation266). E. coli harboring phaABC and phaP of Azotobacter sp. is a suitable host as a heterologous expression background for foreign genes that can be easily manipulated and improved by means of recombinant DNA methodologies. Also, high-cell-density cultivation strategies for numerous E. coli strains are well established ( Citation41, Citation288). E. coli cells that accumulate large amounts of PHB become fragile, facilitating the isolation and purification of the biopolymer, and the bacterium does not express PHA-degrading enzymes ( Citation34, Citation110). However, there are at least two disadvantages in using E. coli. First, E. coli does not contain a PHA precursor-providing pathway, so that a new pathway has to be constructed to provide precursors for PHA synthesis. Second, the metabolic background is quite different between E. coli and native PHA production strains. Many PHA-producing strains can accumulate PHA from fatty acids, while E. coli grows poorly on fatty acid culture medium ( Citation289, Citation290). Therefore, application of E. coli as the host to produce PHA has not been the best option.
At present, the application of defined co-cultures for PHA production is still in its infancy. For single-stage co-culture fermentation systems, one of the main challenges is providing cultivation parameters for efficient and effective bioconversion of carbon substrates into PHA. Parameters such as inoculum concentration, dissolved oxygen, pH, temperature, cultivation time, carbon and nutrients feed rate, and secondary metabolites production rate would need to be fine-tuned in order to maximize the bioconversion process. Another challenge is the harvesting and separation of PHA-containing biomass from non-PHA-containing biomass, particularly for co-cultures comprising PHA-accumulating and non-PHA-forming microorganisms as the presence of non-PHA-containing biomass would increase the extraction cost of PHA. Compared to single-stage fermentation approach, two-stage fermentation approach may be more advantageous as it enables finer control over cultivation parameters and harvesting of PHA-accumulating biomass. However, higher capital and operation cost are associated with two-stage fermentation system. Ultimately, the type of systems chosen would greatly depend on the microbial characteristics of the co-culture as well as the economic viability of the bioprocess ( Citation58).
The cost of PHA using the natural producer A. eutrophus is US$15–30 per Kg ( Citation30, Citation291) which is 18 times more expensive than polypropylene. With recombinant E. coli as a producer of PHA, price can be reduced to US$4 per Kg, which is close to other biodegradable plastic materials such as PLA and aliphatic polyesters. The commercially viable price should come to US$3–5 per Kg ( Citation266). Although there are presently economic disadvantages and limited uses of bacterial plastics, there is currently a high amount of research devoted to improve productivity, to reduce production costs, and, more importantly, to produce specific functionalized PHAs.
Conclusion and future prospects
The intracellular accumulation of PHAs as hydrophobic granules of energy/or carbon storage materials under stress conditions and its mobilization on return of conditions for normal growth is a most widely spread phenomenon among bacterial flora. PHAs are degradable within host cells and also biologically degraded under both aerobic and anaerobic conditions by naturally existing microorganisms in natural environment into carbon dioxide and water, which return to the environment. Bacteria produce a wide range of different PHAs with varying monomer compositions depending on the substrate specificities of PHA synthases, carbon sources and the metabolic pathways. All PHAs extracted from bacterial cells exhibit varying physical properties range from brittle to flexible to elastic which make PHAs attractive for substitution to petrochemical thermoplastics. Due to production from renewable resources, a wide range of mechanical properties, biocompatibility, complete biodegradability and potential chemical pools for useful chiral compounds, PHAs have been drawn much attention for broad commercial applications. But a major problem in the commercialization of PHAs in a wide range of applications is their high production cost which has restricted its potential applications. So, it is desirable to find out new and low-cost ways to produce PHAs with superior qualities and quantities. Also in a world with shrinking petroleum reserves and increasing environmental issues, PHAs are definitely potential candidates that deserve further exploration. The challenge for the future application of the PHA polymers depends mostly on the increase in the production levels of these polymers with the desired various properties in an economical fashion. This needs improvement of the current technology for the whole process from the start to the final step. This suggests the selection and development of bacterial strains that are capable of efficient consumption and transformation of various substrates into a range of PHAs with different properties, at high yield and productivity; high performance fermentations, and efficient extraction and purification to lower the price. The utilization of local cheap substrates such as agri-waste, the cultivation processes combining batch and fed-batch fermentations might give the highest productivity compared to the other reported methods. However, considering the controllable nature of chemostat, it has the greatest potential to provide higher productivities. Therefore, currently much research effort has been devoted to lower the production cost and meet the target pricing by developing more efficient fermentation routes, recovery processes and recombinant organisms. This field of research requires further investigation in future to enhance the productivity and lower the production costs to make it more competitive. All efforts at laboratory scale will need to be validated at pilot scale for future industrial production. The challenges of scale-up process might put a question mark against those procedures and processes that have been proposed to be promising. So, it is expected that the ongoing research all over the world soon shall achieve potential PHAs with acceptable prices in near future.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hayden, K.W.; Arnott, J.; Russell, J.C.; Elena, P.I. Polymers. 2013, 5, 1–18.
- Kalaivani, R.; Sukumaran, V. Eur. J. Exp. Biol. 2013, 3(3), 56–64.
- Gumel, A.M.; Annuar, M.S. M.; Chisti, Y. J. Polym. Environ. 2013, 21, 580–605.
- Dawes, E.A.; Senior, P.J. Adv. Microb. Physiol. 1973, 10, 135–266.
- Hänggi, U.J. Pilot Scale Production of PHB with Alcaligenes latus. In Novel Biodegradable Microbial Polymers: Dawes E.A., Ed.; Kluwer Publishers: Dordrecht, The Netherlands, 1990; pp 65–70.
- Janes, B.; Hollar, J.; Dennis, D. Molecular Characterization of the Poly-β-Hydroxybutyrate Biosynthetic Pathway of Alcaligenes Eutrophus H16. In New Biosynthetic Biodegradable Polymers of Industrial Interest From Microorganisms; Kluwer Publishers: Amsterdam, 1990; pp 175–190.
- Madison, L.L.; Huisman, G.W. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53.
- Berlanga, M.; Montero, M.T.; Fernandez-Borrell, J.; Guerrero, R. Inter. Microbiol. 2006, 9, 95–102.
- Goh, Y.S.; Tan, I.K.P. Microbiol. Res. 2012, 167, 211–219.
- Steinbüchel, A. Acta. Biotechnol. 1991, 11, 419–427.
- Steinbüchel, A. Polyhydroxyalkanoaic Acids. In Biomaterials, Novel Materials from Biological Sources; Byrom, D., Ed.; Stockton Press: New York, 1991; pp 123–213.
- Yu, J. J. Biotechnol. 2001, 86, 105–112.
- Du, G.; Yu, J. Environ. Sci. Technol. 2002, 36, 5511–5516.
- Shilalipi, S.; Nirupama, M. J. Appl. Phycol. 2012, 24(4), 803–814.
- Steinbüchel, A.; Hustede, E.; Liebergesell, M.; Pieper, U.; Timm, A.; Valentin, H. FEMS Microbiol. Rev. 1992, 103, 217–230.
- Byrom, D. Polyhydroxyalkanoates. In Plastic From Microbes, Microbial Synthesis of Polymers and Polymer Precursors; Mobley, D.P., Ed.; Hanser: Munich, Gremany, 1994; pp 5–33.
- Stapp, C. Zentbl. Bakteriol II. 1924, 61, 276–292.
- Lemoigne, M. Bull. Soc. Chimique Biol. 1926, 8, 770–782.
- Lemoigne, M. Ann. Inst. Pasteur. 1927, 41, 148–165.
- Lemoigne, M.; Delaporte, B.; Croson, M. Ann. Inst. Pasteur. 1944, 70, 224–233.
- Macrae, R.M.; Wilkinson, J.R. J. Gen. Microbiol. 1958, 19, 210–222.
- Byrom, D. Trends Biotechnol. 1987, 5, 246–250.
- Poirier, Y.; Dennis, D.E.; Klomparens, K.; Sommerville, C. Science. 1992, 256, 520–523.
- Page, W.J. Can. J. Microbiol. 1995, 41, 1–3.
- de Koning, G.J.M.; Kellerhals, M.B.; van Meurs, C.; Witholt, B. J. Environ. Polym. Degrad. 1996, 4, 243–252.
- Anderson, A.J.; Dawes, E.A. Microbiol. Rev. 1990, 54, 450–472.
- Page, W.J. FEMS Microbiol. Rev. 1992, 103, 149–157.
- Ojumu, T.J.; Solomon, B.O. Afr. J. Biotechnol. 2004, 3(1), 18–24.
- Williams, S.F.; Martin, D.P.; Horowitz, D.M.; Peoples, O.P. Int. J. Biol. Macromol. 1999, 25, 111–121.
- Zinn, M.; Witholt, B.; Egli, T. Adv. Drug Deliv. Rev. 2001, 53, 5–21.
- Reddy, C.S.K.; Ghai, R.; Rashmi, K.V.C. Bioresour. Technol. 2003, 87, 137–146.
- Chen, G.Q.; Wu, Q. Appl. Microbiol. Biotechnol. 2005a, 67, 592–599.
- Steinbüchel, A. Curr. Opin. Biotechnol. 2005, 16, 607–613.
- Nikel, P.I.; deAlmeida, A.; Melillo, E.C.; Galvagno, M.A.; Pettinari, M.J. Appl. Environ. Microbiol. 2006, 72(6), 3949–3954.
- Khanna, S.; Srivastava, A.K. Proc. Biochem. 2005, 40, 607–619.
- Jendrossek, D.; Schirmer, A.; Schlegel, H.G. Appl. Microbiol. Biotechnol. 1996, 46, 451–463.
- Müller, H.M.; Seebach, D. Ang. Chemie. Inter. Eng. 1993, 32, 477–502.
- Hocking, P.J.; Marchessault, R.H. In Chemistry and Technology of Biodegradable Polymers: Griffin, G.J.L., Ed.; Blackie Academic: New York, 1994; pp 48–96.
- Steinbüchel, A. Biotechnology. In PHB and Other Polyhydroxyalkanoic Acids: Rehm, H. J., Reed, G., Eds.; Wiley-VCH: Weinheim, 1996; pp 405–464.
- Steinbüchel, A.; Füchtenbusch, B. Trends Biotechnol. 1998, 16, 419–427.
- Shiloach, J.; Fass, R. Biotechnol. Adv. 2005, 23, 345–357.
- Linko, S.; Vaheri, H.J.S. Enzym. Microb. Technol. 1993, 15, 401–406.
- Sudesh, K.; Abe, H.; Doi, Y. Prog. Polym. Sci. 2000, 25, 1503–1555.
- Platt, D. Biodegradable Polymers; Woodhead Publishing Ltd: Cambridge, 2006.
- Rehm, B.H.A. Biotechnol. Lett. 2006, 28, 207–213.
- Dias, J.M.; Lemos, P.C.; Serafim, L.S.; Oliveira, Eiroa, C.M.; Albuquerque, M.G.E.; Ramos, A.M.; Oliveira, R.; Reis, M.A.M. Macromol. Biosci. 2006, 6, 885–906.
- Full, T.D.; Jung, D.O.; Madigan, M.T. Lett. Appl. Microbiol. 2006, 43, 377–384.
- Li, R.; Zhang, H.; Qi, Q. Bioresour. Technol. 2007, 98, 2313–2320.
- Lee, S.Y.; Lee, Y.; Wang, F. Biotechnol. Bioeng. 1999, 65, 363–368.
- Poirier, A. Macromol. Biosci. 2001, 1, 1–24.
- Doi, Y.; Abe, C. Macromolecules. 1990, 23, 3705–3707.
- Fritzsche, K.; Lenz, R.W.; Fuller, R.C. Macromolecules. 1990, 12, 85–91.
- Fritzsche, K.; Lenz, R.W.; Fuller, R.C. Int. J. Biol. Macromol. 1990, 12, 92–101.
- Fritzsche, K.; Lenz, R.W.; Fuller, R.C. Makromol. Chem. 1990, 191, 1957–1965.
- Kim, B.S.; Lee, S.Y.; Chang, H.N. Biotechnol. Lett. 1992, 14, 811–816.
- deSmet, M.J.; Eggink, G.; Witholt, B.; Kingma, J.; Wynberg, H. J. Bacteriol. 1983, 154, 870–878.
- Hai, T.; Lange, D.; Rabus, R.; Steinbüchel, A. Appl. Environ. Microbiol. 2004, 70, 4440–4448.
- Tan, G.A.; Chen, C.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J. Polymers. 2014, 6, 706–754.
- Brandl, H.; Knee, E.J.; Fuller, R.C.; Gross, R.A.; Lenz, R.W. Int. J. Biol. Macromol. 1989, 11, 49–55.
- Haywood, G.W.; Anderson, A.J.; Williams, G.A.; Dawes, E.A.; Ewing, D.F. Int. J. Biol. Macromol. 1991, 13, 83–88.
- Liebergesell, M.; Hustede, E.; Timm, A.; Steinbüchel, A.; Fuller, R.C.; Lenz, R.W.; Schlegel, H.G. Arch. Microbiol. 1991, 155, 415–421.
- Kato, M.; Bao, H.J.; Kang, C.-K.; Fukui, T.; Doi, Y. Appl. Microbiol. Biotechnol. 1996, 45, 363–370.
- Kato, M.; Fukui, T.; Doi, Y. Bull. Chem. Soc. Japan. 1996, 69, 515–520.
- Lee, S.H.; Oh, D.H.; Ahn, W.S.; Lee, Y.; Choi, J.; Lee, S.Y. Biotechnol. Bioeng. 2000, 67, 240–244.
- Schlegel, H.G.; Lafferty, R.; Krauss, I. Arch. Mikrobiol. 1970, 71, 283–294.
- Reddy, S.V.; Thirumala, M.; Reddy, T.V.K.; Mahmood, S.K. World J. Microbiol. Biotechnol. 2008, 24, 2949–2955.
- Redzwan, G.; Gan, S.-N.; Tan, I.K.P. World J. Microbiol. Biotechnol. 1997, 13, 707–709.
- Ostle, A.G.; Holt, J.G. Appl. Environ. Microbiol. 1982, 44, 238–241.
- Bhuwal, A.K.; Singh, G.; Aggarwal, N.K.; Goyal, V.1.; Yadav, A. Int. J. Biomater. 2013, 2013, 1–10.
- Charen, T.; Vaishal, I.P.; Kaushalya, M.; Amutha, K.; Ponnusami, V.; Gowdhaman, D. Int. J. ChemTech. Res. 2014, 6(5), 3197–3202.
- Gorenflo, V.; Steinbuchel, A.; Marose, S.; Rieseberg, M.; Scheper, T. Appl. Microbiol. Biotechnol. 1999, 51, 765–772.
- Kranz, R.G.; Gabbert, K.K.; Madigan, M.T. Appl. Environ. Microbiol. 1997, 63, 3010–3013.
- Spiekermann, P.; Rehm, B.H.A.; Kalscheuer, R.; Baumeister, D.; Steinbuchel, A. Arch. Microbiol. 1999, 171, 73–80.
- Minghsun, L.; Juan, E.G.; Laura, B.W.; Walker, G.C. Appl. Environ. Microbiol. 1998, 64(11), 4600–4602.
- Degelau, A.; Scheper, T.; Bailey, J.E.; Guske, C. Appl. Microbiol. Biotechnol. 1995, 42, 653–657.
- Müller, S.; Losche, A.; Bley, T.; Scheper, T. Appl. Microbiol. Biotechnol. 1995, 43, 93–101.
- Akerlund, T.; Nordstrom, K.; Bernander, R. J. Bacteriol. 1995, 177, 6791–6797.
- Sheu, D.S.; Wang, Y.T.; Lee, C.Y. Microbiology. 2000, 146, 2019–2025.
- Shamala, T.R.; Chandrashekar, A.; Vijayendra, S.V.N.; Kshama, L. J. Appl. Microbiol. 2003, 94, 369–374.
- Hong, K.; Sun, S.Q.; Tian, W.D.; Chen, G.Q. Appl. Microbiol. Biotechnol. 1999, 51, 523–526.
- Solaiman, D.K.Y.; Ashby, R.D.; Foglia, T.A. Appl. Microbiol. Biotechnol. 2000, 53, 690–694.
- Khan, A.B.; Khattak, M.I.; Tarar, O.M.; Habib, F.; Jamil, K.; Yasmin, A.; Parvez, S. Braz. Arch. Biol. Technol. 2013, 56(4), 645–652.
- Ciesielski, S.; Cydzik-Kwiatkowska, A.; Pokoj, T.; Klimiuk, E. J. Appl. Microbiol. 2006, 101, 190–199.
- Ciesielski, S.; Górniak, D.; Mo-zejko, J.; Świątecki, A.; Grzesiak, J.; Zdanowski, M. Curr. Microbiol. 2014, 69, 594–603.
- Ciesielski, S.; Pokoj, T.; Mo-zejko, J.; Klimiuk, E. Pol. J. Microbiol. 2013, 62(1), 45–50.
- Berlanga, M.; Montero, M.T.; Fernandez-Borrell, J.; Guerrero, R. Int. Microbiol. 2006, 9, 95–102.
- Lenz, R.W.; Kim, Y.B.; Fuller, R.C. FEMS Microbiol. Rev. 1992, 103, 207–214.
- Han, J.; Hou, J.; Liu, H.; Cai, S.; Feng, B.; Zhou, J.; Xiang, H. Appl. Environ. Microbiol. 2010, 76, 7811–7819.
- Matsusaki, H.; Manji, S.; Taguchi, K.; Kato, M.; Fukui, T.; Doi, Y. J. Bacteriol. 1998, 180, 6459–6467.
- Anderson, A.J.; Haywood, G.W.; Dawes, E.A. Int. J. Biol. Macromol. 1990, 12, 102–105.
- Doi, Y.; Kunioka, M.; Nakamura, Y.; Soga, K. Macromolecules. 1988, 21, 2722–2727.
- Friedrich, B.; Hogrefe, C.; Schlegel, H.G. J. Bacteriol. 1981, 147, 198–205.
- Shimamura, E.; Kasuya, K.; Kobayashi, G.; Shiotani, T.; Shima, Y.; Doi, Y. Macromolecules. 1994, 27, 878–880.
- Doi, Y.; Kitamura, S.; Abe, H. Macromolecules. 1995, 28, 4822–4828.
- Steinbüchel, A.; Wiese, S. Appl. Microbiol. Biotechnol. 1992a, 37, 691–697.
- Abe, H.; Doi, Y.; Fukushima, T.; Eya, H. Int. J. Biol. Macromol. 1994, 16, 115–119.
- Boyandin, A.N.; Kalacheva, G.S.; Rodicheva, E.K.; Volova, T.G. Microbiology. 2008, 77(3), 318–323.
- Fukui, T.; Shiomi, N.; Doi, Y. J. Bacteriol. 1998, 180, 667–673.
- Timm, A.; Byrom, D.; Steinbüchel, A. Appl. Microbiol. Biotechnol. 1990, 33, 296–301.
- Preusting, H.; Kingma, J.; Huisman, G.; Steinbüchel, A.; Witholt, B. J. Environ. Polym. Degrad. 1993, 1, 11–21.
- Timm, A.; Steinbuchel, A. Appl. Environ. Microbiol. 1990, 56, 3360–3367.
- Lee, E.Y.; Jendrossek, D.; Schirimer, A.; Choi, C.Y.; Steinbüchel, A. Appl. Microbiol. Biotechnol. 1995, 42, 901–909.
- Liebergesell, M.; Mayer, F.; Steinbüchel, A. Appl. Microbiol. Biotechnol. 1993, 40, 292–300.
- Reusch, R.N.; Sadoff, H.L. Proc. Nat. Acad. Sci. USA. 1988, 85, 4176–4180.
- Reusch, R.N.; Huang, R.P.; Bramble, L.L. Biophys. J. 1995, 69, 754–766.
- Reusch, R.N. Biochemistry. 1999, 38, 15666–15672.
- Lee, S.Y. Biotechnol. Bioeng. 1996, 49, 1–14.
- Du, G.; Si, Y.; Yu, J. Biotechnol. Lett. 2001, 23, 1613–1617.
- Huisman, G.W.; Wonink, E.; Meima, R.; Kazemier, B.; Terpstra, P.; Witholt, B. J. Biol. Chem. 1991, 266, 2191–2198.
- Aldor, I.S.; Keasling, J.D. Curr. Opin. Biotechnol. 2003, 14, 475–483.
- Hoffmann, N.; Amara, A.A.; Beermann, B.B.; Qi, Q.; Hinz, H.J.; Rehm, B.H.A. J. Biol. Chem. 2002, 277, 42926–42936.
- Rehm, B.H.A.; Krüger, N.; Steinbüchel, A. J. Biol. Chem. 1998, 273, 24044–24051.
- Gerngross, T.U.; Martin, D.P. Proc. Nat. Acad. Sci. USA. 1995, 92, 6279–6283.
- Jossek, R.; Steinbüchel, A. FEMS Microbiol. Lett. 1998, 168, 319–324.
- Qi, Q.; Steinbüchel, A.; Rehm, B.H.A. Appl. Microbiol. Biotechnol. 2000, 54, 37–43.
- Rehm, B.H.A.; Qi, Q.; Beermann, B.B.; Hinz, H.J.; Steinbüchel, A. Biochem. J. 2001, 358, 263–268.
- Grage, K.; Jahns, A.C.; Parlane, N.; Palanisamy, R.; Rasiah, I.A.; Atwood, J.A.; Rehm, B.H.A. Biomacromolecules. 2009, 10, 660–669.
- Dunlop, W.F.; Robards, A.W. J. Bacteriol. 1973, 114, 1271–1280.
- Mayer, F.; Madkour, M.H.; Pieper-Fürst, U.; Wieczorek, R.; Liebergesell, M.L.; Steinbüchel, A. J. Gen. Microbiol. 1996, 42, 445–455.
- Tian, J.; Sinskey, A.J.; Stubbe, J. J. Bacteriol. 2005, 187, 3814–3824.
- Dennis, D.; Liebig, C.; Holley, T.; Thomas, K.S.; Khosla, A.; Wilson, D.; Augustine, B. FEMS Microbiol. Lett. 2003, 226, 113–119.
- Peters, V.; Rehm, B.H.A. FEMS Microbiol. Lett. 2005, 248, 93–100.
- Fuller, R.C. Int. J. Biol. Macromol. 1999, 25(1–3), 21–29.
- Mezzina, M.P.; Wetzler, D.E.; Catone, M.V.; Bucci, H.; Di, P.M.; Pettinari, M.J. Plos One. 2014, 9(7), 1–10.
- Kitamura, S.; Doi, Y. Biotechnol. Tech. 1994, 8, 345–350.
- Pierce, L.; Schroth, M.N. Plant Dis. 1994, 78, 683–685.
- Ballard, D.G.H.; Holmes, P.A.; Senior, P.J. Rec. Adv. Mech. Synth. Asp. Pol. 1987, 215, 293–314.
- Klinke, S.; de Roo, G.; Witholt, B.; Kessler, B. Appl. Environ. Microbiol. 2000, 66, 3705–3710.
- Griebel, R.; Smith, Z.; Merrick, J.M. Biochemistry. 1968, 7, 3676–3681.
- Stuart, E.S.; Lenz, R.W.; Fuller, R.C. Can. J. Microbiol. 1995, 41, 84–93.
- Stuart, E.S.; Tehrani, A.; Valentin, H.E.; Dennis, D.; Lenz, R.W.; Fuller, R.C. J. Biotechnol. 1998, 64, 137–144.
- Gerngross, T.U.; Reilly, P.; Stubbe, J.; Sinskey, A.J.; Peoples, O.P. J. Bacteriol. 1993, 175, 5289–5293.
- Handrick, R.; Reinhardt, S.; Jendrossek, D. J. Bacteriol. 2000, 182, 5916–5918.
- Pieper-Fürst, U.; Madkour, M.H.; Mayer, F.; Steinbüchel, A. J. Bacteriol. 1995, 177, 2513–2523.
- Prieto, M.A.; Buhler, B.; Jung, K.; Witholt, B.; Kessler, B. J. Bacteriol. 1999, 181, 858–868.
- Maehara, A.; Taguchi, S.; Nishiyama, T.; Yamane, T.; Doi, Y. J. Bacteriol. 2002, 184, 3992–4002.
- York, G.M.; Stubbe, J.; Sinskey, A.J. J. Bacteriol. 2002, 184, 59–66.
- Lundgren, D.G.; Pfister, R.M.; Merrick, J.M. J. Gen. Microbiol. 1964, 34, 441–446.
- Saegusa, H.; Shiraki, M.; Kanai, C.; Saito, T. J. Bacteriol. 2001, 183, 94–100.
- Wieczorek, R.; Pries, A.; Steinbüchel, A.; Mayer, F. J. Bacteriol. 1995, 177, 2425–2435.
- Hanley, S.Z.; Pappin, D.J.; Rahman, D.; White, A.J.; Elborough, K.M.; Slabas, A.R. FEBS Lett. 1999, 447, 99–105.
- Pötter, M.; Madkour, M.; Mayer, F.; Steinbüchel, A. Microbiology. 2002, 148, 2413–2426.
- Pötter, M.; Müller, H.; Reinecke, F.; Wieczorek, R.; Fricke, F.; Bowien, B.; Friedrich, B.; Steinbüchel, A. Microbiology. 2004, 150, 2301–2311.
- Pötter, M.; Steinbüchel, A. Microbiology. 2005, 151, 825–833.
- Foster, L.J.R.; Lenz, R.W.; Fuller, R.C. FEMS Microbiol. Lett. 1994, 118, 279–282.
- Cai, S.; Cai, L.; Liu, H.; Liu, X.; Han, J.; Zhou, J.; Xiang, H. Appl. Environ. Microbiol. 2012, 78(6), 1946–1952.
- Jurasek, L.; Marchessault, R.H. Appl. Microbiol. Biotechnol. 2004, 64, 611–617.
- Hoffmann, N.; Rehm, B.H.A. FEMS Microbiol. Lett. 2004, 237, 1–7.
- Hoffmann, N.; Rehm, B.H.A. FEMS Microbiol. Lett. 2005, 27, 279–282.
- Maehara, A.; Ueda, S.; Nakano, H.; Yamane, T. J. Bacteriol. 1999, 181, 2914–2921.
- Terada, M.; Marchessault, R.H. Int. J. Biol. Macromol. 1999, 25, 207–215.
- Jacquel, N.; Lob, C-W.; Wei, Y-H.; Wu, H.-S.; Wang, S.S. Biochem. Eng. J. 2008, 39, 15–27.
- Gomaa, E.Z. Braz. Arch. Biol. Technol. 2014, 57(1), 145–154.
- Kessler, B.; Weusthuis, R.; Witholt, B.; Eggink, G. Adv. Biochem. Eng. 2001, 71, 159–182.
- Kim, Y.B.; Lenz, R.W.; Fuller, R.C. Macromolecules. 1991, 24, 5256–5260.
- Mun, H.C.; Song, J.J.; Youn, S.C.B. Can. J. Microbiol. 1995, 41(1), 60–67.
- Page, W.J.; Cornish, A. Appl. Environ. Microbiol. 1993, 59(12), 4236–4244.
- Riedel, S.L.; Brigham, C.J.; Budde, C.F.; Bader, J.; Rha, C.; Stahl, U.; Sinskey, A.J. Biotechnol. Bioeng. 2013, 110(2), 461–470.
- Yao, J.; Zhang, G.; Wu, Q.; Chen, G.Q.; Zhang, R.Q. Anton. Leeuw. 1999, 75, 345–349.
- Lee, S.Y.; Choi, J.; Song, J.Y. Appl. Environ. Microbiol. 1999, 65(6), 2762–2764.
- Sevastianov, V.I.; Perova, N.V.; Shishatskaya, E.I.; Kalacheva, G.S.; Volova, T.G. J. Biomater. Sci. Polym. Ed. 2003, 14(10), 1029–1042.
- Kunasundari, B.; Sudesh, K. Exp. Pol. Lett. 2011, 5(7), 620–634.
- Kapritchkoff, F.M.; Viotti, A.P.; Alli, R.C.; Zuccolo, M.; Pradella, J.G.; Maiorano, A.E.; Miranda, E.A.; Bonomi, A. J. Biotechnol. 2006, 122(4), 453–462.
- Kathiraser, Y.; Aroua, M.K.; Ramachandran, K.B.; Tan, I.K.P. J. Chem. Technol. Biotechnol. 2007, 82(9), 847–855.
- Ramsay, J.A.; Berger, E.; Ramsay, A.; Chaverie, C. Biotechnol. Tech. 1994, 8, 589–594.
- Yang, Y.-H.; Brigham, C.; Willis, L.; Rha, C.; Sinskey, A. Biotechnol. Lett. 2011. DOI: 10.1007/s10529-010-0513-4
- Singh, M.; Patel, S.K.S.; Kalia, V.C. Microb. Cell Fact. 2009, 8, 1–11.
- Morita, M.; Tanji, Y.; Mizoguchi, K.; Soejima, A.; Orito, Y.; Unno, H. J. Biosci. Bioeng. 2001, 91, 469–473.
- Salzberg, L.I.; Helmann, J.D. J. Bacteriol. 2007, 189, 4671–4680.
- Mergaert, J.; Anderson, C.; Wouters, A.; Swings, J.; Kersters, K. FEMS Microbiol. Rev. 1992, 103, 317–321.
- Mergaert, J.; Webb, A.; Anderson, C.; Wouters, A.; Swings, J. Appl. Environ. Microbiol. 1993, 59, 3233–3238.
- Mergaert, J.; Anderson, C.; Wouters, A.; Swings, J. J. Environ. Degrad. 1994, 2, 177–183.
- Mergaert, J.; Wouters, A.; Anderson, C.; Jeans, S. Can. J. Microbiol. 1995, 41(Suppl 1), 154–159.
- Suyama, T.; Tokiwa, Y.; Ouichanpagdee, O.; Kanagawa, T.; Kamagata, Y. Appl. Environ. Microbiol. 1998, 64, 5008–5011.
- Volova, T.G.; Gladyshev, M.I.; Trusova, M.Y.; Zhila, N.O. Microbiology. 2006, 75(5), 593–598.
- Boyandin, A.N.; Prudnikova, S.V.; Karpov, V.A.; Ivonin, V.N.; Ðỗ, N.L.; Nguyễn, T.H.; Le, T.Y.H.; Filichev, N.L.; Levin, A.L.; Filipenko, M.L.; Volova, T.G.; Gitelson, I.I. Int. Biodet. Biodegrad. 2013, 83, 77–84.
- Jain, S.; Singh, A.K.; Tiwari, A. J. Chem. 2014, 3(1), 11–14.
- Doi, Y.; Segawa, A.; Kunioka, M. Polym. Comm. 1989, 30, 169–171.
- Knoll, M.; Hamm, T.M.; Wagner, F.; Martinez, V.; Pleiss, J. BMC Bioinformatics. 2009, 10, 89–97.
- Jendrossek, D.; Handrick, R. Ann. Rev. Microbiol. 2002, 56, 403–432.
- Chowdhury, A.A. Arch. Mikrobiol. 1963, 47, 167–200.
- Philip, S.; Keshavarz, T. J. Chem. Technol. Biotechnol. 2007, 82, 233–247.
- Hippe, H.; Schlegel, H.G. Arch. Microbiol. 1967, 56, 278–299.
- Doi, Y.; Kawaguchi, Y.; Koyama, N.; Nakamura, S.; Hiramitsu, M.; Yoshida, Y.; Kimura, H. FEMS Microbiol. Rev. 1992, 103, 103–108.
- Doi, Y.; Kanesawa, Y.; Tanahashi, N. Polym. Degrad. Stab. 1992, 36, 173–177.
- Saito, T.; Saegusa, H.; Miyata, Y.; Fukui, T. Int. J. Biol. Macromol. 1992, 103(2–4), 333–338.
- Merrick, J.M.; Steger, R.; Dombroski, D. Int. J. Biol. Macromol. 1999, 25(1–3), 129–134.
- Saito, T.; Takizawa, K.; Saegusa, H. Can. J. Microbiol (Suppl). 1995, 41(1), 187–191.
- Hiraishi, A.; Khan, S.T. Appl. Microbiol. Biotechnol. 2003, 61, 103–109.
- Poirier, Y.; Nawrath, C.; Somerville, C. Biotechnology. 1995, 13, 142–150.
- Madbouly, S.A.; Schrade, J.A.; Srinivasan, G.; Liu, K.; McCabe, K.G., Grewell, D.; Graves, W.R.; Kessler, M.R. Green Chem. 2014, 16, 1911–1920.
- Shimao, M. Curr. Opin. Biotechnol. 2001, 12, 242–247.
- Delafield, F.P.; Doudoroff, M.; Palleroni, N.J.; Lusty, C.J.; Contopoulos, R. J. Bacteriol. 1965, 90, 1455–1466.
- Schirmer, A.; Jendrossek, D.; Schlegel, H.G. Appl. Environ. Microbiol. 1993, 59, 1220–1227.
- Jendrossek, D.; Knoke, I.; Habibian, R.H. J. Environ. Polym. Degrad. 1993, 1, 53–63.
- Saito, T.; Suzuki, K.; Yamamoto, J.; Fukui, T.; Miwa, K.; Tomita, K.; Nakanishi, S.; Odani, S.; Suzuki, J.; Ishikawa, K. J. Bacteriol. 1989, 171, 184–189.
- Jendrossek, D.; Müller, B.; Schlegel, H.G. Eur. J. Biochem. 1993, 218, 701–710.
- Müller, B.; Jendrossek, D. Appl. Microbiol. Biotechnol. 1993, 38, 487–492.
- Briese, B.H.; Schmidt, B.; Jendrossek, D. J. Environ. Polym. Degrad. 1994, 2, 75–87.
- Kuruso, Y.; Kohama, K.; Uchida, Y.; Saito, T.; Yukawa, H. Cloning and Nucleotide Sequencing of the Poly(3-hydroxybutyrate) Depolymerases Gene from Pseudomonas Pickettii. In Biodegradable Plastics and Polymers: Doi, Y.; Fukuda, K., Eds.; Elsevier Science BV: Amsterdam, 1994; pp 357–361.
- Schirmer, A.; Jendrossek, D. J. Bacteriol. 1994, 176, 7065–7073.
- Jendrossek, D.; Frisse, A.; Behrends, A. J. Bacteriol. 1995, 177, 596–607.
- Jaeger, K.-E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; van Heuvel, M.; Misset, O. FEMS Microbiol. Rev. 1994, 15, 29–63.
- Mukai, K.; Doi, Y.; Sema, Y.; Tomita, K. Biotechnol. Lett. 1993, 15, 601–604.
- Adams, J.H.; Irving, G.; Koeslag, J.H.; Lochner, J.D.; Sandell, R.C.; Wilkinson, C. J. Physiol(Lond), 1987, 386, 439–454.
- Wiggam, M.I.; O'Kane, M.J.; Harper, R., Atkinson, A.B.; Hadden, D.R.; Trimble, E.R.; Bell, P.M. Diabet Care. 1997, 20, 1347–1352.
- Reusch, R.N. Biochem. Engl. Trans. 2000, 65, 280–295.
- Wu, Q.; Wang, Y.; Chen, G.Q. Biotechnology. 2009, 37(1), 1–12.
- Brigham, C.J.; Sinskey, A.J. Int. J. Biotechnol. Well Ind. 2012, 1, 53–60.
- Shishatskaya, E.I.; Volova, T.G. J. Mater. Sci. Mat. Med. 2004, 15(8), 915–923.
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Biomacromolecules. 2006, 7(8), 2249–2258.
- Wei, X.; Hu, Y.; Xie, W.; Lin, R.; Chen, G. J. Biomed. Mater. Res. 2008, 90A(3), 894–905.
- Ji, G.; Wei, X.; Chen, G. J. Biomater. Sci. 2009, 20, 325–339.
- Jirage, A.S.; Baravkar, V.S.; Kate, V.K.; Payghan, S.A.; Disouza, J.I. Int. J. Pharm. Biol. Arch. 2013, 4(6), 1107–1118.
- Doi, Y. Macromol. Sym. 1995, 98, 585–599.
- Loo, C.Y.; Sudesh, K. Malay. Polym. J. 2007, 2(2), 31–57.
- Chang, H.M.; Wang, Z.H.; Luo, H.N.; Xu, M.; Ren, X.Y.; Zheng, G.X.; Wu, B.J.; Zhang, X.H.; Lu, X.Y.; Chen, F.; Jing, X.H.; Wan, L. Braz. J. Med. Biol. Res. 2014, 47(7), 533–539.
- Barnard, G.N.; Sanders, J.K. J. Biol. Chem. 1989, 264(6), 3286–3291.
- Harrison, S.T.; Chase, H.A.; Amor, S.R.; Bonthrone, K.M.; Sanders, J.K. Int. J. Biol. Macromol. 1992, 14(1), 50–56.
- Sudesh, K.; Fukui, T.; Iwata, T.; Doi, Y. Can. J. Microbiol. 2000, 46, 304–311.
- Lauzier, C.; Marchessault, R.H.; Smith, P.; Chanzy, H. Polymer. 1992a, 33(4), 823–827.
- Sanders, J.K.M. Chem. Soc. Rev. 1993, 22, 1–7.
- de Koning, G.M.; Lemstra, P.J. Polymer. 1992, 33, 3292–3294.
- Holmes, P.A. Phys. Technol. 1985, 16, 32–36.
- Lauzier, C.; Revol, J.F.; Marchessault, R.H. FEMS Microbiol. Rev. 1992, 103, 299–310.
- Gross, R.A.; DeMello, C.; Lenz, R.W. Macromolecules. 1989, 22, 1106–1115.
- He, W.N.; Zhang, Z.M.; Hu, P.; Chen, G.Q. Acta. Polym. Sin. 1999, 6, 709–714.
- Preusting, H.; Nijenhuis, A.; Witholt, B. Macromolecules. 1990, 23, 4220–4224.
- Chen, G.Q.; Wu, Q.; Zhao, K.; Yu, P.H. Chin. J. Polym. Sci. 2000, 18(5), 389–396.
- Poirier, Y. Nat. Biotechnol. 1999, 17, 960–961.
- Renard, E.; Langlois, V.; Guerin, P. Corr. Eng. Sci. Technol. 2007, 42(4), 300–311.
- Zibiao, L.; Xian Jun, L. Chem. Soc. Rev. 2015, 44, 2865–2879.
- Renard, E.; Walls, M.; Guerin, P.; Langlois, V. Polym. Degrad. Stab. 2004, 85, 779–787.
- Shraddha, G.; Yogita, R.; Simanta, S.; Aparna, S.; Kamlesh, S. Int. Multidiscip. Res. J. 2011, 1(9), 30–33.
- Chee, J.-Y.; Yoga, S.-S.; Lau, N.-S.; Ling, S.-C.; Abed, R.M.M.; Sudesh, K. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. In FORMATEX: Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, 2010; pp 1395–1404.
- Brandl, H.; Bachofen, R.; Mayer, J.; Wintermantel, E. Can. J. Microbiol. 1995, 41(Suppl. I), 143–153.
- Andrady, A.L. Mar. Pollut. Bull. 2011, 62, 1596–1605.
- Müller, R.-J.; Kleeberg, I.; Deckwer, W.-D. J. Biotechnol. 2001, 86, 87–95.
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Exp. Poly. Lett. 2014, 8(11), 791–808.
- Beucker, S.; Marscheider-Weidemann, F. Potentials and Challenges of Bioplastics–Insights from a German Survey on “Green” Future Markets. In R'07 World Congress-Recovery of Materials and Energy for Resource Efficiency, Davos, Switzerland. Hilty, L.M., Edelmann, X., Ruf, A., Eds.; Empa materials science and technology: St. Gallen, 2007.
- Brandl, H.; Puchner, P. Biodegradation. 1992, 2, 237–243.
- Byrom, D. Miscellaneous Biomaterials. In Biomaterials: Byrom, D., Ed.; Basingstoke MacMillan: London, 1991; pp 333–359.
- deMarco, S. Bas. Biotechnol. eJ. 2005, 1, 1–4.
- Steinbūchel, A. Macromol. Biosci. 2001, 1, 1–24.
- Bourbonnais, R.; Marchessault, R.H. Biomacromolecules. 2010, 11(4), 989–993.
- Martini, F.; Perazzo, L.; Vietto, P. 1989, US patent 9 No: 4, 826,493.
- Zhang, L.L.; Deng, X.M.; Zhao, S.J.; Huang, Z.T. Polym. Int. 1997, 44, 104–110.
- Sharma, R.; Ray, A.R. J. Macromol. Sci. 1995, C35, 327–359.
- Urakami, T.; Imagawa, S.; Harada, M.; Iwamoto, A.; Tokiwa, Y. Polymer. Kob. Ronb. 2000, 57, 263–270.
- Maekawa, M.; Pearce, R.; Marchessault, R.H.; Manley, R.S.J. Polymer. 1999, 40, 1501–1505.
- Woodruff, M.A.; Hutmacher, D.W., Ed.; Resorbable Composite Scaffolds for Bone Tissue Engineering. In Tissue and Cell Engineering Society (TCES); Manchester. 2010.
- Rai, R.; Keshavarz, T.; Roether, J.; Boccaccini, A.; Roy, I. Mater. Sci. Eng., R. 2011, 72(3), 29–47.
- Sudesh, K.; Doi, Y. Polyhydroxyalkanoates. In Handbook of Biodegradable Polymers: Bastioli, C., Ed.; Rapra Technologies Ltd: Shrewsbury England, 2005; pp 219–256.
- Senior, P.J.; Dawes, E.A. Biochem. J. 1973, 134, 225–238.
- Chen, G.Q. Polyhydroxyalkanoates. In Biodegradable Polymers for Industrial Applications: Smith, R., Ed.; CRC press: Cambridge, 2005; pp 32–56.
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Canc. Prev. Res. 2011, 4, 1158–1171.
- Sudesh, K.; Ching-Yee, L.; Lay-Koon, G.; Tadahisa, I.; Mizou, M. Mater. Macromol. Biosci. 2007, 7, 1119–1205.
- Voinova, O.N.; Kalachevam, G.S.; Grodnitskayam, I.D.; Volova, T.G. Appl. Biochem. Microbiol. 2009, 45(4), 384–388.
- Wang, J.G.; Bakken, L.R. Microb. Ecol. 1998, 35, 94–101.
- Nhan, D.T.; Wille, M.; De, Schryver, P.; Defoirdt, T.; Bossier, P.; Sorgeloos, P. Aquaculture. 2010, 302(1–2), 76–81.
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Curr. Opin. Microbiol. 2011, 14(3), 251–258.
- Khan, S.T.; Horiba, Y.; Yamamoto, M.; Hiraishi, A. Appl. Environ. Microbiol. 2002, 68, 3206–3214.
- Zhang, X.; Wei, C.; He, Q.; Ren, Y. J. Haz. Mater. 2010, 177(1–3), 508–515.
- Lee, S.Y.; Choi, J.; Wong, H.H. Int. J. Biol. Macromol. 1999, 25, 31–36.
- Williams, S.F.; Peoples, O.P. Chemtech. 1996, 26, 38–44.
- Lee, S.Y. TIBTECH. 1996, 14, 431–438.
- Amache, R.; Sukan, A.; Safari, M.; Roy, I.; Keshavarz, T. Chem. Eng. Tran. 2013, 32, 931–936.
- Koller, M.; Gasser, I.; Schmid, F.; Berg, G. Eng. Life Sci. 2011, 11(3), 222–237.
- Thirumala, M.; Reddy, S.-V.; Mahmood, K. J. Ind. Microbiol. Biotechnol. 2010, 37, 271–278.
- Sangkharak, K.; Prasertsan, P. J. Gen. Appl. Microbiol. 2012, 58, 173–182.
- Prasad, M.P.1.; Sethi, R. Int. J. Adv. Biotechnol. Res. 2013, 4(4), 527–532.
- de Koning, G.J.M.; Witholt, B. Bioprocess. Eng. 1997, 17, 7–13.
- de Koning, G.J.M.; Kellerhals, M.B.; Witholt, B. Bioprocess. Eng. 1997, 17, 15–21.
- Park, S.J.; Ahn, W.S.; Green, P.R.; Lee, S.Y. Biomacromolecules. 2001, 2, 248–254.
- Yilmaz, M.; Beyatli, Y. Zuckerindustrie. 2005, 130, 109–112.
- Jiang, Y.; Song, X.; Gong, L.; Li, P.; Dai, C.; Shao, W. Enzym. Microb. Technol. 2008, 42, 167–172.
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M.G. Bioresour. Technol. 2009, 100, 5996–6009.
- Santimano, M.C.; Prabhu, N.N.; Garg, S. Res. J. Microbiol. 2009, 4(3), 89–96.
- Rebah, F.B.; Prévost, D.; Tyagi, R.D.; Belbahri, L. Appl. Biochem. Biotechnol. 2009, 158, 155–163.
- Du, C.; Sabirova, J.; Soetaert, W.; Carol, S.K.L. Trends Biotechnol. 1996, 6, 14–25.
- Wang, F.; Lee, S.Y. Appl. Environ. Microbiol. 1997, 63(9), 3703–3706.
- Sangkhara, K.; Prasertsan, P. Electron J. Biotechnol. 2008, 11(3), 1–12.
- Manjunath, R.; Gunasekaran, S.; Pingale, B.N. Bioinfolet. 2011, 8(2), 194–195.
- Subin, R.S.; Varghese, S.M.; Bhat, S.G. J. Sci. Ind. Res. 2013, 72, 228–235.
- Belal, E.B. Curr. Res. J. Biol. Sci. 2013, 5(6), 273–284.
- Saleem, F.; Aslam, R.; Saleem, Y.; Naz, S.; Syed, Q.; Munir, N.; Khurshid, N.; Shakoori, A.R. Pakistan J. Zool. 2014, 46(5), 1337–1344.
- Muthezhilan, R.1.; Kaarthikeyan, C.P.; Jayaprakashvel, M.; Hussain, A.J. Pak. J. Biotechnol. 2014, 11(2), 59–66.
- Lee, S.Y. Trends Biotechnol. 1996, 14, 98–105.
- Lu, X.Y.; Zhang, W.J.; Jian, J.; Wu, Q.; Chen, G.Q. FEMS Microbiol. Lett. 2005, 243(1), 149–155.
- Ouyang, S.-P.; Liu, Q.; Fang, L.; Che, G.-Q. Macromol. Biosci. 2007, 7, 227–233.
- Witholt, B.; Kessler, B. Curr. Opin. Biotechnol. 1999, 10, 279–285.