ABSTRACT
Anodic inhibition of steel in 8 mol L−1 H3PO4 was investigated in the absence and presence of different concentrations of extracts of Lawsonia inermis. An experimental measurement, including galvanostatic polarization studies, was done. The anodic corrosion rate and the inhibition efficiencies of the extract were calculated. The results obtained show that the inhibition was found to increase with increasing concentration of Lawsonia inermis extract. The inhibition actions of extracts are discussed on the basis of adsorption of Lawsonia inermis at the steel surface. Theoretical fitting of different isotherms were tested to clarify the nature of adsorption. Polarization curves revealed that Lawsonia inermis inhibitor acts as a corrosion inhibitor. The activation energy (Ea) as well as other thermodynamic parameters (ΔH*, ΔS*, ΔG*) for the inhibition process were calculated. These thermodynamic parameters show strong interaction between the inhibitor and the steel surface. The scanning electron microscope analysis study confirmed the adsorption of inhibitor molecules on the steel surface. The social organization and morphology of the extract were characterized by Fourier transform infrared spectroscopy.
1. Introduction
Electropolishing is widely employed in industry for microfinishing and deburring of different metallic components. A large number of electrolytic baths with different operating conditions and electrolyte compositions are reported in the literature ( Citation1– Citation4). In spite of much advancement in the field of corrosion science and technology, the phenomenon of corrosion remains a major concern to industries around the world. Steels are the most commonly used construction materials for pipelines in the oil and gas industry, but they are very susceptible to both a high general corrosion rate and severe localized corrosion ( Citation5).
Inhibitors are generally applied to protect materials against deterioration from corrosion. The choice of optimal inhibitor should be based on three considerations: (a) it should have a convenient synthesis of inexpensive raw materials, (b) the presence of nitrogen, oxygen, sulfur and multiple bonds in the inhibitor molecule is required for its efficiency and (c) its toxicity in the environment must be negligible ( Citation6– Citation9).
It is generally accepted that organic molecules inhibit corrosion via adsorption at the metal-solution interface ( Citation10– Citation11). Two primary mechanisms of adsorption are associated with organic compounds; act by blocking the reaction sites or generating a physical barrier to reduce the diffusion of corrosion species to the metal surface. The mode of absorption is dependent on the following factors: inhibitor concentration, electrochemical potential at the interface, nature and surface charge of the metal surface, solution chemistry and temperature of the corrosion reaction. Temperature has a great effect on the rate of metal corrosion and its variation is a very useful tool for studying and clarifying the adsorption mechanism of an inhibitor. A detailed understanding of the mechanism of adsorption for corrosion inhibitors requires the characterization of corrosion inhibitor films by using surface analytical techniques such as X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR) spectroscopy, etc. ( Citation12).
Mechanistic studies of electropolishing have revealed that electropolishing is a diffusion controlled reaction which takes place at the limiting current; the limiting current is attained most probably when the diffusion layer becomes saturated with Fe2+ ions ( Citation2– Citation3). Therefore the value of the limiting current which determines the polishing rate depends on the rate of mass transfer of Fe2+ from the diffusion layer to the solution bulk.
Henna leaves, flowers, seeds, stem bark and roots are used in traditional medicine to treat a variety of ailments such as rheumatoid arthritis, headache, ulcers, diarrhea, leprosy, fever, leucorrhoea, diabetes and cardiac disease and as a hepatoprotective and coloring agent. They are also used since antiquity to dye skin, hair and fingernails, as well as fabrics including silk, wool and leather ( Citation13– Citation14).
The aim of this work was to investigate the role played by the extract of Lawsonia inermis in retarding the dissolution of carbon steel in 8 mol L−1 H3PO4 solution employing the galvanostatic polarization method. Moreover, different reaction conditions such as temperature, concentration of the extract, thermodynamic of adsorption and activation energy of this extract were considered in order to go forward to clarify how this inhibitor works in acid media.
2. Experimental methods
2.1. Preparation of plant extract
The leaves of Lawsonia inermis were dried for 6 h in an oven at 70°C and ground into powder and 50 gm of the powder of Lawsonia inermis was refluxed in 500 ml double distilled water for 1 h. The aqueous solution was filtered and concentrated to 100 ml. This concentrated solution was used to prepare a solution of different concentrations by dilution method. The Lawsonia inermis plant extract was characterized by FTIR spectroscopy.
2.2. Galvanostatic measurement of the rate of corrosion
The cell used in the present work consists of a rectangular plastic container having the dimensions 10 × 5 × 10 cm with electrodes fitting the whole cross-section. The electrodes were rectangular steel sheets of 10 cm height and 5 cm width. Electrode separation was 10 cm; a porous PVC diaphragm was used to prevent the stirring effect due to H2 bubbles evolved at the cathode. The electrical circuit consists of 6 V DC power supply, a variable resistance and a multi range ammeter connected in series with cell. A high impedance voltammeter was connected in parallel with the cell to measure its potential. 8 mol L−1 of phosphoric acid was prepared from analar grade H3PO4 (95%). Five concentrations of Lawsonia inermis solutions with 8 mol L−1 H3PO4 are used. The steady-state anode potential was measured against a reference electrode consisting of steel wire immersed in a cup of luggin tube filled with a solution similar to that in the cell. The tip of the luggin tube was placed 0.5–1 mm from the anode wall. Polarization curves, from which the limiting current was determined, were plotted by increasing the applied current stepwise and measuring the corresponding steady-state potential. Before each run, the back part of the anode was insulated with polystyrene lacquer and the active surface of the anode was polished with a fine grade paper, degreased with trichloroethylene, washed with alcohol and finally rinsed in distilled water. The temperature was regulated by placing the cell in the thermostatic water bath at different temperatures (25°C, 30°C, 35°C and 40°C). The steel used had the following chemical composition (wt %): 0.2 C, 0.04 S, 2.6 Mn, 0.039 P, 0.36 Si and 96.761 Fe.
2.3. Scanning electron microscope analysis
The scanning electron microscope (SEM) images were taken using a JEOL, Analytical Scanning electron microscope, JSM-6360 LA. Sample for SEM experiments where iron sheet anode was (1 cm × 1 cm).
3. Results and discussion
3.1. Characterization of plant extract
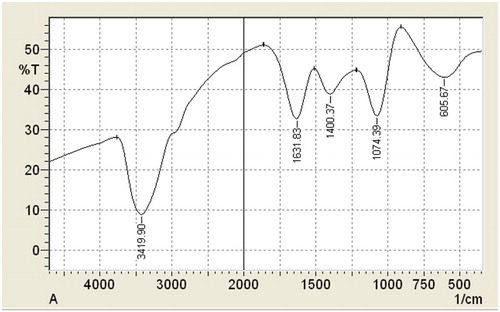
Infrared spectroscopy is a powerful analytical tool to reveal the functional groups in a molecule. More specifically, it provides information about the nature of the bonds in the analyzed sample. Chemical bonds absorb electromagnetic waves at certain energy levels and convert them into rotational and vibrational kinetic energy forms. The FTIR spectrum of Lawsonia inermis extract is shown in to exhibit a strong band at 3419.9 cm−1 due to ν (OH) mode of the alcoholic phenolic group. The appearance of strong bands at 1631.8 cm−1 is related to ν (C=O) mode stretching vibration and to a C=C alkene. The adsorption bands at 1400 and 1074 cm−1 could be assigned to the framework vibration of the aromatic ring or aromatic ring breathing ( Citation15). The adsorption bands at 605 cm−1 could be assigned to the alkene (=C–H). Finally, it is clear that Lawsonia inermis extract contains many active groups that have the ability to be adsorbed on the steel surface.
The IR spectrum analysis confirmed that the Lawsonia inermis extract contained functional groups such as OH and O that may be responsible for the decrease in the corrosion rate, since they can react with the Fe+2 ions.
3.2. Galvanostatic polarization studies
The effect of Lawsonia inermis extract on the corrosion rate of carbon steel in 8 mol L−1 H3PO4 solution was studied by galvanostatic polarization curves. shows the anodic polarization curves with the addition of various concentrations of Lawsonia inermis extract at 303 K. Applied potential vs. the current was plotted and an extrapolation of the linear portion of the corrosion potential gives the limiting current (IL). The values of IL are given in .
Figure 2. Polarization curves of steel dissolution in 8 mol L−1 H3PO4 in the absence and presence of different concentrations of Lawsonia inermis extract at 303 K.
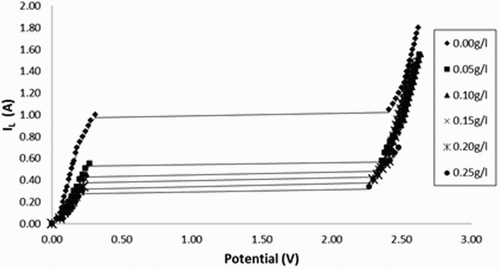
Table 1. Values of limiting current IL (A), inhibition efficiency percentage (IE%) for dissolution of steel in 8 mol L−1 H3PO4 in the absence and presence of Lawsonia inermis extract at different temperatures and activation energy Ea (kJ mol−1).
It is observed that the limiting current values decreased when Lawsonia inermis extract was added. This was attributed to the presence of Lawsonia inermis particles deposited on the surface of carbon steel electrode, which acts as a protective layer and reduces the interaction between H3PO4 and the carbon steel surface. From , it is also clear that the inhibition increases with growth in concentration, which means that Lawsonia inermis extract may be classed as a corrosion inhibitor in 8 mol L−1 H3PO4 ( Citation16– Citation17). The inhibition efficiency ranged from 36.6% to 67.6% depending on the operating condition and inhibitor adsorption on steel is most likely to take place through the polar groups (=O and OH) present in the inhibitor structure.
If IL(blank) is the limiting current in the absence of extract and IL(extract) in the presence of extract, then percentage inhibition efficiency (IE%) was calculated using the following equation:(1)
It is clear that IL decreases and IE% increases by increasing extract concentration. This conduct is the compactness of the extract molecules on the electrode solution interface may increase the interfacial viscosity with consequent reduction in the diffusivity of electroactive ions. This led to a hindrance in the flow of solution past the electrode surface and consequent decrease in the values of limiting current ( Citation18).
3.3. Effect of temperature and activation parameters of the dissolution process
The effect of temperature on the inhibitory action of the inhibitor was determined at various concentrations and at different temperatures (25–40°C). The tabulated data () reveal that as the concentration of the inhibitor increases, the dissolution rate (IL) increases with increasing temperature both in the absence and presence of Lawsonia inermis, while IE% decreases with temperature increase (). This increase could be explained on the basis that increasing temperature enhances the transfer rate of the PO4−3 ions and increases the conductivity. Also the increase in temperature reduces solution viscosity with a consequent increase in Fe2+ ion diffusivity which leads to increase in the dissolution rate ( Citation19). The decrease in IE% is due to desorption of Lawsonia inermis molecules of the Fe surface by increasing temperature. The decrease in inhibition efficiency with increasing temperature is suggestive of physical adsorption of the used Lawsonia inermis on the steel surface ( Citation20).
Some relevant information about the adsorption mechanism of the inhibitor can be obtained by comparing Ea, both in the absence and presence of the corrosion inhibitor. Activation energy Ea and thermodynamic data, such as change in free energy of activation ΔG*, enthalpy of activation ΔH* and entropy of activation ΔS* for carbon steel in 8 mol L−1 H3PO4 in the absence and in the presence of the inhibitor were calculated and are listed in . The activation energy at different concentrations of the inhibitor in H3PO4 is calculated by plotting ln IL vs. 1/T ().
Figure 3. Arrhenius plots of ln IL versus 1/T for steel corrosion in 8 mol L−1 H3PO4 solutions in the absence and presence of different concentrations of Lawsonia inermis extract.
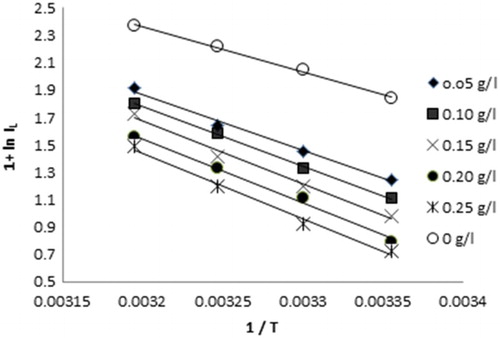
Table 2. Thermodynamic parameters of the dissolution of steel in 8 mol L−1 H3PO4 solution in the absence and presence of different concentrations of Lawsonia inermis extract.
The dissolution reaction can be seen as an Arrhenius type process and transition-state equations:(2)
(3)
(4)
where IL is the limiting current obtained from the polarization method, R is the universal gas constant, T is the absolute temperature, A is the Arrhenius pre-exponential factor, N is the Avogadro's number and h is Plank's constant.
Ea is lower in the absence than in the presence of the inhibitor (). The increase in activation energy in the presence of inhibitor compared to its absence jointly with a decrease in inhibition efficiency with increase in temperature is often ascribed in literature to the formation of an inhibitor film physically adsorbed ( Citation21– Citation23). The values of activation energy Ea increase in the presence of the extract which suggested that the inhibitors reduce anodic corrosion processes by creating a physical barrier to charge and mass transfer through the adsorption process ( Citation24).
A plot of ln (IL/T) vs. 1/T gave a straight line with the slope of −ΔH*/R and intercept ln (R/Nh) + ΔS*/R (not shown) from which the values of ΔS* and ΔH* were calculated and are given in , which indicates that the addition of extracts to the dissolution medium leads to an increase in the ΔH* values, so these molecules increase the height of the energy barrier for the dissolution process. The negative value of entropy implies that the activated complex in the rate-determining step represents an association rather than dissociation step meaning that a decrease in disorder takes place on going from reactants to the activated complex ( Citation25).
3.4. Adsorption isotherms
A determination of the type of adsorption isotherm itself provides information on the adsorption process such as surface coverage, adsorption equilibrium constant and information on the interaction between the Lawsonia inermis extract and the electrode surface. The surface coverage (θ) values for different concentrations of the inhibitor in 8 mol L−1 H3PO4 were calculated using the following equation (
Citation26).(5)
The data were tested graphically to find a suitable adsorption isotherm. The adsorption mechanism of the extract on steel surface was determined by fitting the θ values to different adsorption isotherms such as Langmuir, Flory–Huggins and Kinetic-adsorption models by the following equations (
Citation27–
Citation28).(6)
(7)
(8)
where K is the equilibrium constant of the adsorption process, x is the number of active sites occupied by one inhibitor molecule or number of water molecules replaced by one molecule of the adsorbate and y is the number of extract molecules occupying an active site ().
Table 3. Adsorption parameters, calculated values of free energy of adsorption −ΔGads (kJ mol−1) at different temperatures and thermodynamic functions of the adsorption process.
A plot of C/θ against C ((a)) gave a straight line with a higher correlation coefficient (R2) but the gradient is never unity, contrary to what is expected for an ideal Langmuir adsorption isotherm equation. Extract molecules having polar atoms or groups which are adsorbed on the metal surface may interact by mutual repulsion or attraction and this may be advocated as the reason for the departure of the slope values from unity.
Figure 4. Adsorption isotherm plots for the adsorption of Lawsonia inermis in 8 mol L−1 H3PO4 on the surface of steel at 298 K (a) Langmuir isotherm, (b) Flory–Huggins isotherm, (c) Kinetic-adsorption isotherm.
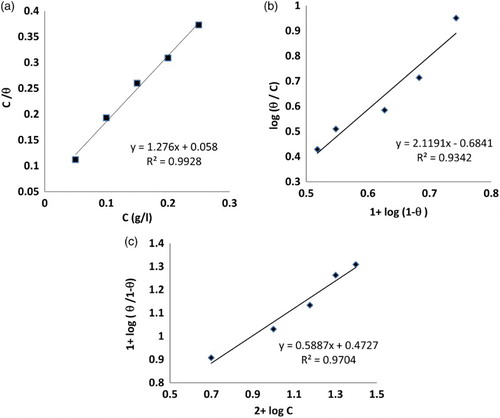
The plot of log θ/C against log (1−θ) is shown in ((b)). The value of x > 1 implied that one inhibitor molecule replaces more than one water molecule ( Citation29).
A linear relationship can be obtained on plotting log θ/1−θ as a function of log C as shown in ((c)). It is obvious that the values of 1/y greater than unity imply that the inhibitor molecule will occupy more than one active site ( Citation30).
The adsorption equilibrium constant Kads is related to the free energy of adsorption (ΔGads) by the equation (
Citation31).(9)
where the value 55.5 is the water concentration in the solution expressed in mol L−1 and T is the absolute temperature.
Generally, the change in free energy values of adsorption ΔGads up to −20 kJ mol−1 is consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption), while those more negative than −40 kJ mol−1 involve charge sharing or transfer from the inhibitor molecules to the metal surface to form a co-ordinate type of bond (chemisorption). The ΔGads values are less negative than −40 kJ mol−1; so ΔGads values designate that the adsorption is a physical type, the spontaneity of the adsorption process and stability of the adsorbed layer on the steel surface ( Citation32).
Thermodynamically, the change in free energy values of adsorption (ΔGads) is related to enthalpy and entropy of adsorption, ΔHads and ΔSads, respectively, by the following equation:(10)
The obtained values of ΔHads and ΔSads are −30.36 kJ mol−1 and −46.4 J mol−1 K−1, respectively. The ΔHads value in the presence of the Lawsonia inermis inhibitor is large and negative, indicating that the adsorption of inhibitor molecules on the steel surface is an exothermic process. For the chemisorption process ΔHads approaches 100 kJ mol−1 while for the physisorption process, it is less than 40 kJ mol−1 ( Citation5). In the present study, the ΔHads is −30.36 kJ mol−1; this suggested that the adsorption mechanism of Lawsonia inermis on steel is physisorption. The obtained negative values of ΔSads indicate a reduction of degree of freedom. This means that an increase of an order takes place in going from the free bulky Lawsonia inermis molecules to the more orderly inhibitor molecules onto the metal surface.
3.5. Scanning electron microscope
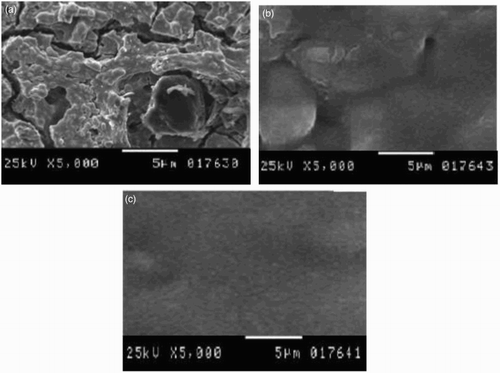
SEM was applied to study the surface morphology of the iron sheet. Micrographs of the specimens before and after electropolishing in the absence and in the presence of different concentrations of Lawsonia inermis extract are shown in . (a) shows the morphology of steel after electropolishing in 8 mol L−1 H3PO4; the surface was seriously damaged as a great deal of deep cavities and drawbacks were found. The surface roughness of the steel appears to be lower with a few small notches by the addition of the inhibitor than that without the addition ((b) and 5(c)). Inhibition of steel by Lawsonia inermis extract can be explained on the basis of adsorption. By increasing the concentration of the extract, the SEM micrograph ((c)) shows that smoothness and brightness increase. The surface appearance is enhanced in the presence of extract in which a smooth and completely uniform surface is obtained. This behavior is attributed to the involvement of extract molecules in the reaction sites of the steel surface and this result indicates that the extract molecules hinder the dissolution of steel by formation of a protective film on the steel surface ( Citation33).
4. Conclusions
The present study leads to the following conclusions in controlling the anodic corrosion of steel by extracts of Lawsonia inermis; the results obtained from the polarization study revealed that the extract under study behaved as a type of inhibitor. FTIR and SEM prove that the inhibition is due to the formation of an insoluble stable film through the process of adsorption of the inhibitor molecules on the metal surface. Electropolishing of steel in a bath containing Lawsonia inermis extract could increase the ability of the bath to produce continuous polishing over an anode. Inhibition efficiency increases with inhibitor concentration and the maximum inhibition efficiency was 67.1% of the inhibitor concentration 0.25 g L−1. Lawsonia inermis extract increases the value of the activation energy of the dissolution process and consequently decreases the rate of dissolution of steel in the H3PO4 solution. The improvement produced in electropolishing by the studied Lawsonia inermis extract was due to the adsorption of such agent on the anode surface. The adsorption of Lawsonia inermis extract on the steel surface does not obey Langmuir isotherm, but obey Flory–Huggins isotherm and Kinetic-adsorption isotherm. The adsorption process is spontaneous and exothermic accompanied by a decrease in entropy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Karthikaiselvi, R.; Subhashini, S.; Rajalakshmi, R. Arab. J. Chem. 2012, 5 (4), 517–522.
- Atta, A.M.; El-Azabawy, O.E.; Ismail, H.S.; Hegazy, M.A. Corros. Sci. 2011, 53 (5), 1680–1689.
- Bentiss, F.; Traisnel, M.; Lagrenee, M. Corros. Sci. 2000, 42 (1), 127–146.
- Abd El-Maksoud, S. Mater. Corros. 2003, 54 (2), 106–112.
- Priya, S.V.; Saratha, R. Elixir Corros. 2011, 37, 3617–3622.
- Soror, T.Y.; El-Ziady, M.A. Mater. Chem Phys. 2002, 77 (3), 703–697.
- Fuchs-Godec, R. Electrochim. Acta. 2007, 52 (15), 4974–4981.
- Olivares-Xometl, O.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Arce, E.; Dorantes, H.; Arellanes-Lozada, P. Mater. Chem. Phys. 2008, 110 (2–3), 344–351.
- Quartarone, G.; Battilana, M.; Bonaldo, L.; Tortato, T. Corros. Sci. 2008, 50 (12), 3467–3474.
- Amin, M.A.; Ahmed, M.A.; Arida, H.A.; Arslan, T.; Saracoglu, M.; Kandemirli, F. Corros. Sci. 2011, 53 (2), 540–548.
- Desimone, M.P.; Gordillo, G.; Simison, S.N. Corros. Sci. 2011, 53 (12), 4033–4043.
- Bastidas, J.M.; Pinilla, P.; Cano, E.; Polo, J.L.; Miguel, S. Corros. Sci. 2003, 45 (2), 427–449.
- Sedahmed, G.H.; Abdo, M.S.; Kamal, M.A.; Fadaly, O.A.; Osman, H.M. Int. Commun. Heat Mass Tran. 2001, 28 (2), 257–265.
- Huang, C.A.; Chen, Y.C.; Chang, J.H. Corros. Sci. 2008, 50 (2), 480–489.
- Bhandari, H.; Choudhary, V.; Dhawan, S.K. Synthetic Met. 2011, 161 (9–10), 753–762.
- Abdel-Rahman, H.H.; Hafez, A.M.; Helmy, A.A. Electrochemistry. 2015, 83, 440–444.
- Negm, N.A.; Kandile, N.G.; Badr, E.A.; Mohammed, M.A. Corros. Sci. 2012, 65, 94–103.
- Taha, A.A. J. Colloid. Interface. Sci. 2004, 275 (1), 235–242.
- Lalitha, A.; Ramesh, S.; Rajeswari, S. Electrochim. Acta. 2005, 51 (1), 47–55.
- Kumar, A. Inter J Phys Sci. 2008, 3, 140–143.
- Hassan, H.H. Electrochim. Acta. 2007, 53 (4), 1722–1730.
- Núñez López, P.J.; García Plaza, E.; Hernando Prada, M.; Trujillo Coronel, R. Mater. Sci. Forum. 2014, 797, 133–138.
- Conde, A.; de Damborenea, J.J. Corros. Sci. 2002, 44 (7), 1555–1567.
- Hosseini, S.M.A.; Salari, M.; Ghasemi, M. Mater. Corros. 2009, 60 (12), 968–963.
- Quraishi, M.A.; Rafiquee, M.Z.A.; Khan, S.; Saxena, N. J. Appl. Electrochem. 2007, 37 (10), 1153–1162.
- Hegazy, M.A.; Zaky, M.F. Corros. Sci. 2010, 52 (4), 1333–1341.
- Yadav, M.; Sharma, U., J. Mater. Environ. Sci. 2011, 2 (4), 407–414.
- El-Awady, A.A.; Abd-El-Nabey, B.A.; Aziz, S.G. J. Electrochem. Soc. 1992, 139 (8), 2149–2154.
- Murthy, Z.V.P.; Vijayaragavan, K. Green Chem. Lett. Rev. 2014, 7 (3), 209–219.
- Karthikaiselvi, R.; Subhashini, S. J. Assoc. Arab Univ. Basic Appl, Sci. 2014, 16, 74–82.
- Yurt, A.; Ulutas, S.; Dal, H. Appl. Surf. Sci. 2006, 253 (2), 919–925.
- Zhang, S.; Tao, Z.; Liao S.; Wu, F. Corros. Sci. 2010, 52 (9), 3126–3132.
- Li, L.; Wang, C.; Lu, H. Electrochim. Acta. 2013, 104, 295–301.