ABSTRACT
The present work reports a method for green synthesis of silver nanoparticles (AgNPs) by reducing Ag ions from silver nitrate solution using alcoholic, aqueous and alcoholic-aqueous Stevia rebaudiana extracts. Depending on a particular extract different sizes of AgNPs could be observed – 40 nm in aqueous and aqueous-alcoholic extracts and significantly bigger 170 nm in case of alcoholic extract. Synthesis of AgNPs was analyzed within spectra range of 300–700 nm. The performed research provided information that aqueous and aqueous-alcoholic extracts are excellent sources for synthesis of AgNPs; however AgNPs were unstable in the case of alcoholic extract. The aqueous-alcoholic extract showed the best properties during studies. Antioxidant potential, antimicrobial properties and cytotoxicity were determined. The 2,2-diphenyl-1-(2,4,6-triphenyl-hydrazyl) method showed that antioxidant activity of an extract containing AgNPs was higher compared to Stevia extract alone. Bacterial inhibition studies had shown substantial antibacterial properties of AgNPs, which was much higher than Stevia extract alone and silver nitrate.
Introduction
Nanomaterials (nanoparticles, NPs) include particles with diameter from 1 nm up to 100 nm. Nanomaterials share several properties with macromolecules but they have also a range of unique chemical, physical and optical properties, which are not observed for larger particles ( Citation1–3).
Silver nanoparticles (AgNPs), due to their strongly developed, specific surface area, have a high adsorption ability, causing their high reactivity, catalytic properties and antimicrobial activity ( Citation1–2). Effective antibacterial and antifungal properties of the AgNPs have found application in many branches of industry ( Citation4–7). These properties are used in medicine (implants modified with nanosilver, surgical instruments coated with AgNPs, dental fillings), cosmetics (antibacterial and anti-acne cosmetics), pharmacy (patch with nanosilver-supportive treatment for difficult to heal wounds and burns) and also in the production of antimicrobial paints, lacquers, and household and industrial detergents. A wide range of practical applications of AgNPs has caused recent popularity of silver nanocomposites, representing the combination of AgNPs with various materials, for example, natural and synthetic polymers, fabrics or glass. The silver nanocomposites are used in the textile industry (antibacterial clothing, neutralizing the bacteria present in the sweat) and in the production of antimicrobial elements used in fridges, washing machines and medical devices ( Citation1–4, Citation8–9).
AgNPs are produced by three different methods: chemical, physical and biological (biotechnological) ( Citation1–23). In chemical methods, silver ions (Ag+) are reduced to silver atoms (Ag0) in nanoscale by the reducing agent. Many different substances may be used as the reducer – ascorbic acid, sodium citrate, galus acid, hydoquinone, hydrogen peroxide, glucose, sodium bromohydride, formaldehyde or hydrasin ( Citation1–4, Citation8–10). AgNPs obtained by this method are usually unstable and have tendency to agglomerate and sedimentate. To prevent the connection of small NPs with larger aggregates, the stabilizing agents are used, such as synthetic or natural polymers, for example, polyvinylpyrrolidne, polyvinyl alcohol, polyethylene glycols, chitosan, hydroxyethyl cellulose. Literature reports show that some reducing agents, for example, sodium citrate, act as both the reducing and the stabilizing agent ( Citation11–17).
Physical methods of AgNP synthesis require usage of the UV light, ultrasounds, microwaves or electricity. Similar to the chemical reduction method, physical methods require the use of stabilizers to prevent agglomeration of NPs ( Citation8).
Despite the simplicity of AgNP synthesis methods and relatively simple apparatus requirements, many of them require the usage of hazardous chemicals, which are unacceptable in medicine, pharmaceutical and cosmetics industry, as well as large amounts of energy. The residue of the reducing agent in the reaction mixture requires removal, which significantly increases the cost of the manufacturing process and generates wastes that are hazardous for the health and the environment ( Citation1–4, Citation8, Citation11–12).
Due to the many disadvantages of the chemical and physical AgNP synthesis methods, increased attention is paid to the biological methods. In the biological synthesis of AgNPs, herbal extracts and various species of bacteria and fungi can be used. Compared to the chemical and physical methods, biological synthesis does not require the usage of hazardous chemicals, large amounts of energy, high temperature or pressure. Biological methods do not generate hazardous waste and the product usually does not need purification. For that reason, the biological methods are classified as “green chemistry” and are named as the “green synthesis” methods ( Citation2–3, Citation9, Citation18–24).
The biological methods use the ability of natural reducing agents present in plant extracts for the conversion of Ag+ to Ag0. It was shown that several plant extract ingredients, including saccharides, terpenoids, polyols, amino acids, ascorbic acid, geraniol and polyphenols, may act as natural reducers ( Citation2–3, Citation9, Citation18–24). Furthermore, literature reports show that the NPs synthesized using plant extracts display greater stability over a prolonged period of time and do not require the addition of stabilizing agents, probably due to the presence of natural stabilizers like proteins and polyols. Modification of the reaction parameters, such as time and concentration of the reagents, allows to control the size and shape (morphology) of the obtained NPs. Nanomaterials produced by biological methods do not require the usage of complicated and expensive apparatus and purification following the synthesis. The overall costs of the production are low and the AgNPs synthesized by “green methods” may be directly used in pharmaceutical and cosmetic products ( Citation2–3, Citation9, Citation18–24). According to Varshney et al. ( Citation25), one of the plant-derived chemicals with potential application in AgNP synthesis is glycosides obtained from Stevia rebaudiana. The described glycosides are responsible for the reduction of silver ions and act as natural stabilizers of synthesized NPs. According to Huang and Yang ( Citation26), the reduction of silver ions is caused by polysaccharides, whereas the stabilization of NPs is maintained by negatively charged heparin. Among several polysaccharides found in S. rebaudiana, the most effective in the reduction of silver ions and forming the NPs are rebaudiosides A, B, C, D, E and dulcoside A ( Citation27).
Literature reports describing the “green synthesis” of NPs proved that the AgNPs were successfully synthetized using various aqueous plant extracts, including green tea (Camelia sinensis) ( Citation28), coffee ( Citation29), Vitex negundo ( Citation30), pelargonium ( Citation31), rose ( Citation21), mangosteen ( Citation32), Acalypha indica ( Citation33) and S. rebaudiana ( Citation26). The presented study evaluates the usage of S. rebaudiana leaf extracts, prepared using three different solvents, in the “green synthesis” of AgNPs. The btained AgNPs were analyzed for their physiochemical as well as biological properties, including free radical scavenging and antimicrobial activity. Due to the reports indicating the toxic effect of AgNPs, cytotoxicity of AgNPs synthesized using S. rebaudiana extract was evaluated in vitro using the human skin fibroblast model.
Materials and methods
Materials
The extracts used for the synthesis of AgNPs were prepared using dried leaves of S. rebaudiana grown in Poland, purchased in the local market. Silver nitrate (0.001 M standard solution) was obtained from Chempur, ethyl alcohol (98%) from Honeywell and DPPH (2,2-diphenyl-1-(2,4,6-triphenyl-hydrazyl) from Sigma-Aldrich. All extracts were prepared using ultra-pure water, obtained from the Milli-Q Intergral Water Purification System (Merck Millipore). Antimicrobial activity of AgNPs was evaluated using Mueller-Hinton broth (Sigma-Aldrich) and granulated agar (Sigma-Aldrich). Staphylococcus aureus (ATCC 25323), Escherichia coli (ATCC 23724) and Enterococcus faecalis (ATCC 700802) strains were obtained from LGC Standards. In vitro toxicity of AgNPs was analyzed using human skin fibroblast cell line BJ (ATCC®CRL-2522), purchased from LGC Standards. Eagle's essential minimum medium (EMEM) with l-glutamine was purchased from LGC Standards, foetal bovine serum (FBS) was obtained from Invitrogen, whereas Dulbecco's phosphate buffered saline (DPBS) and 3.3 mg/ml neutral red solution were purchased from Sigma-Aldrich. All reagents were of analytical grade.
Preparation of extracts
Three different extracts of S. rebaudiana leaves were prepared using a solvent extraction method. Solvents were: distilled water, ethyl alcohol and a mixture of water and ethyl alcohol in a 1:1 ratio. The procedure was the same for each extract. About 5 g of Stevia leaves were transferred into a mortar containing 20 ml of solvent and pounded for 5 min. The content of the mortar was transferred into a 250 ml beaker and mixed with additional 80 ml of the solvent. The extraction process was carried out in darkness using a mechanical stirrer (OS20 PRO, Chemland, Poland) with a rotational speed of 750 rpm and mixing time of 4 h. Afterwards extracts were filtered twice trough Whatmann filter paper No 1. The extracts were stored in dark glass bottles at 4°C.
Synthesis of AgNPs
In order to synthesize AgNPs, 50 ml of the silver nitrate standard solution (0.001 M) was transferred into an Erlenmeyer flask with stopper. About 0.5 ml of Stevia extract was added and mixed at room temperature (25°C) for 24 h in a magnetic stirrer (250 rpm) in light. Following 5, 60, 120, 180, 360 min, 24 h and 7 days of the reduction synthesis the conversion of Ag ions to AgNPs was monitored spectrometrically. About 0.5 ml of the sample was diluted in 2.5 ml of Mili-Q water and the UV spectrum was measured. The same procedure was repeated for each type of extracts.
Monitoring of AgNP synthesis
By using UV-VIS spectroscopy the properties of Stevia extracts used for the reduction of Ag ions to AgNPs were monitored. In different time intervals after the beginning of the AgNPs synthesis (0, 5, 15, 30, 60, 120, 240 min, 24 h and 6 days) 0.5 ml of the reaction solution was diluted in 2.5 ml Mili-Q water and UV spectra were measured in the range from 350 to 600 nm with spectra resolution of 2 nm, using an AquaMate spectrophotometer (Thermo Scientific).
Determination of the AgNP size
The size distribution and polydispersity index (PDI) of synthetized AgNPs were determined by the dynamic light scattering (DLS) technique using a Zetasizer Nano (Malvern) instrument. The size of the particles was measured 24 h and 6 days after the beginning of the synthesis. The measurements were carried out in the range between 1 and 10,000 nm, at a scattering angle of 137° and temperature of 25°C. The presented values are the average from 3 runs with at least 10 measurements.
Stability of the AgNPs
The stability of the AgNPs synthesized using S. rebaudiana extracts was determined by the multiple light scattering technique, based on the transmission and backscattering measurements. The analysis was performed using a Turbiscan analyzer (Formulation, USA) that scans the samples placed in glass cylindrical cells. The samples are scanned through the entire height by the detection heads, acquiring transmission and backscattering data for every 40 µm. Samples of AgNPs were tested 24 h and 6 days after the beginning of reaction.
DPPH• scavenging activity
Antioxidant activity of AgNPs synthesized using stevia extracts was analyzed using DPPH free radical scavenging assay, according to the method described by Brand-Williams et al. (
Citation34). DPPH is a stable free radical, which accepts an electron and forms a stable molecule. About 50 µM ethanol solution of DPPH was mixed with the analyzed samples or appropriate solvent in a 1:1 ratio. The reaction mixture was mixed and incubated at room temperature for 30 min, in darkness. The absorbance was measured against the blank (96% ethanol) at λ = 517 nm, using a UV–VIS AquaMate Spectrophotometer (Thermo Scientific). The DPPH solution, mixed with an equal volume of distilled water, served as a control. The percentage of the DPPH radical scavenging was calculated using the equation:
where As is the absorbance of the sample, Ac is the absorbance of the control. Each assay was performed in triplicate. The concentration of antioxidants needed to obtain the scavenge of 50% was calculated based on linear regression of the percent inhibition against the concentration of samples, for points located in nearby interested area.
Antibacterial activity
Antimicrobial activity of stevia extracts with AgNPs was analyzed using three different bacteria strains commonly occurring in the environment – E. coli, S. aureus and E. faecalis. The inoculum of each bacteria strain was diluted to 0.5 McFarland. About 100 μl of diluted inoculum was transferred onto Petri dishes containing Mueller-Hinton broth with 2% agar and spread with a Drigalski spatula. After 15 min of incubation, 4 wells with a 7 mm-wide cavity were punctured onto each Petri dish. Petri dishes were made in three series where each set contained a puncture well. The wells in the petri dishes were filled with 50 μl of: nanosilver particles solution, stevia extract solution, sterilized water and silver nitrate and incubated at 36°C for 16 h. The diameter of the inhibition zones was measured using a caliper.
In vitro cytotoxicity assay
Human skin fibroblasts BJ were maintained in EMEM supplemented with 10% FBS at 37°C in a humidified incubator with 5% CO2. For the assay, 3500 of the cells were seeded per well on a 96-well plate and grown overnight. The next day, the cell culture medium was replaced with EMEM supplemented with 1% FBS and different concentrations of aqueous-alcoholic stevia extract, AgNO3 or stevia extract with AgNPs. Control cells were grown in the presence of EMEM + 1% FBS. Following 48 h of culture, the number of viable cells in each well was estimated using the neutral red uptake test ( Citation35). The cell culture medium was removed from the wells and 100 µl of EMEM supplemented with 1% FBS and 33 µg/ml neutral red (Sigma-Aldrich) was added per well. The cells were incubated for 2 h at 37°C followed by washing once with 150 µl DPBS. Neutral red was extracted from the viable cells using an acidified ethanol solution (50% ethanol, 1% acetic acid) and the absorbance of a solubilized dye was quantified at 540 nm using a FilterMax F5 Multi-Mode microplate reader (Molecular Devices). The mean optical density of control cells was set to 100% viability and used to calculate the percentage of viable cells in each experimental condition. The experiments were performed three times using three wells for each extract tested. Statistical analysis was assessed using GraphPad Prism 5.0 software using one-way ANOVA and the differences of the means between samples were determined using the Tukey test. p-Values below .05 were considered significant.
Results and discussion
Stevia extracts, depending on the solvent used in order to obtain them, had different coloring. Aqueous extract was characterized by dark-brown color, alcohol extract by dark-green and extract based on water and alcohol mixture (1:1) had a color between brown and green. Extracts remained stabile, showed no color changes and did not precipitate any solid compounds when stored with and without light exposure.
In order to synthetize the AgNPs, Stevia extracts were mixed with 0.001 M aqueous solution of silver nitrate. Five minutes after the addition of the extracts to an AgNO3 solution, a gradual darkening of the reaction mixture was observed. In the first minutes of the synthesis, mixtures of aqueous and aqueous-ethanol extract of Stevia with silver nitrate became bright red. After 60 min the mixtures became dark red; changes in the color indicated formation of AgNPs. With usage of the aqueous extract of Stevia, after three days from the start of synthesis, sedimentation of a dark pellet in the bottom of the flask was observed. The particles flocculated slowly, formed large aggregates and sedimentated from the reaction mixture. The amount of precipitated aggregates had increased during synthesis time. The changes in suspensions colors when using aqueous-ethanol extract from Stevia leaves were the same as with aqueous extract, but AgNPs prepared in the aqueous-ethanol extract proved to be more stable. According to literature ( Citation25–27) steviol glicosydes exhibited best solubility in the water-ethanol mixture, but weaker solubility both in water as well as in ethanol. Better stability of NPs obtained in the water-ethanol extract may be due to better solubility of steviol glicosydes in this mixture. In their case, sedimentation of large aggregates was observed after five days of synthesis. In the case of AgNP synthesis when using the ethanol extract from Stevia in the first minutes of reduction reaction the mixture became violet in color. However, after 1 h turbidity of the reaction mixture was observed as well as change in color to gray. After 3 h, large agglomerates of AgNPs sedimentated from the suspension. The AgNPs prepared in alcoholic extract were not stable. The cause of instability of the AgNPS may be the denaturation process of proteins. As proved via performed research ( Citation36) extract of stevia leaves contained proteins which can stabilize NPs and get denatured in ethyl alcohol.
Ethyl alcohol used as the extractant in the extraction process caused denaturation of proteins, which are suggested to be the stabilizer agents of AgNPs in green synthesis methods.
As blanks were used, the aqueous solution of AgNO3 and the extracts of Stevia in three different solvents. No changes in the color and precipitation for the blanks during their storage were observed. The UV–VIS spectra after 0, 15, 30, 60, 120, 180, 240 min, 24 h and 7 days of synthesis of AgNPs are shown in (a)–(c).
Figure 1. The influence of ethanolic (a), aqueous (b) and aqueous-ethanolic S. rebaudiana leaf extracts on the synthesis of AgNPS.
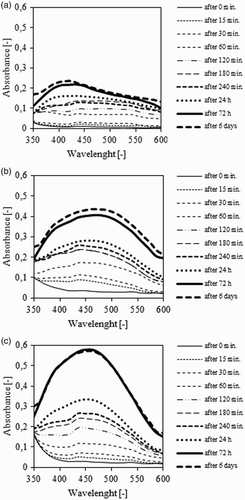
For the samples in which the reduction of Ag ions to AgNPs was induced by aqueous and aqueous-ethanol Stevia extracts with sunlight exposure, a strong characteristic for the AgNPs was observed, with absorbance peak at around 435 nm. The maximum absorbance underwent a slight shift to about 450 nm during synthesis time. In the first 180 min of reduction, the absorbance value was comparable for both types of extracts. After 24 h of synthesis the absorbance for the aqueous-ethanol extract with NPs was higher (about 20–30%) in comparison to that of the aqueous extract. The reason for the difference in the synthesis of AgNPs between the aqueous and the aqueous-ethanol extracts may be distinction in the stability of the Stevia solutions. Definitely lower values of the absorbance were observed for the AgNPs prepared with ethanol extract of Stevia leaves. After 24 h and 6 days of synthesis the absorbance was classified on a similar level to those observed for synthesis of AgNPs in aqueous and aqueous-alcohol extracts after 180 and 240 min. The absorbance peak was observed at around 430 nm.
Size of AgNPs
(a) shows the average size of the synthesized AgNPs, 24 h and 6 days after the beginning of the reduction reaction. (b)–(d) show an example of the size distribution of AgNPs prepared in aqueous ((b)), aqueous-alcoholic ((c)) and alcoholic ((d)) Stevia extracts after the 24-h synthesis.
Figure 2. The analysis of the average size of synthesized AgNPs (a) and particle size distribution after 24 h of synthesis: aqueous-ethanolic extract (b), aqueous extract (c), ethanolic extract (d).
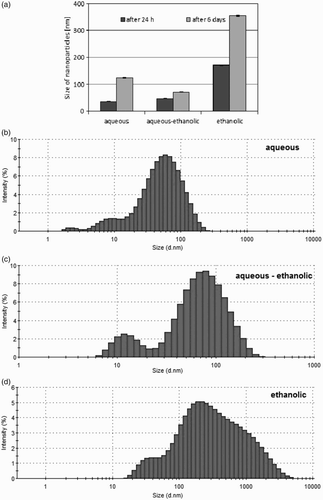
The z-average size of AgNPs synthesized using aqueous extract of Stevia leaves was the lowest after 24 h from the beginning of the reduction reaction. The value obtained for this sample is 35.3 ± 1 nm. After the same time, a slightly higher particle size (46.4 ± 0.9 nm) was observed for AgNPs prepared in aqueous-alcoholic Stevia extract. The largest particles were observed for alcoholic extract. The size of these particles was 170.5 ± 1.6 nm. Six days after the beginning of the synthesis the size of the NPs increased. The smallest increase of the particle size was observed for the AgNPs obtained in the aqueous-alcoholic extract. The particle size after this time was 70 ± 1.1 nm. An increased growth of particle size was noted for aqueous extract of Stevia and the particle size was placed at the level 123.8 ± 1.5 nm. The largest particles (353.6 ± 2.2 nm) were observed for the AgNPs prepared using ethanol extract. AgNPs synthesized in this extract were not stable. Flocculation of small particles results the formation of large agglomerates which precipitate out of the solution and sedimentate.
The PDI of the particles prepared using aqueous and aqueous-alcohol Stevia extract was relatively low. The values for both types of extracts were similar and 24 h after the beginning of the reaction were both approximately 0.40. The PDI decreases slightly to a value of about 0.37, 6 days after the beginning of the AgNP synthesis. The PDI of NPs synthesized in alcoholic extract was higher and amounted to about 0.75 after 24 h and 0.55 after 6 days. This result showed that AgNPs prepared using alcoholic extract have a very broad size distribution.
Stability of AgNPs
The multiple light scattering technique was used in order to measure AgNPs' stability. Mechanism of the Turbiscan analyzer is based on measuring the transmission (T) and backscattering (BS) of the light beam, passing through the sample located in the measuring chamber. The results are collected for every 40 µm. Analysis of results allows to determine the changes in the sample, such as particles' flocculation, size increase (aggregation), sedimentation or creaming, which are invisible to the naked eye. The measurement result is shown as a graph of the transmission and backscattering as a function of sample height.
If the transmission or backscattering increases in the upper part of the graph, it indicates an increase in the number of particles in the upper part of the sample (creaming). Increase in the lower part of the diagram indicates a fall of the particles (sedimentation). An increase or decrease of the analyzed parameters during the measurement in all sample heights indicates an increase in particle size due to flocculation. (a)–(c) shows the results of the measurements of the analyzed samples, 24 h and 6 days after the beginning of synthesis.
Figure 3. The stability of AgNPs synthesized using aqueous (a), aqueous-ethanolic (b) or ethanolic (c) S. rebaudiana leaf extracts, determined by the Multiple Light Scattering technique.
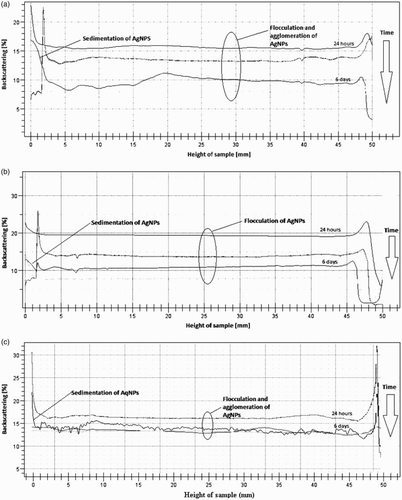
The results show that all samples were not stable. AgNPs synthesized using aqueous, aqueous-alcoholic and alcoholic extracts of Stevia had flocculated and agglomerated. It caused an increase in particle size. Only for alcoholic extract was this phenomenon visible by the naked eye. The measurements of transmission and backscattering also show that synthesized NPs sedimentate on the bottom of the measuring cells. Sedimentation was observed by the naked eye only for the alcoholic extract.
Measurements using the multiple light scattering technique turned out to be an effective, fast and simple method for determination of stability of the obtained NPs.
DPPH● scavenging activity
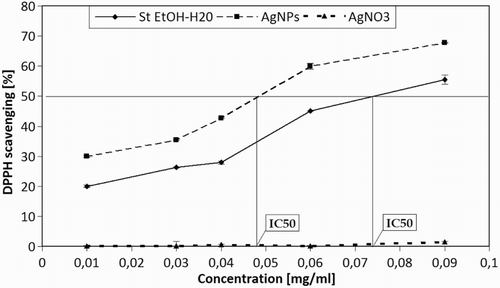
In order to measure the anti-radical activity, DPPH● assay was used. Silver nanoparticles, obtained by synthesis involving an alcoholic-aqueous solution of Stevia showed a concentration-dependent ability to scavenge free radicals determined by DPPH technique. The aqueous-alcoholic extract of Stevia also demonstrated antioxidant activity.. shows antioxidant activity in Stevia aqueous-alcoholic extracts. The highest antioxidant activity was observed for AgNPs (0.01–0.09 mg/ml) with observed values in a range of 31.22–67.80%. In this case, the value of IC 50 regarded as the concentration of antioxidants needed to obtain a scavenge of 50% equaled 0.05. Lower values of DPPH scavenging were observed for aqueous-alcoholic extracts, with values in the range of 18.21–55.54% and IC 50 = 0.072. In the case of AgNO3, no antioxidant properties were observed. This suggests that the increase in antioxidant capacity is a result of the creation of AgNPs. Stronger antioxidant properties of the mixture of AgNO3 compared to the pure extract of Stevia were an additional confirmation of NPs' synthesis. NPs with smaller dimensions and larger interfacial area showed higher antioxidant properties, in comparison to larger particles. Results of the research carried out were consistent with the current state of knowledge ( Citation37).
Antimicrobial activity
All petri dish inhibition zone series for all of three bacteria were measured, averaged and pictured on a graph ().
Figure 5. Inhibition of S. aureus, E. coli and E. faecalis growth in the presence of AgNPs synthesized using aqueous-ethanolic S. rebaudiana extract, aqueous-ethanolic Stevia extract or appropriately diluted AgNO3. Inhibition zones were measured following 16 h of bacteria growth in the presence of analyzed solutions or sterile H2O as a negative control. The histogram shows average values from three experiments ± SD. ***p < .001.
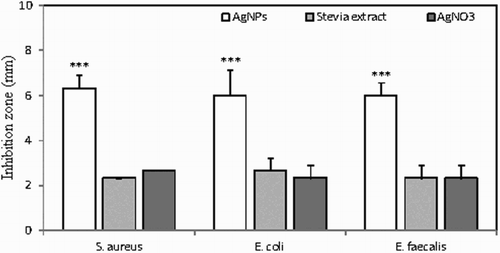
Results were clear that AgNPs have larger inhibition zones than silver nitrate or stevia extracts by themselves in all of the studied microorganisms. AgNPs had the highest inhibition ability in S. aureus, a gram-positive coccal bacterium found mainly on skin (average inhibition zone of 15.3 ± 0.58 mm) whereas using stevia extract it was significantly smaller (9.3 ± 1.15 mm average), similar to silver nitrate (9.7 ± 0.57 mm average). Inhibition in the presence of nanosilver with E. coli, gram-negative, facultative anaerobic, rod-shaped bacterium found in the lower intestine of a majority of mammals was slightly lower (12.7 ± 0.58 mm average), while inhibition similar to the rest of the studied organism was observed in stevia extract (9.3 ± 0.57 mm average) and silver nitrate (9.7 ± 0.57 mm average). In our studies the lowest inhibition ability of nanosilver was shown with E. faecalis gram-positive, commensal bacterium inhabiting the gastrointestinal tracts of humans (average inhibition zone of 10.7 ± 1.15 mm), and even smaller zones were reported on samples of stevia extract (8.7 ± 0.57 mm average) as well as silver nitrate (9.0 ± 0.0 mm average). Deionized, sterilized water which was used as control, showed no inhibition in all tested samples. Inhibition zones were equal to the cavity diameter (7 ± 0.0 mm). Studies conducted at Islamic Azad University also showed similar effects in microbiological inhibition of E. coli and S. aureus. Inhibition zones were measured using 6 mm discs. AgNPs were compared to silver nitrate and V. negundo extract from which AgNPs were obtained. Results showed that AgNPs had the highest inhibition zones in both bacterial models (12.0 ± 0.7mm for E. coli and 11.0 ± 0.3 for S. aureus) while silver nitrate had significantly smaller inhibition zones (9.5 ± 0.5mm for both bacteria) and V. negundo showed no inhibition at all (6.0 ± 0mm for both microorganism) (Citation30). Different studies of the Division of Environmental Science and Nanobiotechnology produced results of AgNPs' (obtained from Plumeria alba) influence on S. aureus and E. coli. Results show that all three bacteria were susceptible for AgNPs activity, and showed large inhibition zones: 15 ± 0.2mm (S. aureus) and 12 ± 0.3mm (E. coli) ( Citation38). Results clearly show that AgNPs have increased antibacterial activity. Stevia extract and silver nitrate also possess such activity but it is lower and may be caused by inferior antibacterial abilities and smaller diffusion of those compounds in agar. Further studies should be conducted to analyze this mechanism. NPs show good diffusion in agar medium and may be tested on other bacterial models.
In vitro cytotoxicity of AgNPs synthesized using aqueous-alcoholic stevia extract
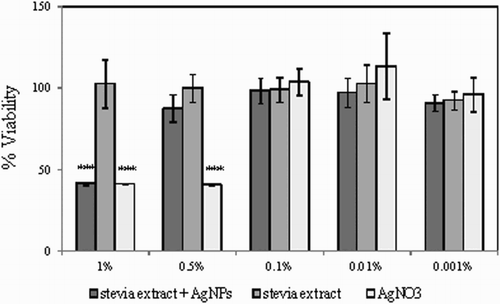
AgNPs synthesized using aqueous-alcoholic extract from S. rebaudiana leaves showed significant free radical scavenging ability () and antimicrobial activity (). However, the application of AgNPs in household products and cosmetics is often limited due to its high toxicity. For that reason the influence of AgNPs synthesized in the presence of stevia extract on human skin fibroblasts was analyzed in vitro. Aqueous-alcoholic extract of stevia as well as 0.001 M AgNO3 solution were filter-sterilized, mixed in a 1:100 ratio and incubated for 24 h in light. In order to compare the cytotoxic effect of AgNPs with the potential toxicity of AgNO3 and stevia extract alone, appropriate control mixtures were prepared (100 µl H2O + 10 ml 0.001 AgNO3 or 100 µl stevia extract + 10 ml H2O). The prepared reaction mixtures were then added to human skin fibroblasts grown on 96-wells in EMEM supplemented with 1% FBS. The addition of 1% FBS allowed to maintain healthy cells without significant stimulation of cell proliferation and helped to evaluate the biological effect of AgNPs. Following 48 h of culture in the presence of 1–0.001% of AgNPs and stevia extract of AgNO3, the number of viable fibroblasts in each experimental condition was evaluated using the neutral red uptake test ( Citation35). As shown in , at a concentration of 1% AgNPs as well as AgNO3 there was a significant reduction in the number of viable fibroblasts to about 40%, indicating their high cytotoxicity and thus skin irritating potential. Interestingly, at the 0.5% concentration AgNPs did not impair the viability of fibroblasts, whereas the analogous concentration of AgNO3 was still highly cytotoxic. At concentrations from 0.1% to 0.001% none of the tested solutions displayed significant in vitro toxicity against fibroblasts.
Figure 6. Viability of human skin fibroblasts in vitro following 48 h of culture in the presence of stevia extract + AgNPs, stevia extract or AgNO3. The viability of untreated cells was set to 100% and used to calculate the percentage of viable cells in each experimental condition. The histogram shows the mean values from three experiments with three repeats per condition ± SD. ***p < .001.
Conclusion
The presented study describes a fast and simple “green synthesis” method of AgNPs, using S. rebaudiana leaf extracts. The influence of the solvent type used for the extraction of Stevia on the reduction efficiency of Ag ions to Ag NPs was analyzed. Also the size of NPs and their stability were measured.
In the present study, it was found that aqueous and aqueous-alcoholic extracts from Stevia leaves can be adequate sources for synthesis of AgNPs. AgNPs synthesized in alcoholic extract were not stable. The average size of NPs was observed to be about 40 nm for aqueous and aqueous-alcoholic extracts and 170 nm for alcoholic Stevia extracts. Six days after the beginning of reduction, the particle size had grown. The largest increase of AgNPs' size was observed for alcoholic and aqueous extracts, the smallest for the aqueous-alcoholic extract. The multiple light scattering method showed that AgNPs prepared in all type of extracts flocculate and agglomerate, but even after several days of synthesis sedimentation of AgNPs only for alcoholic extracts was observed by the naked eye.
For further analysis (evaluation of antioxidant capacity, antimicrobial properties and cytotoxicity) AgNPs obtained using aqueous-alcoholic extract of Stevia were chosen, due to their best properties. It was demonstrated that AgNPs have better antioxidant properties than Stevia extract alone.
Microbial studies had proven substantial inhibition properties of AgNPs obtained from aqueous-alcoholic Stevia extracts. It was shown that in all studied models, organism NPs had blocked bacterial growth better than both silver nitrate and stevia extract. Inhibition zones of studied AgNPs were 2–6 mm wider in diameter than compared substances, thus proving the activity of NPs.
The results obtained from the in vitro cytotoxicity assay showed that in addition to significant antioxidant potential and antimicrobial activity, AgNPs synthesized using aqueous-alcoholic extract from S. rebaudiana possess lower cytotoxicity than AgNO3 alone, strongly suggesting the potential for the application of the described AgNPs' “green synthesis” method in the pharmaceutical or cosmetic industry. Presented results show that Stevia aqueous-alcohol extract is a donor of natural substances with good reduction properities, such as glicozydes. It is also characterized by moderatate stabilization potencial. In addition this extract may be used in fast, simple and eco-friendly synthesis of AgNPs. Further research of ecological stabilizers for this system should be conducted.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Suber, L.; Sondi, I.; Matijevi, E.; Goia, D.V. J. Colloid Interface Sci. 2005, 288, 489–495.
- Raj, S.A.; Divya, S.; Sindhu, S.; Kasinathan, K. Int. J. Pharm. Pharm. Sci. 2014, 6, 453–455.
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. J. Nanopart. Res. 2008, 10, 507–517.
- Chaloupka, K.; Malam, Y.; Seifalian, A. Trends Biotechnol. 2010, 28, 580–588.
- Cho, K.H.; Park, J.E.; Osaka, T.; Park, S.G. Electrochim. Acta. 2005, 51, 956–960.
- Sondi, I.; Salopek-Sondi, B. J. Colloid Interface Sci. 2004, 275, 177–182.
- Jung, W.K. Appl. Environ. Microbiol. 2008, 74, 2171–2178.
- Panigrahi, S.; Kundu, S.; Ghosh, S.; Nath, S.; Pal, T. J. Nanopart. Res. 2004, 6, 411–414.
- Albrecht, M.A.; Evans, C.; Raston, C. Green Chem. 2006, 8, 417–432.
- Chou, K.S.; Ren, Ch.Y. Mater. Chem. Phys. 2000, 64, 241–246.
- Chou, K.S.; Lai, Y.S. Mater. Chem. Phys. 2004, 83, 82–88.
- Pencheva, D.; Bryaskova, R.; Kantardjiev, T. Mater. Sci. Eng. C. 2012, 32, 2048–2051.
- Yu, D.G. Colloids Surf. B. 2007, 59, 171–178.
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. J. Colloid Interface Sci. 2005, 288, 444–448.
- Smetana, A.B.; Klabunde, K.J.; Sorensen, C.M. J. Colloid Interface Sci. 2005, 284, 521–526.
- Huang, H.; Yang, Y. Compos. Sci. Technol. 2008, 68, 2948–2953.
- Sun, Ch.; Qu, R.; Chen, H.; Wang, Ch. Carbohydrate Res. 2008, 343, 2595–2599.
- Park, Y.; Hong, Y.; Weyers, A.; Kim, Y.; Linhardt, R. IRT Nanobiotech. 2011, 5, 69–78.
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. J. Nanopart. Res. 2008, 10, 507–517.
- Bar, H.; Bhui, D.; Sahoo, G.P.; Sarkar, P.; Misra, A. Colloids Surf. A. 2009, 339, 134–139.
- Dubey, S.P.; Lahtinen, M.; Sillanpä, M. Colloids Surf. A. 2010, 364, 34–41.
- Narayanan, K.B.; Sakthivel, N. Adv. Colloid Interface Sci. 2010, 156, 1–13.
- Song, J.; Kim, B. Bioprocess Biosyst. Eng. 2009, 32, 79–84.
- Raveendran, P.; Fu, J.; Wallen, S.L. Green Chem. 2005, 8, 34–38.
- Varshney, R.; Bhadauria, S.; Gaur, M.S. Adv. Mat. Lett. 2010, 1, 232–237.
- Huang, H.; Yang, X. Carbohydr. Res. 2004, 339, 2627–2631.
- Kinghorn, A.D. Stevia: The Genus Stevia; Taylor & Francis: London, 2002.
- Loo, Y.Y.; Chieng, B.W.; Nishibuchi, M.; Radu, S., Int. J. Nanomedicine 2012, 7, 4263–4267.
- Nadagouda, M.N.; Varma, R.S. Green Chem. 2008, 10, 859–862.
- Zargar, M.; Hamid, A.A.; Bakar, F.; Shamsudin, M.N.; Shameli, K.; Jahanshiri, F.; Farahani, F. Molecules. 2011, 16, 6667–6676.
- Safaepour, M.; Shahverdi, A.R.; Shahverdi, H.R.; Khorramizadeh, M.R.; Gohari, A.R. Avicenna J. Med. Biotechnol. 2009, 1, 111–115.
- Veerasamy, R.; Xin, T.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. J. Saudi Chem. Soc. 2011, 15, 113–120.
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Colloids Surf. B. 2010, 76, 50–56.
- Brand-Williamis, W.; Cuvelier, M.; Berset, C. LWT – Food Sci. Technol. 1995, 28, 25–30.
- Repetto, G.; del Peso, A.; Zurita, J.L. Nat. Protoc. 2008, 3, 1125–1131.
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Molecules. 2015, 20, 5468–5486.
- Bunghez, I.R.; Barbinta-Patrascu, M.E.; Badea, N.; Doncea, S.M.; Popescu, A.; Ion, R.M. J. Optoelectron. Adv. Mater. 2012, 14, 1016–1022.
- Patil, B.M.; Hooli, A.A. J. Nanosci. NanoEng. Appl. 2013, 3, 13–18.