ABSTRACT
Among a variety of microbial materials employed for biosorption, algae have added advantages of non-toxic and autotrophic nature. In this study, biosorption of Hg(II) was studied with red algal biomass of Porphyridium cruentum. The parameters affecting biosorption such as dosage of biosorbent, pH, contact time, initial metal concentration, temperature and effect of foreign metal cations in binary system were evaluated. Kinetic data were described with the help of pseudo-first-order and pseudo-second-order kinetic models. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherm models were applied to adsorption equilibrium data. According to the results, the maximum removal capacity (qmax) was 2.62 mg/g observed at pH 7 with 0.25 g/L of biosorbent dosage for Hg(II) solution containing 10 mg/L of metal ions. The Langmuir isotherm model fits best to the adsorption data while the kinetic data followed the pseudo-second-order model. Thermodynamics studies showed that the biosorption process of Hg(II) on P. cruentum was exothermic in nature.
GRAPHICAL ABSTRACT
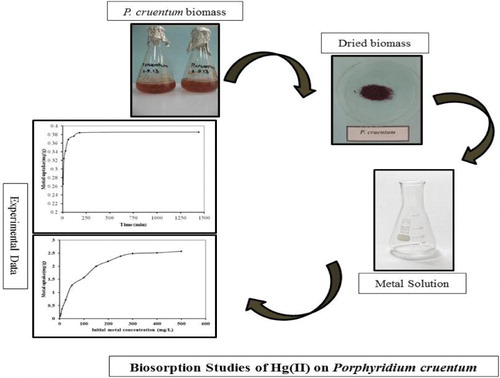
1. Introduction
Mercury has been declared among top-priority toxic heavy metals due to its persistent nature, long transport ranges in atmosphere and ability to bioaccumulate in food chain (Citation1). Naturally mercury exists in earth crust and metal is released into our environment through weathering of rocks and volcanic activities (Citation2). Re-emission from soil, vegetation or sea surface is also considered an important natural source (Citation3). In addition to these natural sources, mercury is anthropogenically released through combustion of coal and oil, cement production, manufacture of pig iron, steel and non-ferrous metals, batteries, lamps, measuring devices (barometer, thermometer, etc.), chlor alkali industry, dental amalgam, vinyl monomer production and artisanal gold mining (Citation4). Mercury is a neurotoxin and mainly affects the nervous system. After entrance into the human body, mercury compounds can be oxidized to inorganic mercury state. Mercuric cations have a strong tendency to bind with proteins due to their affinity for sulfhydryl groups and cause visual constriction, loss of motor coordination, memory, hearing, speech, taste and ultimately total damage to the brain and central nervous system (Citation5). In history two notorious cases of mercury poisoning occurred, one in Japan known as Minamata disease (Citation6) and other in Iraq (Citation7). Therefore, in industrial wastewater, water for domestic and agricultural-use mercury level should be within the permissible limit (0.001 mg/L) (Citation8). In the perspective of mercury removal, several methods are available including chemical precipitation, oxidation, filtration, electrochemical treatment and evaporation (Citation9). Though these methods are globally efficient however, they are noncompetitive in case of wastewater containing lower metal concentration (Citation10). On the other hand, biosorption has emerged as an effective alternative to the above-mentioned methods due to low operating cost, short operation time and absence of toxic by-product production (Citation11). Among a number of biological materials available for biosorption, including moss (Citation12), lichen (Citation13) and fungus (Citation14), algae have been recognized as a potent biosorbent material due to its easy and cheap availability, greater surface area and high metal binding capacity(Citation15, Citation16). Over the time period, brown and green algae have been exploited for heavy metal removal in comparison to red algae (Citation17). However, red algae have played significant role in the removal of Pb(II) and Cd(II) ions (Citation18). In algae, biosorption occurred through electrostatic interaction, complexation or ion exchange with functional moieties of the algal cell wall structure such as carboxyl, hydroxyl, amino and sulfate (Citation19) and particularly in red algae, metal binding capacities are attributed to sulfated polysaccharides, made of galactans (Citation17).
Marine red unicellular micro algae from the genus Porphridium (Porphyridiales, Rhodophyta) are characterized by extracellular sulfated polysaccharides with acidic characteristics and found potential application as therapeutic and nutraceutical agent (Citation20). Porphridium cruentum is unicellular marine red micro algal (Rhodophyta) species (Citation21). In P. cruentum, the presence of starch, carbohydrates and sugar monomers (glucose, xylose, methyl glucaronic acid) of polysaccharides makes it a potential carbon source (Citation22) and the biomass consists of 32% (w/w) available carbohydrates and 34% (w/w) crude proteins (Citation23). This showed that it could be a potent agent for removal of heavy metals. To the best of our knowledge, this red micro algal species has not been yet employed for mercury removal.
In the present study, various parameters were performed to assess, characterize and quantify uptake of Hg(II) by Porphyridium cruentum using batch biosorption studies. The study is aimed to have an insight into the biosorption mechanism and the effects of various cations on the biosorption of Hg(II).
2. Materials and methods
2.1. Metal solution
Stock solution of Hg(II) was prepared from its nitrate salt (Merck, Germany) and subsequent solutions were prepared on daily basis by dilution of stock solution. All the chemicals used to prepare the nutrient medium were also of analytical grade (Merck, Germany) and used without further purification. Deionized water was used for all kinds of solution preparation and dilutions.
2.2. Cultural condition for P. cruentum
P. cruentum strain (CCAP 1380/1A) was collected from the Culture Collection of Algae and Protozoa (CCAP) UK. The nutrient medium for this algal specie was prepared as reported elsewhere (Citation21). P. cruentum biomass was grown in Erlenmeyer flasks (250 mL) containing the nutrient medium (100 mL). After inoculation, cultures were maintained at 25°C with continuous shaking in an incubator and illuminated with fluorescent cool-white lamps (color code 840) at an irradiance of 78 µE m−2 s−1 (app.7000 lux) for 28 days. At the end of the growth cycle, biomass was harvested through centrifugation and freeze-dried. Freeze-dried biomass was ground, sieved through mesh of 250 µm and stored in air tight desiccator till further use.
2.3. Characterization of biosorbent
Point of zero charge is considered an important characteristic of biosorbent due to its role in ionization and interaction between sorbate and sorbent. In case of point of zero charge determination, initial pH of a series of KNO3 (0.1 M, 50 mL) solution was adjusted from 2 to 12. Algal biomass (0.1 g) was added to above solutions and equilibrated for 48 h. After this, final pH of each solution was noted and point of zero charge was determined from the plot drawn between initial pH (pHi) and ΔpH (pHi−pHf) (Citation24).
Fourier Transformed Infrared Spectroscopy (FTIR) with attenuated total reflectance (ATR) in solid phase was used for detection of functional moieties on unloaded and Hg(II)-loaded P. cruentum in the range of 4000–400 wavenumbers cm−1 (Alpha Bruker, Germany). The data obtained from FTIR-ATR spectra were evaluated with OPUS® 5.5 software. Hg(II)-loaded P. cruentum was prepared by adding 0.1 g/L of biosorbent to Hg(II) solution (100 mg/L) and left on shaking for 24 h. Afterwards, biomass was separated through centrifugation and oven-dried.
The effective surface area of P. cruentum and Hg-loaded P. cruentum was determined using Chemisorb 2750 (Micromeritics, USA) analyzer. This method utilized the low-temperature nitrogen adsorption procedure with further analysis by Brunauer-Emmett-Teller (BET) model (Citation25).
2.4. Biosorption batch studies
Batch studies were performed to optimize the biosorption process variables. Batch experiments were conducted by adding an appropriate amount of biomass (g/L) in metal solution (100 mL) in an Erlenmeyer flask and agitating on an orbital shaker at a speed of 110 rpm and temperature 25°C. The effect of pH was studied in the range of 2–10 at fixed operating conditions. The initial pH of the solution was adjusted by HCl (0.1 M) or NaOH (0.1 M). Different biosorbent dosages (0.01–1 g/L) were tested for a fixed metal solution concentration at predetermined experimental conditions. The effect of different initial metal concentrations was studied in the range of 2.5–500 mg/L at a predetermined pH and biosorbent dosage value. Similarly effect of contact time was evaluated in the range of 5–1440 min and effect of temperature at 298, 308 and 318 K, respectively.
The residual Hg(II) concentration was estimated in filtered samples using a UV-Vis spectrophotometer at an absorbance wavelength of 650 nm after complexation with 2-(2-benzothiazolylazo)-p-cresol (Citation26). The metal uptake capacity (q, mg/g) and metal percentage removal were calculated as follows:(1)
(2)
‘Ci’ and ‘Cf’ are the initial and final metal concentrations (mg/L), ‘V’ is the volume of metal solution (L) and ‘W’ is the biosorbent amount (g), respectively.
3. Results and discussion
3.1. Characterization of biosorbent
The characterization of P. cruentum was performed using available techniques, in order to have an idea about the possible binding of Hg(II) ions with it. The isoelectric point or point of zero charge was determined so that the possible behavior of biomass towards Hg(II) biosorption might be explained as a function of solution pH. As shown in (a), pHpzc was found to be 5. This indicated the presence of weak acidic functional groups in P. cruentum. A pH of solution, less than 5, protonates the material resulting in positively charged surface and a decreased biosorption in an acidic medium. On the other hand, a pH above 5 will result in the negatively charged surface and increased biosorption of Hg(II) ions(Citation27). Hence, P. cruentum has an acidic surface that might be due to dissociation of carboxyl groups in alginic acid as well as some contribution from aspartic acid and glutamic acid present in algal proteins (Citation28).
Figure 1. (a) Determination of point of zero charge of P. cruentum and (b) FTIR spectra of unloaded and Hg(II)-loaded P. cruentum.
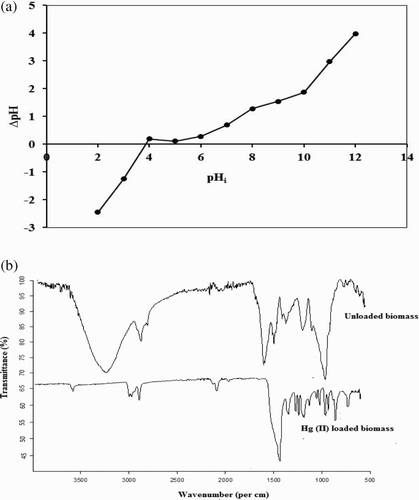
The presence of a number of functional groups in P. cruentum was confirmed by FTIR analysis. The results demonstrated ((b)) showed the FTIR spectra of unloaded and Hg(II)-loaded biomass. These results illustrated the presence of various functional moieties on the cell wall structure of algal biomass and the possible interaction between the metal ion and these functional groups. From the data, it can be seen that in the unloaded biomass, the broadband at 3394 cm−1 was due to H bonded –OH group stretching vibrations (Citation26). Band at 2795 cm−1 was attributed to –CH stretching of methoxy group and 1614 cm−1 could be assigned to asymmetric –C=O stretching vibration (Citation27). Asymmetric and symmetric SO3 stretching vibrations gave bands at 1381 and 1173 cm−1, respectively (Citation28). Intense peak at 981 cm−1 arises due to the presence of galactose. Bands on 845 and 805 cm−1 are due to –C–O–SO3 bond on C4 of galactose and C2 of 3,6-anhydrogalactose (Citation18). The presence of both –OH and –C=O groups indicated the possible presence of carboxylic acid groups. Since, the pHpzc is 5 (slightly acidic), it can be inferred that these acid groups are part of long chains and thus are weak acids.
In order to have an insight into binding sites, FTIR spectrum of Hg(II)-loaded P. cruentum was also with that of unloaded one. In Hg(II)-loaded biomass, the bands appeared at 3520, 2850, 2920, 2087, 1401, 1310, 1180, 930 and 845 cm−1 wave numbers. It suggested the presence of –OH stretching, symmetric and asymmetric stretching of aliphatic chains (–CH), terminal alkyne (≡C) mono-substituted group vibration, asymmetric –C=O stretching vibration, asymmetric and symmetric SO3 stretching vibrations, –C–O–SO3 bond vibration on galactose and –C–O group vibration of 3,6-anhydrogalactose, respectively.
From the comparison of both FTIR spectra, it can be concluded that major shifts appeared in the wavenumbers for functional groups at absorption band of –OH, –C=O and –C–O–SO3 (3,6-anhydrogalactose) groups that underwent a shift from 3394 to 3520 cm−1, from 1614 to 1401 cm−1 and from 805 to 930 cm−1. This showed that these groups have actively participated in the binding of Hg(II). Point of zero charge determination has indicated the acidic nature of P. cruentum biomass. This is further confirmed by FTIR spectra studies.
BET surface area is a useful indicator for the adsorption potential of the biosorbent. The chemisorption BET surface area of P. cruentum was 6110.21 m2/g and that of Hg-loaded P. cruentum was 1731.79 m2/g. The monolayer volume for P. cruentum was 1403.61 cm3/g and that of Hg-loaded biosorbent was 397.82 cm3/g. The significant decrease in the BET parameters indicated the high potential of the biomass under study for the adsorption of Hg(II) ions from aqueous solutions.
The characterization indicated that the biomass from red algae could be a potential candidate for the biosorption of Hg(II) ions in slightly acidic or basic solutions.
3.2. Effect of biosorbent dosage
Biosorbent dosage has a great influence on the biosorption process and defined the potential of biosorbent through available binding sites for fixed initial metal concentration. showed the effect of biosorbent dosage studied in the range of 0.01–1 g/L at constant experimental conditions of 10 mg/L Hg(II) solution and temperature 25°C. The biosorbent dosage and metal removal percentage are directly related to each other as increment in biosorbent amount from 0.01 to 0.25 g/L increased metal removal percentage from 37% to 88%.
Figure 2. Effect of biosorbent dosage on biosorption studies of Hg(II) by P. cruentum (Co = 10 mg/L, contact time = 1440 min, solution pH).
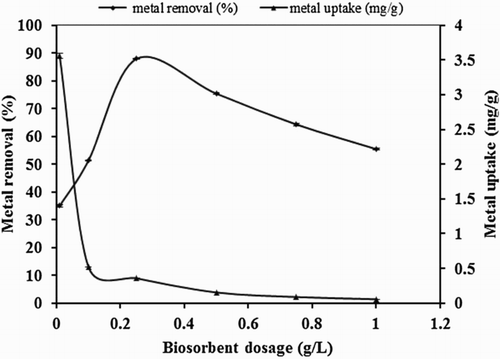
On further increasing the dosage quantity (0.25–1 g/L), metal removal percentage was decreased. Increase in percentage removal in the initial set of dosage quantity increment was due to availability of greater surface area. Decrease in percentage removal at a later stage was due to the reason that biomass formed aggregates at higher dosage quantity which reduced the effective biosorption surface area (Citation18). On the other hand, an inverse relation was found to exist between biosorbent dosage and metal uptake capacity. The metal uptake capacity decreased from a value of 3.56–0.056 mg/g when the biomass amount increased from 0.01 to 1 g/L. The decrease in metal uptake capacity might be attributed to the split in the flux and aggregation of sites (Citation 29).
3.3. Effect of pH
pH of the metal solution reflects the hydrogen ion concentration as it affects the charge network of biomass’s cell wall structure in addition to chemistry and hydrolysis of metal ions. The effect of pH on removal capacity of P. cruentum for 10 mg/L Hg(II) solution was studied in the range of 2–10 (). An increment in metal uptake was observed with an increase in pH and a maximum value was obtained at pH 7 while no detectable change was noticed between pH 7 and 10. The increase in the uptake capacity with pH of solution can be explained on the basis of electrostatic interactions between metal ions and the ‘charged’ biomass surface. Hg(II) ions speciation is given in inset in .
Figure 3. Effect of pH on biosorption studies of Hg(II) by P. cruentum (Co = 10 mg/L, biosorbent dosage = 0.25 g/L, contact time = 1440 min). Inset graph indicates the speciation of Hg(II) with pH.
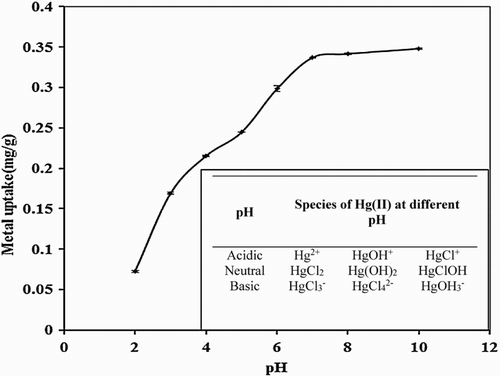
Point of zero charge for P. cruentum was calculated to be 5. At pH < 5, it had positive surface charge and electrostatic repulsion from a number of H+ ions and mercury cationic species (Hg2+, HgCl+, Hg[OH]+) caused a lower attachment of metal ions. At pH > 5, the biosorbent sites carried negative charges, thus favoring the attachment of metal cationic complexes. Between pH 7 and 10, no significant change was observed. On the other hand, in basic conditions, Hg(II) ions mostly either precipitated out or were present in negatively charged moieties. Such a precipitation was unwanted for the biosorption studies since mutual binding of metal ‘ions’ with biomass was no more present. Thus pH 7 was taken as the optimum pH value for further biosorption studies.
3.4. Effect of contact time
In the biosorption process with the passage of time, the process attains equilibrium and kinetics of the process can be studied. Thus the effect of contact time is very important for process optimization. The effect of contact time on the mercury removal capacity of P. cruentum was depicted in . With increasing the contact time, the metal removal capacity of Hg(II) with P. cruentum also increased. The sharp increase in the initial interval indicated a high affinity of P. cruentum biomass for Hg(II) ions. Equilibrium was established at 120 min. Further studies were performed at optimum 120 min of contact time.
Figure 4. Effect of contact time on biosorption studies of Hg(II) by P. cruentum (Co = 10 mg/L, biosorbent dosage = 0.25 g/L, pH = 7). Inset graph shows the fitting of PFO and pseudo-second-order kinetic models.
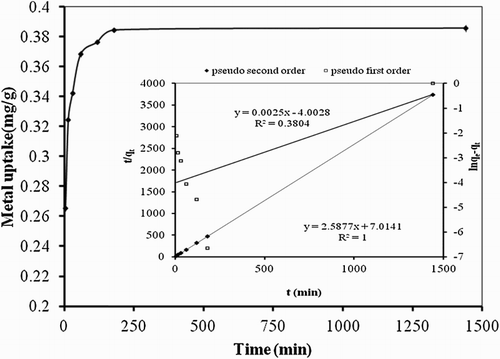
The biosorption of Hg(II) on P. cruentum gradually attained equilibrium as three mass transport steps involved in the biosorption process. During the early stage of biosorption process, there was an external surface mass transport or film diffusion process (process I) followed by a constant rate stage (process II) and ultimately by a diffusion stage (process III) where the biosorption process slowed down considerably (Citation30). The very small difference between the vertical error bars, a measure of standard deviation, showed very stable and reproducible results. Smoothness of the curve was indicative of very stable biosorption of Hg(II) ions. depicted that time profile of Hg(II) was single, smooth and continuous curve eventually leading to the monolayer coverage of Hg(II) on P. cruentum.
3.5. Biosorption kinetics
Kinetic models were used to investigate biosorption data in terms of order and rate constant for the biosorption process. Kinetic data were treated with the Lagergren pseudo-first-order (PFO) rate equation (Citation31) given below in linear form:(3)
where qe (mg/g) is the amount of Hg(II) adsorbed at equilibrium, qt (mg/g) is the amount of Hg(II) adsorbed at time t, and k1 (1/min) is the Lagergren’s PFO rate constant. The process will be considered to follow PFO if the straight line is obtained. A linear fit is shown when R2 is equal to or greater than 0.98. Another tool is when the calculated qe is equal to experimental qe. This can be shown by the percent deviation () value. Values of k1 and qe were calculated from slope and intercept of ln(qe−qt) and t (inset graph in with right-hand side y-axis). Data given in showed that coefficient of determination carried a significantly low value and calculated qe value differed remarkably from the experimental one. All these values suggested that biosorption of Hg(II) on P. cruentum did not follow the PFO reaction.
Table 1. PFO and second-order kinetics parameters for the biosorption of Hg(II) on P. cruentum.
Kinetic data were further investigated with Ho’s pseudo-second-order kinetic model (Citation32). The linear equation for this model is given below:(4)
From the slope and intercept of linear plot of t/qt and t, values of k1 and qeq were calculated (inset graph in with left-hand side y-axis). The value of qe calculated from the pseudo-second-order equation was quite close to the experimental qe value (a very small D% value). Moreover, R2 value of 1 was obtained indicating a true linear relationship between the independent and dependent parameter for Equation (4) (). Thus this study followed the pseudo-second-order kinetics and depicted the involvement of chemisorption process in its rate determining step (Citation33). This is in accordance with the biosorption of most of the divalent metal ions ().
3.6. Effect of initial metal concentration
The effect of initial metal concentration (2.5–500 mg/L) in the biosorption studies of mercury with P. cruentum is shown in . With the increase in initial concentration, the amount of metal adsorbed increased from 0.10 to 2.57 mg/g for a constant amount of biosorbent. However, the percentage removal decreased from 97% to 12.9%.
Figure 5. Effect of initial metal concentration on biosorption studies of Hg(II) by P. cruentum (biosorbent dosage = 0.25 g/L, pH = 7, contact time = 120 min).
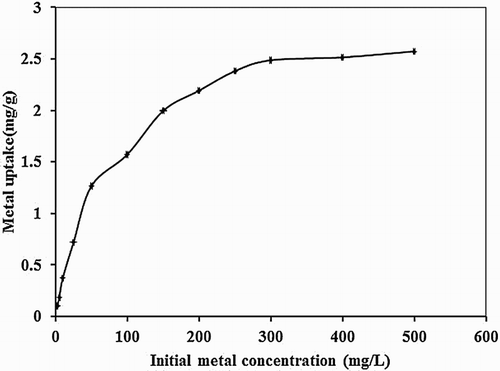
The decrease in percentage removal at higher concentration attributed to lack of sufficient binding sites for increasing number of metal ions in solution. At low concentration of metal, all ions interacted with active sites resulting in high removal efficiency. On the other hand, metal uptake capacity showed a low value at low concentration of metal ion due to a decrease in diffusion coefficient and mass transfer coefficient (Citation34). With an increment in initial concentration, mass transfer driving forces of metal species between the aqueous phase and biosorbent phase increased leading to the increase in biosorption efficiency.
3.7. Biosorption isotherm
In order to optimize the biosorption process, different isotherms were applied using their linear forms. The purpose was to have an insight into the biosorbate–biosorbent relationship.
Langmuir isotherm (Equation (5)) assumes a monolayer coverage of adsorbate on adsorbent having finite number of adsorption sites of uniform energies with no transmigration of adsorbate into the plane of the surface. The linear form is given below:(5)
where qeq (mg/g) is the amount of Hg(II) adsorbed at equilibrium, qmax (mg/g) is the maximum adsorption capacity and b (L/mg) is the Langmuir constant calculated from slope and intercept of linear plot between Ceq/qeq and Ceq. The essential features of Langmuir isotherm is expressed by a separation factor RL (Equation (6)).(6)
It indicates shape of the isotherm and favorability of the biosorption procedure.
Freundlich isotherm (Equation (7)) is an empirical equation based on sorption on a heterogeneous surface or surface containing sites of varied affinities. It is assumed that stronger binding sites are occupied first and binding strength decreases with the increase in site occupation. The linearized form is:(7)
where KF and 1/n are the Freundlich constants calculated from intercept and slope of plot between ln qe and ln Ce. KF (mg/g) is the adsorption diffusion coefficient and represents the quantity of Hg(II) adsorbed on P. cruentum per unit equilibrium concentration, 1/n represents the adsorption intensity or surface heterogeneity.
In Temkin isotherm it is assumed that heat of adsorption falls in a linear way rather than logarithmically, as implied in Freundlich isotherm. As shown in Equation (8), its derivation is characterized by uniform distribution of binding energies and is carried out by plotting a graph between ln Ce and qe.(8)
where AT (L/mg) is the Temkin isotherm equilibrium binding constant and B (J/mol) is the constant related to the heat of sorption.
The Dubinin–Radushkevich (D–R) isotherm (Equation (9)) is considered an analogue of Langmuir, as it describes adsorption on a single type of uniform pores. However, it is more general as it does not assume homogeneous surface or constant sorption potential. The linear form of this isotherm is:(9)
where ε (=RT ln (1 + 1/Ce)) is Polanyi potential, qs is the adsorption capacity, K (mol2/kJ2) is a constant related to adsorption energy, R (8.314 J/mol K) is universal gas constant and T (K) is temperature in absolute units. The slope and intercept of plot between ln qe and ε2 gave the values of constants. This approach is usually employed to distinguish between physical and chemical adsorption of metal ions with mean free energy ‘E (=(−2K)−1/2)’ per molecule of adsorbate (Citation35).
The constants calculated for the various adsorption isotherm models are given in . It can be seen that highest coefficient of determination was obtained for the Langmuir model and theoretical maximum adsorption capacity given by the Langmuir model was quite close to the experimental one in comparison to adsorption capacity calculated from the D–R isotherm suggesting the monolayer coverage of Hg(II) on P. cruentum. The separation factor RL was also in the range 0.0008–0.1389, indicating the favorable adsorption. The high potential for Hg(II) biosorption was indicative from the very high surface area. The value of 1/n from the Freundlich model indicated that the relative distribution of energy sites depended on the nature and strength of the biosorption process. As the value of 1/n for Hg(II) on P. cruentum was 0.38, this suggested that 38% of active sites have equal energy where the biosorption took place (Citation36). According to Temkin model parameters, the heat of adsorption (B) is 0.32 suggesting the weak interaction between the sorbate and sorbent. Moreover, this demonstrated that this biosorption process was a physical process in nature further confirmed from the D–R isotherm. The value of ‘E’ given by D–R isotherm is 2.67 kJ/mol (less than 8 KJ/mol) demonstrating the physical nature of biosorption process for Hg(II) on P. cruentum (Citation37).
Table 2. Parameters for equilibrium models for the biosorption of Hg(II) onto P. cruentum.
3.8. Biosorption thermodynamics
Temperature plays an important role in the biosorption studies, so the changes in standard free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) were calculated from the experimental data run at different temperature (298 K, 308 K, 318 K), as shown in and .
Figure 6. Effect of temperature studies on biosorption studies of Hg(II) by P. cruentum (biosorbent dosage = 0.25 g/L, pH = 7, contact time = 120 min). Inset graph shows thermodynamics of the process.
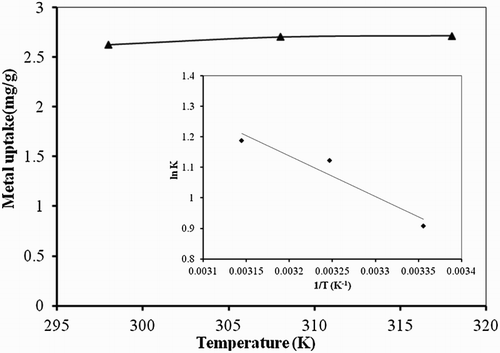
Table 3. Thermodynamics parameters for Hg(II) biosorption on P. cruentum.
The negative values of ΔG° and decrease with the increase in temperature implied the feasibility and spontaneity of the biosorption process with temperature (Citation38). A negative value of enthalpy (ΔH°) illustrated the exothermic nature of Hg(II) biosorption on P. cruentum. While the positive value of (ΔS°) showed the increased randomness at the solid–solution interface and also exhibited the affinity of P. cruentum for Hg(II).
3.9. Comparison of Hg(II) biosorption with other red algal species
Hg(II) uptake capacity for P. cruentum was compared with that of some other reported biosorbents (). It can be seen that P. cruentum has greater biosorption capacity than a number of other reported red algal species. Biosorbents other than red algae showed higher qmax values, however, with a much greater biosorbent amount. The amount of red algae used in the present studies was very small as compared to other reported studies. In addition, the initial Hg(II) concentration in the present studies was also very small.
Table 4. Comparison of Hg(II) biosorption efficiency of P. cruentum with other red algal species.
3.10. Effect of foreign metal ions in binary system
The interactive effects of a mixture of a metal ions on a biomass is a complex process and depends upon biosorbent type, metal combination factor, level of metal concentration and other experimental parameters. Based on all these factors, a response of synergism, antagonism or non-interaction may occur. The sorption of mercury was studied in the presence of binary system (1:1 concentration ratio) of different metal cations in order to evaluate their effect on the biosorption capacity of P. cruentum. As shown in (a), the presence of other metal ions affected the interaction between the algal biomass and Hg(II).
Figure 7. Effect of foreign metal ions in the binary system on biosorption studies of Hg(II) by P. cruentum (a) (Co(each) = 10 mg/L, biosorbent dosage = 0.25 g/L, pH = 7, contact time = 120 min) (b) represents the increased removal percentage and (c) indicates the decreased removal percentage due to the presence of interfering metal ions.
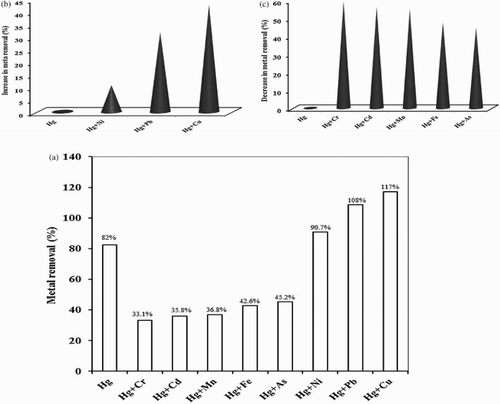
Percentage increase (%) in removal capacity of P. cruentum for Hg(II) is illustrated in (b). It is clear that Cu (II) has caused the maximum augmentation in metal removal capacity up to a level of 42.68% followed by Pb(II) (31.7%) and Ni(II) (10.6%). Thus, Pb(II), Cu(II) and Ni(II) appeared to have synergistic impact on Hg(II) biosorption. This could be explained in accordance with the Pearson’s soft and hard acid–base (SHAB) concept that Hg(II) is a class B metal ion and preferably complexes covalently with sulfur and nitrogen-containing ligands. The borderline acids Pb(II), Cu(II) and Ni(II) form intermediately strong covalent bonds with sulfur or nitrogen-containing ligands (Citation49). The soft acid-soft base pair has greater probability being stronger. On the other hand, the presence of As(III), Cd(II), Cr(III), Fe(III) or Mn(II) cations imparts an antagonistic effect on percentage removal of Hg(II), as shown in (c). Greatest decrement was observed in the binary system of Hg(II):Cr(III) (59.6%) followed by Hg(II):Cd(II) (56.3%), Hg(II):Mn(II) (55.12%), Hg(II):Fe(III) (48.04%) and Hg(II):As(III) (44.8%) at 1:1 concentration ratio of analyte and interferent metal ion, respectively. The reason for antagonistic behavior could be the competition for similar binding sites or the screening effect caused by other metal ions (Citation16).
Conclusion
The present study showed that P. cruentum contained a number of functional groups and Hg(II) bound mostly with oxygen and nitrogen-containing groups. The point of zero charge also confirmed the presence of large amounts of acidic groups. The kinetic study predicted the chemisorption process as the rate determining step, as it followed the pseudo-second-order rate equation. The binding was initially fast and attained equilibrium, later, at slower rates in 120 min. The adsorption data fitted to the isotherm models in the following order: Langmuir > Freundlich > Temkin > Dubinin–Radushkevich. According to thermodynamics studies, it was a feasible, exothermic and spontaneous biosorption process. Thus, it can be concluded that P. cruentum can be used as promising biosorbent to bind Hg(II) present in low concentrations from waters due to its high affinity and non-toxic production in comparison to commercial adsorbents.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Maria Zaib earned her Ph.D. degree from Institute of Chemistry, University of the Punjab, Lahore, Pakistan in 2015. Her research interests include modified working electrodes and their application in detection of environmental pollutants. She is currently working as Assistant Professor at the Government College Women University, Sialkot, Pakistan.
M. Makshoof Athar, currently Director of Institute of Chemistry, University of the Punjab Lahore, has post-doctoral experience from Ryerson University Canada. He has more than 100 research articles on his credit including a number of reviews. His field of interest is applied / analytical chemistry especially associated with the Environmental issues. He is also interested in the development of low cost chromatographic and spectroscopic methods of analysis and polymer chemistry.
Asma Saeed, currently, she is Principal Officer at Food and Biotechnology Center, PCSIR Laboratories Complex, Lahore, Pakistan. She has been elected as member of Pakistan Academy of Sciences in 2013. Her research interest includes bioremediation, phytochemical and antioxidant studies.
Umar Farooq, currently working as Assistant Professor of Analytical Chemistry in Institute of Chemistry, University of the Punjab, earned his M.Sc. Chemistry with distinction from The Islamia University of Bahawalpur in 2003. He did PhD in 2011 under HEC Indigenous Fellowship Program. His area of specialization is Analytical/Environmental Chemistry. He has 30 publication is International Impact Factor Journals, including 3 review articles. His research interests include development of environmentally friendly modified biosorbents for the treatment of polluted water layers.
Muhammad Salman got his M.Sc. and M.Phil in Applied Chemistry from the Institute of Chemistry, University of the Punjab in 2006 and 2011, respectively. He had completed his Ph.D in December 2015. He worked as Student Demonstrator and Scientific Officer in 2006-07 in the Institute of Chemistry. He joined the Institute of Chemistry as Lecturer in November, 2007. Now he is working as Assistant Professor in the same institute since October 2014. His field of interest is Applied Chemistry especially associated with environmental issues and production of chemicals. He has published 57 research articles including a book chapter in the field of Applied Chemistry.
Muhammad Nouman Makshoof acquired his Bachelors in Chemical Engineering degree from Institute of Chemical Engineering and Technology, University of the Punjab, Lahore. He has been working as research assistant in ICET during his course of study. Currently he is working in research and development section of a private organization. He owns more than two publications in his credit. His research area includes chemical synthesis and environmental engineering.
Additional information
Funding
References
- El Aroui, F.; Lahrich, S.; Farahi, A.; Achak, M.; El Gaini, L.; Manoun, B.; Bakasse, M.; Bouzidi, A.; El Mhammedi, M. J. Taiwan Inst. Chem. E. 2014, 45, 2725–2732. doi: 10.1016/j.jtice.2014.05.003
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.; Friedli, H.; Leaner, J.; Mason, R.; Mukherjee, A.; Stracher, G.; Streets, D. Atmos. Chem. Phys. 2010, 10, 5951–5964. doi: 10.5194/acp-10-5951-2010
- Schroeder, W.H.; Munthe, J. Atmos. Environ. 1998, 32, 809–822. doi: 10.1016/S1352-2310(97)00293-8
- Pacyna, E.G.; Pacyna, J.; Sundseth, K.; Munthe, J.; Kindbom, K.; Wilson, S.; Steenhuisen, F.; Maxson, P. Atmos. Environ. 2010, 44, 2487–2499. doi: 10.1016/j.atmosenv.2009.06.009
- Duruibe, J.; Ogwuegbu, M.; Egwurugwu, J. Int. J. Phys. Sci. 2007, 2, 112–118.
- Harada, M. CRC Crit. Rev. Toxicol. 1995, 25, 1–24. doi: 10.3109/10408449509089885
- Bakir, F.; Damluji, S.; Amin-Zaki, L.; Murtadha, M.; Khalidi, A.; Al-Rawi, N.; Tikriti, S.; Dhahir, H.; Clarkson, T.; Smith, J. Science 1973, 181, 230–241. doi: 10.1126/science.181.4096.230
- Kumar, M.; Puri, A. Indian J. Occup. Environ. Med. 2012, 16, 40. doi: 10.4103/0019-5278.99696
- Fu, F.; Wang, Q. J. Environ. Manage. 2011, 92, 407–418. doi: 10.1016/j.jenvman.2010.11.011
- Ahluwalia, S.S.; Goyal, D. Bioresource Technol. 2007, 98, 2243–2257. doi: 10.1016/j.biortech.2005.12.006
- Sarı, A.; Uluozlü, Ö.D.; Tüzen, M. Chem. Eng. J. 2011, 167, 155–161. doi: 10.1016/j.cej.2010.12.014
- Sari, A.; Tuzen, M. J. Hazard Mater. 2009, 171, 500–507. doi: 10.1016/j.jhazmat.2009.06.023
- Tuzen, M.; Sari, A.; Mendil, D.; Soylak, M. J. Hazard Mater. 2009, 169, 263–270. doi: 10.1016/j.jhazmat.2009.03.096
- Sari, A.; Tuzen, M.; Citek, D. Sep. Sci. Technol. 2012, 47, 1167–1176. doi: 10.1080/01496395.2011.644615
- Sarı, A.; Tuzen, M. J. Hazard Mater. 2008, 160, 349–355. doi: 10.1016/j.jhazmat.2008.03.005
- Abdel-Aty, A.M.; Ammar, N.S.; Ghafar, H.H.A.; Ali, R.K. J. Adv. Res. 2013, 4, 367–374. doi: 10.1016/j.jare.2012.07.004
- He, J.; Chen, J.P. Bioresource Technol. 2014, 160, 67–78. doi: 10.1016/j.biortech.2014.01.068
- Yipmantin, A.; Maldonado, H.J.; Ly, M.; Taulemesse, J.M.; Guibal, E. J. Hazard Mater. 2011, 185, 922–929. doi: 10.1016/j.jhazmat.2010.09.108
- Hamdy, A. Curr. Microbiol. 2000, 41, 232–238. doi: 10.1007/s002840010126
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Life Sci. 2014, 101, 56–63. doi: 10.1016/j.lfs.2014.02.013
- Jones, R.F.; Speer, H.L.; Kury, W. Physiol. Plantarum 1963, 16, 636–643. doi: 10.1111/j.1399-3054.1963.tb08342.x
- John, R.P.; Anisha, G.; Nampoothiri, K.M.; Pandey, A. Bioresource Technol. 2011, 102, 186–193. doi: 10.1016/j.biortech.2010.06.139
- Soeprobowati, T.R.; Hariyati, R. Int. J. Mar. Sci. 2013, 3, 212–218.
- Saeed, A.; Iqbal, M.; Höll, W.H. J. Hazard. Mater. 2009, 168, 1467–1475. doi: 10.1016/j.jhazmat.2009.03.062
- Iqbal, J.; Wattoo, F.H.; Watoo, M.H.S.; Malik, R.; Trimizi, S.A.; Imran, M.; Ghangro, A.B. Arab. J. Chem. 2011, 4, 389–395. doi: 10.1016/j.arabjc.2010.07.007
- Lemos, V.A.; dos Santos, L.O.; Silva, E.S.; Vieira, E.V. J. AOAC Int. 2012, 95, 227–231. doi: 10.5740/jaoacint.11-198
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Bioresource Technol. 2010, 101, 5043–5053. doi: 10.1016/j.biortech.2010.02.030
- Li, Y.-H.; Du, Q.; Peng, X.; Wang, D.; Wang, Z.; Xia, Y.; Wei, B. Colloids Surf. B: Biointerfaces 2011, 85, 316–322. doi: 10.1016/j.colsurfb.2011.03.003
- Rajamohan, N.; Rajasimman, M.; Dilipkumar, M. J. Taiwan Inst. Chem. E. 2014, 45, 2622–2627. doi: 10.1016/j.jtice.2014.07.004
- Yavuz, H.; Denizli, A.; Güngüneş, H.; Safarikova, M.; Safarik, I. Sep. Purif. Technol. 2006, 52, 253–260. doi: 10.1016/j.seppur.2006.05.001
- Lagergren, S. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39.
- Ho, Y.-S.; McKay, G. Process Biochem. 1999, 34, 451–465. doi: 10.1016/S0032-9592(98)00112-5
- Rao, R.A.K.; Kashifuddin, M. Appl. Water Sci. 2014, 4, 371–383. doi: 10.1007/s13201-014-0153-2
- Salinas, E.; de Orellano, M.E.; Rezza, I.; Martinez, L.; Marchesvky, E.; de Tosetti, M. S. Bioresource Technol. 2000, 72, 107–112. doi: 10.1016/S0960-8524(99)00111-X
- Soltani, R.D.C.; Khorramabadi, G.S.; Khataee, A.; Jorfi, S. J. Taiwan Inst. Chem. Eng. 2014, 45, 973–980. doi: 10.1016/j.jtice.2013.09.014
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N. J. Hazard Mater. 2010, 176, 510–520. doi: 10.1016/j.jhazmat.2009.11.059
- Kiruba, U.P.; Kumar, P.S.; Prabhakaran, C.; Aditya, V. J. Taiwan Inst. Chem. Eng. 2014, 45, 2957–2968. doi: 10.1016/j.jtice.2014.08.016
- Daneshvar, E.; Sohrabi, M. S.; Kousha, M.; Bhatnagar, A.; Aliakbarian, B.; Converti, A.; Norrström, A.-C. J. Taiwan Inst. Chem. Eng. 2014, 45, 2926–2934. doi: 10.1016/j.jtice.2014.09.019
- Schmitt, D.; Müller, A.; Csögör, Z.; Frimmel, F. H.; Posten, C. Water Res. 2001, 35, 779–785. doi: 10.1016/S0043-1354(00)00317-1
- Esmaeili, A.; Saremnia, B.; Kalantari, M. Arabian J Chem. 2012.
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. J. Taiwan Inst. Chem. Eng. 2013, 44(2), 266–269. doi: 10.1016/j.jtice.2012.09.004
- Tamilselvan, N.; Saurav, K.; Kannabiran, K. Pharmacol. Online 2011, 1, 518–528.
- Huang, S.; Lin, G. J. Environ. Health Sci. Eng. 2015, 13, 21. doi: 10.1186/s40201-015-0180-4
- Wang, X. S.; Li, F. Y.; He, W.; Miao, H. H. CLEAN–Soil, Air, Water 2010, 38, 44–48.
- Sari, A.; Tuzen, M. J. Hazard Mater. 2009, 171, 500–507. doi: 10.1016/j.jhazmat.2009.06.023
- Tuzen, M.; Sari, A.; Mendil, D.; Soylak, M. J. Hazard Mater. 2009, 169, 263–270. doi: 10.1016/j.jhazmat.2009.03.096
- Sari, A.; Tuzen, M.; Citek, D. Sep. Sci. Technol. 2012, 47, 1167–1176. doi: 10.1080/01496395.2011.644615
- Cancer, D.; Sari, A.; Tuzen, M. Ind. Eng. Chem. Res. 2015, 54, 7524–7533. doi: 10.1021/acs.iecr.5b01293
- Al Rmalli, S. W.; Dahmani, A. A.; Abuein, M. M.; Gleza, A. A. J. Hazard Mater. 2008, 152, 955–959. doi: 10.1016/j.jhazmat.2007.07.111
