ABSTRACT
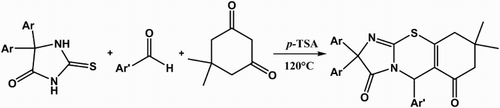
A simple and eco-friendly method for the synthesis of novel imidazo[2,1-b][1,3]thiazin derivatives has been developed via the one-pot, three-component and solvent-free reaction of thiohydantoins, aromatic aldehyde and 5,5-dimethylcyclohexane-1,3-dione in the presence of catalytic amount of p-toluene sulfonic acid, in good and excellent yields.
GRAPHICAL ABSTRACT
Introduction
It is known that heterocycle rings are one of the most important substructures found in a large number of natural products and pharmacologically active compounds. Thus, in the past several decades, researchers have been challenged to find and produce (in the form of drug) biologically active compounds for the treatment of a wide range of illness (Citation1–3). As an important kind of these heterocycles, hydantoins and 2-thiohydantoins are an important class of therapeutic agents in medicinal chemistry and their biological activity has been known for a long time (Citation4). The development of the synthesis of new conjugates of small bioactive molecules derived from well-known organic compounds, to produce compounds with new biological properties different from those of the parent material, is an attractive goal in the medicinal chemistry area (Citation5). As part of our current studies to develop new synthetic methods in modern heterocyclic chemistry continued, with design of new multi-component reactions involving the use of the imidazole ring (Citation6–13), we would like to report a novel three-component reaction for the synthesis of imidazo[2,1-b][1,3]thiazins ().
Results and discussion
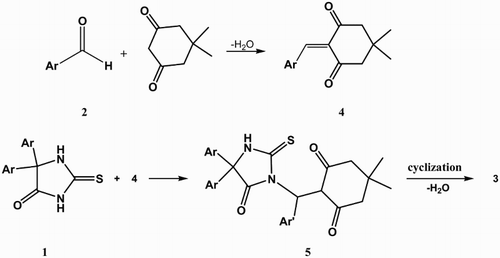
As indicated in and , NH-acid 1, aldehyde 2 and dimedone undergo a smooth 1:1:1 addition reaction in the presence of catalytic amount of p-toluene sulfonic acid (p-TSA) at 120°C to produce imidazo[2,1-b][1,3]thiazin derivatives. The 1H- and 13C-NMR spectra of the target products clearly indicated the formation of 3. The structures of compounds 3a–3j were deduced from their elemental analyses and from their IR, 1H-NMR, and 13C-NMR spectra.
Table 1. Synthesis of imidazo[2,1-b][1,3]thiazins in the presence of p-TSA under solvent-free conditions.
The 1H-NMR spectrum of 3a exhibited three-type picks readily recognized as arising from methyl and methylene groups for dimedone and CH group (δ = 6.80 ppm) protons, along with a multiplet for the aromatic (δ = 7.00–7.91 ppm) protons. The 13C-NMR spectrum of 3a showed 31 signals in agreement with the proposed structure. The 1H- and 13C-NMR spectra of 3b–5j are similar to those of 3a except for the aromatic moieties, which exhibited characteristic signals with appropriate chemical shifts. The structural assignments of compounds 3a–3j made on the basis of their NMR spectra were supported by their IR spectra. In this study was reported an efficient synthetic procedure for the preparation of imidazo[2,1-b][1,3]thiazins 3 in the presence of p-TSA as a catalyst. To choose the best catalyst among various common acidic catalysts, the results showed that p-TSA has the best yield in the shortest reaction time, so that it was selected as catalyst for subsequent experiments.
A rationale for the formation of product is shown in . According to these results, the reaction can be mechanistically considered to proceed through the intermediate 4 through a Knoevenagel condensation (formed in situ by reaction of the aldehyde with dimedone). The subsequent Michael-type addition of thiohydantoin to the intermediate 4 affords complex 5. Finally, cyclization and a water elimination from 5 gives corresponding target molecules 3a–j in good yields.
Conclusions
In conclusion, we have developed a one-pot, simple, useful and environment-friendly method for the synthesis of functionalized imidazo[2,1-b][1,3]thiazines. The present method carries the advantage that not only is the reaction performed under mild conditions, but also the starting materials and reagents can be mixed in a solvent-free condition as a green reaction.
Experimental
Aldehydes and 5,5-dimethylcyclohexane-1,3-dione were obtained from Merck and were used without further purification. 5,5-diaryl thiohydantoins were prepared by known methods (Citation14, Citation15). Melting points (uncorrected) were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H, N and S were performed using a Heraeus CHN-S-Rapid analyzer. The experimental data were in good agreement with the calculated values. 1H- and 13C-NMR spectra (DMSO-d6) were measured with a Bruker DRX-360 Avance spectrometer. IR spectra were recorded on a Shimadzu IR-460 spectrometer.
General procedure for the preparation of 8,8-dimethyl-2,2,5-triaryl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6 (5H,7H)-diones 3
A mixture of the aldehyde (1 mmol), thiohydantoin (1 mmol), dimedone (1 mmol) and catalytic amount of p-TSA (0.1 mmol) was stirred at 120°C for 3 h. Completion of the reaction was indicated by thin layer chromatography. The reaction was cooled to room temperature, then ethylacetate (5 mL) was added and the mixture stirred for 5 min. The solid obtained was removed by filtration and recrystallized from ethanol.
5-(4-methoxyphenyl)-8,8-dimethyl-2,2-diphenyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3a)
Red crystals; m.p.: >400°C; yield: 0.45 g (89%). IR (KBr) (νmax/cm−1): 1703 (C=O), 1681 (C=O), 1655 (C=N). Anal. Calcd (%) for C31H28N2O3S (508.63): C, 73.20; H, 5.55; N, 5.51; S, 6.30. Found: C, 73.07; H, 5.66; N, 5.47; S, 6.25. 1H-NMR (360 MHz, DMSO-d6): δ = 1.17 (3H, s, CH3), 1.31 (3H, s, CH3), 2.31 (2H, s, CH2), 2.69 (2H, s, CH2), 3.59 (3H, s, OCH3), 6.80 (1 H, s, CH), 7.00–7.91 (14 H, m, Ar). 13C-NMR(90 MHz,DMSO-d6): δ = 22.9 (CH3), 25.6 (CH3), 28.4 (C), 38.4 (CH2), 48.5 (CH2), 53.3 (CH), 57.2 (CH3O), 71.7 (C), 107.3 (C-S), 113.2, 125.9, 126.2, 128.7, 129.2, 129.4, 132.4, 132.5, 133.9, 138.0, 138.8, 153.8, 157.6, 168.5 (C=O), 178.6 (C=N), 196.3 (C=O).
5-(4-hydroxyphenyl)-8,8-dimethyl-2,2-diphenyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3b)
Red crystals; m.p.: 346–348°C; yield: 0.40 g (82%). IR (KBr) (νmax/cm−1): 3451 (OH), 1710 (C=O), 1690 (C=O), 1671 (C = N). Anal. Calcd (%) for C30H26N2O3S (494.60): C, 72.85; H, 5.30; N, 5.66; S, 6.48. Found: C, 73.02; H, 5.26; N, 5.47; S, 6.39. 1H-NMR (360 MHz, DMSO-d6): δ = 1.12 (3H, s, CH3), 1.24 (3H, s, CH3), 2.34 (2H, s, CH2), 2.74 (2H, s, CH2), 7.16 (1 H, s, CH), 7.41–8.03 (14 H, m, Ar), 8.19 (1 H, br s, OH).13C-NMR(90 MHz, DMSO-d6): δ = 22.9 (CH3), 25.7 (CH3), 28.6 (C), 38.5 (CH2), 48.9 (CH2), 53.4 (CH), 67.9 (C), 107.4 (C–S), 111.0, 125.9, 126.2, 128.5, 129.2, 129.6, 132.4, 133.9, 138.0, 138.8, 153.7 (C), 157.5 (C), 169.1 (C=O), 176.3 (C=N), 203.0 (C=O).
5-(4-chlorophenyl)-8,8-dimethyl-2,2-diphenyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3c)
Colorless crystals; m.p.: 327–330°C; yield: 0.38 g (75%). IR (KBr) (νmax/cm−1): 1707 (C=O), 1688 (C=O), 1662 (C=N), 760 (C=Cl). Anal. Calcd (%) for C30H25ClN2O2S (513.05): C, 70.23; H, 4.91; N, 5.46; S, 6.25. Found: C, 70.34; H, 4.96; N, 5.37; O, 6.15. 1H-NMR (360 MHz, DMSO-d6): δ = 1.18 (3H, s, CH3), 1.31 (3H, s, CH3), 2.35 (2H, s, CH2), 2.73 (2H, s, CH2), 7.17 (1 H, s, CH), 7.38–7.60 (14 H, m, Ar).13C-NMR(90 MHz,DMSO-d6): δ = 24.2 (CH3), 27.4 (CH3), 31.5 (C), 38.9 (CH2), 47.8 (CH2), 53.7 (CH), 73.1 (C), 105.5 (C–S), 125.2, 125.8, 126.3, 128.7, 129.2, 129.4, 130.5, 130.8, 135.3, 140.0, 140.8, 141.5, 156.4 (C), 169.2 (C=O), 177.8 (C=N), 194.8 (C=O).
5-(4-nitrophenyl)-8,8-dimethyl-2,2-diphenyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3d)
Colorless crystals; m.p.: >400°C; yield: 0.41 g (78%). IR (KBr) (νmax/cm−1): 1705 (C=O), 1680 (C=O), 1653 (C=N), 1354 (NO2). Anal. Calcd (%) for C30H25N3O4S (523.60): C, 68.82; H, 4.81; N, 8.03; S, 6.12. Found: C, 68.90; H, 4.76; N, 8.11; S, 6.05. 1H-NMR (360 MHz, DMSO-d6): δ = 1.10 (3H, s, CH3), 1.22 (3H, s, CH3), 2.34 (2H, s, CH2), 2.74 (2H, s, CH2), 6.96 (1 H, s, CH), 7.15–8.29 (14 H, m, Ar). 13C-NMR(90 MHz,DMSO-d6): δ = 23.3 (CH3), 25.6 (CH3), 29.8 (C), 37.2 (CH2), 47.4 (CH2), 54.3 (CH), 72.7 (C), 109.3 (C–S), 120.6, 126.2, 126.8, 127.5, 128.3, 129.0, 129.3, 131.8, 132.3, 142.0, 143.2, 146.5, 150.1, 154.4, 167.4 (C=O), 175.2 (C=N), 196.1 (C=O).
5-(4-methylphenyl)-8,8-dimethyl-2,2-di-p-tolyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3e)
Orange crystals; m.p.: >400°C; yield: 0.44 g (72%). IR (KBr) (νmax/cm−1): 1702 (C=O), 1682 (C=O), 1658 (C=N). Anal. Calcd (%) for C33H32N2O2S (520.68): C, 76.12; H, 6.19; N, 5.38; S, 6.16. Found: C, 76.20; H, 6.06; N, 5.27; S, 6.12. 1H-NMR (360 MHz, DMSO-d6): δ = 1.15 (3H, s, CH3), 1.28 (3H, s, CH3), 1.91 (3H, s, CH3), 2.30 (6H, s, CH3), 2.36 (2H, s, CH2), 2.73 (2H, s, CH2), 7.10 (1 H, s, CH), 7.15–7.62 (12 H, m, Ar). 13C-NMR(90 MHz,DMSO-d6): δ = 21.2, 22.4, 22.7, 26.5 and 27.8 (4 CH3), 29.5 (C), 38.0 (CH2), 47.2 (CH2), 52.9 (CH), 71.5 (C), 108.1 (C–S), 125.9, 127.1, 127.4, 127.7, 128.3, 128.5, 135.0, 135.8, 136.4, 140.0, 140.8, 141.5, 157.1 (C), 168.3 (C=O), 174.6 (C=N), 195.2 (C=O).
5-(4-methoxyphenyl)-8,8-dimethyl-2,2-di-p-tolyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3f)
Brown crystals; m.p.: >400°C; yield: 0.45 g (85%). IR (KBr) (νmax/cm−1): 1709 (C=O), 1686 (C=O), 1668 (C=N). Anal. Calcd (%) for C33H32N2O3S (536.68): C, 73.85; H, 6.01; N, 5.22; S, 5.97. Found: C, 73.70; H, 6.06; N, 5.29; S, 5.91. 1H-NMR (360 MHz, DMSO-d6): δ = 1.13 (3H, s, CH3), 1.24 (3H, s, CH3), 2.29 (6H, s, 2CH3), 2.33 (2H, s, CH2), 2.67 (2H, s, CH2), 3.58 (3H, s, OCH3), 7.07 (1 H, s, CH), 7.10–7.48 (12 H, m, Ar). 13C-NMR (90 MHz, DMSO-d6): δ = 19.0, 21.2, 27.5 and 29.3 (3 CH3), 31.4 (C), 38.9 (CH2), 48.5 (CH2), 54.1 (CH), 56.5 (CH3O), 65.8 (C), 96.2 (C–S), 100.6, 125.8, 127.5, 128.1, 128.7, 128.8, 135.2, 135.8, 136.0, 140.6, 141.2, 153.5 (C), 156.4 (C), 166.4 (C=O), 175.9 (C=N), 196.4 (C=O).
5-(4-hydroxyphenyl)-2,2-bis(4-methoxyphenyl)-8,8-dimethyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3g)
Brown crystals; m.p.: >400°C; yield: 0.48 g (87%). IR (KBr) (νmax/cm−1): 3448 (OH), 1707 (C=O), 1681 (C=O), 1667 (C=N). Anal. Calcd (%) for C32H30N2O5S (554.66): C, 69.29; H, 5.45; N, 5.05; S, 5.78. Found: C, 69.20; H, 5.52; N, 5.09; S, 5.63. 1H-NMR (360 MHz, DMSO-d6): δ = 1.10 (3H, s, CH3), 1.24 (3H, s, CH3), 2.33 (2H, s, CH2), 2.67 (2H, s, CH2), 3.62 (3H, s, OCH3), 3.70 (3H, s, OCH3), 6.96 (1 H, s, CH), 7.09–7.48 (12 H, m, Ar), 8.75 (1 H, br s, OH). 13C-NMR (90 MHz, DMSO-d6): δ = 27.2 (CH3), 29.0 (CH3), 32.2 (C), 38.6 (CH2), 47.8 (CH2), 55.5 (CH), 57.8 (O–CH3), 58.6 (O–CH3), 70.8 (C), 101.6 (C–S), 112.8, 113.7, 113.9, 127.6, 129.0, 129.2, 132.3, 132.5, 134.8, 153.4, 155.4, 156.6, 157.1, 164.0 (C=O), 171.6 (C=N), 196.3 (C=O).
5-(2,4-dihydroxyphenyl)-2,2-bis(4-methoxyphenyl)-8,8-dimethyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3h)
Brown crystals; m.p.: 325–328°C; yield: 0.47 g (82%). IR (KBr) (νmax/cm−1): 3455 (OH), 1712 (C=O), 1685 (C=O), 1669 (C=N). Anal. Calcd (%) for C32H30N2O6S (570.66): C, 67.35; H, 5.30; N, 4.91; S, 5.62. Found: C, 67.20; H, 4.96; N, 5.77; S, 5.70. 1H-NMR (360 MHz, DMSO-d6): δ = 1.15 (3H, s, CH3), 1.31 (3H, s, CH3), 2.38 (2H, s, CH2), 2.62 (2H, s, CH2), 3.58 (3H, s, OCH3), 3.79 (3H, s, OCH3), 6.44 (1 H, br s, OH), 6.82 (1 H, s, CH), 6.90–7.23 (11 H, m, Ar), 9.12 (1 H, br s, OH). 13C-NMR (90 MHz, DMSO-d6): δ = 24.3 (CH3), 26.5 (CH3), 31.4 (C), 37.4 (CH2), 47.5 (CH2), 56.2 (CH), 57.6 (O–CH3), 59.0 (O–CH3), 72.7 (C), 103.2 (C–S), 107.3, 110.2, 114.4, 114.6, 115.5, 128.9, 129.2, 129.7, 133.9, 134.1, 153.4, 155.4, 156.6, 156.6, 157.1, 165.7 (C=O), 172.5 (C=N), 199.1 (C=O).
2,2-bis(4-chlorophenyl)-5-(4-hydroxyphenyl)-8,8-dimethyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3i)
Dark red crystals; m.p.: >400°C; yield: 0.45 g (80%). IR (KBr) (νmax/cm−1): 3460 (OH), 1705 (C=O), 1684 (C=O), 1668 (C=N), 756 (C=Cl). Anal. Calcd (%) for C30H24Cl2N2O3S (563.49): C, 63.94; H, 4.29; N, 4.97; S, 5.69. Found: C, 63.80; H, 4.22; N, 5.00; S, 5.74. 1H-NMR (360 MHz, DMSO-d6): δ = 1.07 (3H, s, CH3), 1.24 (3H, s, CH3), 2.33 (2H, s, CH2), 2.67 (2H, s, CH2), 7.06 (1 H, s, CH), 7.08–7.90 (12 H, m, Ar), 9.17 (1 H, br s, OH). 13C-NMR (90 MHz, DMSO-d6): δ = 24.5 (CH3), 28.7 (CH3), 31.5 (C), 36.2 (CH2), 46.4 (CH2), 56.3 (CH), 72.1 (C), 108.8 (C–S), 113.8, 128.2, 128.9, 129.0, 129.5, 129.6, 131.8, 132.0, 136.4, 144.5, 144.7, 153.6 (C), 157.3 (C), 168.5 (C=O), 178.1 (C=N), 196.7 (C=O).
2,2-bis(4-chlorophenyl)-5-(4-chlorophenyl)-8,8-dimethyl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-dione (3j)
Yellow crystals; m.p.: 332–333°C; yield: 0.44 g (77%). IR (KBr) (νmax/cm−1): 1708 (C=O), 1686 (C=O), 1670 (C=N), 762 (C=Cl). Anal. Calcd (%) for C30H23Cl3N2O2S (581.94): C, 61.92; H, 3.98; N, 4.81; S, 5.51. Found: C, 61.80; H, 3.92; N, 4.77; S, 5.60. 1H-NMR (360 MHz, DMSO-d6): δ = 1.16 (3H, s, CH3), 1.29 (3H, s, CH3), 2.34 (2H, s, CH2), 2.74 (2H, s, CH2), 7.12 (1 H, s, CH), 7.15–7.53 (12 H, m, Ar). 13C-NMR (90 MHz, DMSO-d6): δ = 25.0 (CH3), 28.2 (CH3), 32.0 (C), 36.9 (CH2), 47.1 (CH2), 60.2 (CH), 70.3 (C), 109.6 (C–S), 127.6, 127.8, 127.9, 128.9, 129.2, 129.5, 131.9, 132.2, 133.2, 144.1, 144.2, 146.0, 155.4 (C), 167.7 (C=O), 179.0 (C=N), 195.2 (C=O).
Notes on contributor
Mohammad Mehdi Ghanbari is an assistant professor and PhD in organic chemistry and research on organic synthesis and dynamic NMR.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Silva, L.R.; Cimas, Á.; Vale, N.; Gomes, P.; Monte, M.J.S.; Ribeiro da Silva, M.D.M.C. J. Chem. Thermodyn. 2013, 58, 158–165. doi: 10.1016/j.jct.2012.10.010
- Hines, L.E.; Murphy, J.E. Am. J. Ger. Pharm. 2011, 9, 364–377.
- Muccioli, G.G.; Poupaert, J.H.; Wouters, J.; Norberg, B.; Poppitz, W.; Scriba, G.K.E.; Lambert, D.M.A. Tetrahedron. 2003, 59, 1301–1307. doi: 10.1016/S0040-4020(03)00033-4
- Incesua, Z.; Benklib, K.; Akalina, G.; Kaplanciklib, Z.A. Cell Biol. Int. 2004, 28, 267–272. doi: 10.1016/j.cellbi.2004.01.003
- Talhi, O.; Fernandes, J.A.; Pinto, D.C.G.A.; Almeida Paz, F.A.; Silva, A.S.M. Tetrahedron. 2013, 69, 5413–5420. doi: 10.1016/j.tet.2013.04.111
- Ghanbari, M.M. Monatsh. Chem. 2011, 142, 749–752. doi: 10.1007/s00706-011-0512-8
- Ghanbari, M.M.; Yavari, I.; Emadi, A. J. Sulf. Chem. 2014, 35, 57–61. doi: 10.1080/17415993.2013.789516
- Ghanbari, M.M.; Jamali, M.; Batta, G. J. Sulf. Chem. 2014, 35, 394–398. doi: 10.1080/17415993.2014.885031
- Ghanbari, M.M.; Yavari, I.; Batta, G. J. Sulf. Chem. 2014, 35, 312–317. doi: 10.1080/17415993.2013.879308
- Landreau, C.; Deniaud, D.; Meslin, J.C. J. Org. Chem. 2003, 68, 4912–4917. doi: 10.1021/jo034381l
- Heindel, N.D.; Reid, J.R. J. Org. Chem. 1980, 45, 2479–2482. doi: 10.1021/jo01300a043
- Andreani, A.; Burnelli, S.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R., Rambaldi, M.; Varoli, L.; Calonghi, N.; Cappadone, C.; Farruggia, G.; Zini, M.; Stefanelli, C.; Masotti, L.; Radin, N.S.; Shoemaker, R.H. J. Med. Chem. 2008, 51, 809–816. doi: 10.1021/jm701246g
- Lamberth, C.; Querniard, F. Tetrahedron Lett. 2008, 49, 2286–2288. doi: 10.1016/j.tetlet.2008.02.014
- Safari, J.; Arani, N.M.; Isfahani, A.R. Chin. J. Chem. 2010, 28, 255–258. doi: 10.1002/cjoc.201090062
- Safari, J.; Naeimi, H.; Ghanbari, M.M.; Sabzi-Fini, O. Russ. J. Org. Chem. 2009, 45, 477–479. doi: 10.1134/S1070428009030270

![Scheme 1. Synthesis of 8,8-dimethyl-2,2,5-triaryl-8,9-dihydro-2H-benzo[e]imidazo[2,1-b][1,3]thiazine-3,6(5H,7H)-diones.](/cms/asset/e60bbf0f-a086-4708-a864-559fad12e86a/tgcl_a_1231841_f0001_b.gif)