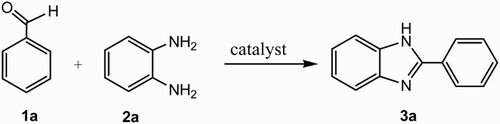
ABSTRACT
An easy synthetic protocol for the synthesis of biologically active benzimidazole, benzothiazole and benzoxazole derivatives has been demonstrated using a hybrid crystal NH3(CH2)4NH3SiF6 as a mild and efficient heterogeneous catalyst. Short reaction times, solvent-free conditions, good to excellent yields, easy reusability and use of an eco-friendly catalyst are some of the significant attributes of the present method.
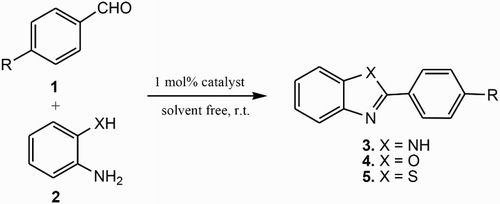
GRAPHICAL ABSTRACT
Introduction
The benzimidazole, benzoxazole and benzothiazole skeletons may be found in numerous pharmaceutical agents with a diverse spectrum of biological properties (Citation1–4). They are considered as privileged structures in the medicinal chemistry field (Citation5) and are found in a large variety of natural products. They have been used as antiviral (Citation6), antimicrobial (Citation7), anti-tumor (Citation8), antibiotic, antifungal (Citation9), anticonvulsant (Citation10), anti-inflammatory (Citation11), antiulcer (Citation12 a), antihelminthic (Citation12 a), anti-hypertensive (Citation12 b) and anti-analgesic (Citation12 c) agents. Moreover, their application in the field of advanced materials is worthy of note (Citation13). The protocols usually followed for their synthesis involve condensation of ortho-esters (Citation14–15), nitriles (Citation16), aldehydes (Citation17–18), carboxylic acids (Citation19), amides (Citation20) and esters (Citation21) with ortho-substituted aminoaromatics, in the presence of different acids or catalysts such as Pd-catalyzed oxidative cyclization (Citation22), base-assisted reaction of 1,1-dibromoethanes (Citation23), different hetero-polyacid catalysts (Citation24), In(OTF)3 (Citation25), Cu-np/SiO (Citation26), iron phthalocyanine (Citation27), nanoCeO2 (Citation28), nanosolid acids (Citation29), CuFe2O4 (Citation30), Fe(NO3)3/Tempo (Citation31), RHA-SO3H (Citation32) and Fe(III)-Schiff base/SBA-15 (Citation33). Therefore, there is a strong demand for a more effective, simple and environmentally friendly process for the synthesis of these heterocycles.
In order to develop such processes, it was used in the present synthetic protocols, the butane-1,4-diammonium hexafluorosilicate NH3(CH2)4NH3SiF6 as a solid catalyst; that is a novel hybrid compound (Citation34) that belongs to the family of alkylenediammonium halogenometallate salts of the general formula NH3(CH2)nNH3MX6 (IV) (M: Sn, Si, Te; X: Cl, Br, I and F) that have recently attracted considerable attention due to their important chemical and physical properties (Citation35–37). Generally, the hexafluorosilicate NH3(CH2)nNH3SiF6 salts were less studied in comparison with other hexahalogenometallate NH3(CH2)nNH3MX6 salts (M: Sn, Te; X: Cl, Br, I) (Citation38–42). But, the monoalkylammonium hexafluorosilicate compounds have extensively been studied and some of them have been found to be of interest for the study of crystal dynamic and phase transition involving hydrogen bonds as well as the reorientation motions of alkylammoniums cations (Citation39–42). The synthesis of complex NH3(CH2)4NH3SiF6 is a practical way to produce heterogeneous catalyst with the associated advantages such as easy catalyst separation, possible recycling, high activity and selectivity.
In the present study, we report the synthesis of benzimidazole, benzoxazole and benzothiazole derivatives by condensation of 1,2-phenylenediamine, ortho-aminophenol and ortho-aminothiophenol with various aromatic aldehydes using NH3(CH2)4NH3SiF6 as an efficient catalyst in free solvent conditions.
Results and discussion
The NH3(CH2)4NH3SiF6 crystal has been recently synthesized and characterized by X-ray diffraction (Citation34). The crystal structure of this compound has been determined in the triclinic system with (Z = 1) as space group and a = 5.796 (1) Å, b = 5.889 (1) Å, c = 7.774 (2) Å and α = 87.02 (1)°, β = 82.15 (1)° and γ = 61.87 (1)°, V = 231.79 (8) Å3 as parameters of the crystal unit cell.
The molecular structure of the NH3(CH2)4NH3SiF6 compound is given in . The crystal structure of this little compound has been found to be built up from inorganic anions linked to the organic cations +NH3(CH2)4NH3+ through N–H … .F hydrogen bonds producing an infinite two-dimensional layer parallel to (0 1 1) ().
Figure 1. The molecular structure of the NH3(CH2)4NH3SiF6 compound with the atom-labeling scheme as has been determined. The symmetry codes are: (i) −x−1, −y+1, −z+1; (ii) −x+1, −y, −z.
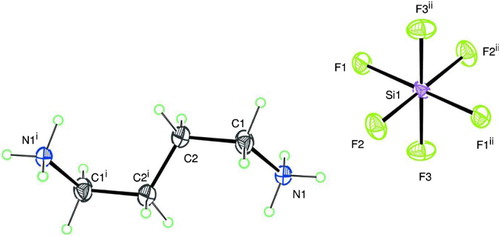
Figure 2. The three-dimensional plot of the NH3(CH2)4NH3SiF6 compound, showing inorganic sheets linked through N–H … .F hydrogen bonds to the organic layers (dashed lines) (Citation34).
The intermolecular hydrogen bonding contacts N–H … .F types provide a linkage between cationic entities +NH3(CH2)4NH3+ and anionic complexes. The hydrogen bonds involved (donors and acceptors) ensure the three-dimensional cohesion of the atomic arrangement. The unit cell of the NH3(CH2)4NH3SiF6 compound contains only one organic cation +NH3(CH2)4NH3+ and one slightly distorted
anion. In this structure, all atoms have been found to be in general positions, except the silicon atom Si which has been located at a crystallographic center of inversion (1/2, 0, 0) of the
space group. The silicon atom was surrounded by six fluorine atoms building a slightly distorted
octahedron. In addition, the center of the bond C2–C2 (i) [with (i) being a symmetry code: −x−1, −y+1, −z+1] was also situated on another crystallographic center of inversion.
Working on a series of new alkylenediammonium halogenometallate compounds, we found it interesting to study the efficiency of the hybrid compound NH3(CH2)4NH3SiF6 as heterogeneous solid catalysts for the preparation of benzoxazoles, benzothiazoles and benzimidazoles. At first, 1,2-phenylenediamine 1a and benzaldehyde 2a were selected as the model substrates to investigate the best reaction conditions (). Then several reaction conditions were tried to accomplish this reaction and the efficiency of the reaction was found to be influenced by the quantity of the catalyst and solvent at room temperature. In the absence of a catalyst, only 12% yield of the desired product was obtained even after longer reaction time (, entry 1). However, in the presence of the catalyst the product 3a was obtained in 93% yield and only after 14 min (, entry 2). Next, the optimization of reaction conditions was undertaken to increase the yield of the product using various solvents, which showed a prominent influence on reaction time and yields to obtain the desired product (, entries 2–8). It was also found that the reaction proceeded efficiently without solvent and resulted in high yields of the desired product (, entries 9–13). Furthermore, the catalytic efficiency of NH3(CH2)4NH3SiF6 was examined using different amounts of this catalyst. Interestingly, the yield of 3a was significantly increased to 98% by employing 1 mol% of the catalyst, without solvent, at room temperature (, entry 13). The opportunity of applying solvent-free conditions is an environmentally significant benefit of the procedure.
Table 1. Screening of the reaction conditions for the reaction of 1,2-phenylenediamine 1a and benzaldehyde 2aa.
The generality of the procedure was evaluated for the synthesis of benzimidazole 3, benzoxazole 4 and benzothiazole 5 derivatives by condensation of different 1,2-phenylene diamine, 1,2-aminophenol and 1,2-aminothiophenol with substituted aldehydes (). As shown in , good to excellent yields were obtained for these reactions. The reactivity of 1 with aldehyde derivatives 2 appear to be not controlled by electronic effects. Indeed, the reaction with both electron-rich and electron-deficient aldehydes is good and affording benzimidazole 3, benzothiazole 4 and benzoxazole 5 in high yields (). The prepared products are known compounds and were confirmed by comparing the 1H NMR and 13C NMR spectral data with authentic samples reported in the literature (Citation33, Citation43).
Table 2. Substrate scope for synthesis of desired heterocycles derivatives.
The recycling of the catalyst was also studied. For this, the catalyst was filtered, separated, washed with methanol and dried at 80°C for 45 min and then the residual catalyst as such was reused without loss of any significant catalytic activity. The structure and aspect of the catalyst remains unchanged after recovery and reuse. In the case of the model reaction, the catalyst was recovered and reused six times without any significant changes in the yield and the reaction time ().
Table 3. Reusability of the catalyst in the synthesis of 2-phenyl benzimidazole 3a.
In order to compare the capability and efficiency of our catalyst with respect to the previously reported catalysts in the literature (Citation26, Citation29, Citation33, Citation43–44), the results for the synthesis of benzimidazoles, benzothiazoles and benzoxazoles employing these catalysts are tabulated in . As it is clear from the Table, NH3(CH2)4NH3SiF6 is more efficient than the others.
Table 4. Comparative synthesis of desired heterocycles using the reported catalysts versus NH3(CH2)4NH3SiF6.
Conclusion
In conclusion, an efficient and mild one-pot protocol for the synthesis of benzimidazoles, benzothiazoles and benzoxazoles using a crystal NH3(CH2)4NH3SiF6 as a catalyst in free solvent has been developed. The use of the crystal as reusable heterogeneous catalyst, solvent-free conditions, excellent product yields and shorter reaction time make this protocol practical and environment-friendly. In addition, the preliminary toxicity evaluation has showed that the catalyst is non-toxic at low concentration levels. The study on the exact mechanism of NH3(CH2)4NH3SiF6 applicable to the preparation of these heterocycle derivatives is underway in our laboratory.
Experimental
Chemicals and apparatus
All the chemicals used were purchased from Sigma-Aldrich and were used as such. All products are known, and were identified by comparison of spectral and physical data with the literature. Melting points were taken on a KOFLER hot stage apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Brucker 300-MHz spectrometer in DMSO-d6.
General procedure for the synthesis of catalyst
The catalyst single crystal has been recently synthesized by slow evaporation of aqueous solution containing NH2(CH2)4NH2 and H2SiF6 by the following method: the solid butane-1,4-diamine NH2(CH2)4NH2 (percentage ≥99%) was primarily dissolved in the necessary distilled water and then was mixed with the stoichiometric amounts (1:1) to H2SiF6 (percentage = 34% in weight). The obtained solution was taken under room temperature for evaporation. The colorless single crystals were so obtained
General procedure for the preparation of benzimidazoles, benzothiazoles and benzoxazoles
Aldehyde (1 mmol), o-phenylenediamine, o-amino thiophenol or o-amino phenol (1 mmol) and 1 mol % (2.32 mg) of NH3(CH2)4NH3SiF6 were stirred, in free solvent conditions, at room temperature for the appropriate time (). The progress of the reaction was monitored by TLC hexane/ethyl acetate (70:30) as eluent. After completion of the reaction, the crude reaction mixture was dissolved in EtOH, and the catalyst was separated out by simple filtration. The product was recrystallized from ethanol to give respectively benzimidazole 3, benzothiazole 4 and benzoxazole 5 in high yields (90–99%, ).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary_Material.doc
Download MS Word (1.9 MB)Notes on contributors
Zakaria Benzekri received a master’s degree in Organic Chemistry from Faculty of Sciences, Ibn Tofail University, Morocco, in 2013 and is now a Ph.D. student in organic chemistry at Ibn Tofail University. His research has focused on the organic synthesis, catalysis and application of natural and synthetic compounds as catalyst in the organic transformations.
Houda Serrar received a master’s degree in Organic Chemistry from Faculty of Sciences, Ibn Tofail University, Morocco, in 2011. She is currently a Ph.D. student at Ibn Tofail University. His research has focused on the new synthetic methodologies to synthesize the heterocyclic compounds and and the study of their inhibitory power of the corrosion of metals (iron, copper). She is mainly investigating the biological activity of these compounds.
Sara Sibous received a master’s degree in Organic Chemistry from Faculty of Sciences, Ibn Tofail University, Morocco, in 2013. She is now a Ph.D. student at Ibn Tofail University. His research has focused on the heterogeneous catalysis and application of functionalized compounds as catalyst in the multi-component reactions.
Said Boukhris received a Ph.D. degree in Organic Chemistry from Faculty of Sciences, Ibn Tofail University, Morocco, in 2004. He is currently professor in the Department of Chemistry, Faculty of Sciences, Ibn Tofail University. He focuses his research in organic synthesis, heterocyclic chemistry, the new synthetic methodologies to synthesize the heterocyclic compounds, catalysis and application of natural and synthetic compounds as catalyst in the organic transformations and multi-component reactions.
Ali Ouasri received a Ph.D. degree in Inorganic Chemistry from Faculty of Sciences, Ibn Tofail University, Morocco, in 2002. He is currently professor in the Regional Centre of the Education and Training Trades, Rabat. His research interests lie in the synthesis of novel hybrid crystals, the determination of their crystal structures by X-ray diffraction on single crystals, their spectroscopic study by means of IR and Raman techniques and the study of the structural phase transitions and dielectric properties.
Ali Rhandour received a Ph.D. degree in Solid Sate Chemistry from Faculty of Sciences, University of Bordeaux 1, France, in 1985. He is currently professor in the Department of Chemistry, Faculty of Sciences, Ibn Tofail University. His research interests lie in the synthesis of novel hybrid crystals, the determination of the crystal structures by X-ray diffraction on single crystals, the spectroscopic study by means of IR and Raman techniques, and the study of the phase transitions and dielectric properties. He also focuses his research in the synthesis in the synthesis of the solid solutions and glass compounds, their structural and vibrational studies as well as their ionic conductivity properties.
Abdelaziz Souizi received a Ph.D. degree in Organic Molecular Chemistry from the University of Rennes 1, France, in 1985. He is currently professor in the Department of Chemistry, Faculty of Sciences, Ibn Tofail University. He focuses his research on the synthesis of novel molecules with potential biological activities, heterocyclic compounds, organic synthesis, medicinal and pharmaceutical chemistry and application of functionalized compounds as catalyst in the organic transformations and multi-component reactions. He also focuses his research in drug synthesis, Nuclear Magnetic Resonance, spectrometry of high and low resolution mass, design and manufacture of detergents, design and manufacture of cosmetics and perfumes and design and manufacture of various fertilizer foliar fertilizer.
References
- Chen, C.; Chen, Y.J. Tetrahedron Lett. 2004, 45, 113–115. doi: 10.1016/j.tetlet.2003.10.095
- Siddiqui, N.; Rana, A.; Khan, S.A.; Bhat, M.A.; Haque, S.E. Bioorg. Med. Chem. Lett. 2007, 17, 4178–4182. doi: 10.1016/j.bmcl.2007.05.048
- Lion, C.J.; Matthews, C.S.; Wells, G.; Bradshaw, T.D.; Stevens, M.F.G.; Westwell, A.D. Bioorg. Med. Chem. Lett. 2006, 16, 5005–5008. doi: 10.1016/j.bmcl.2006.07.072
- Huang, S.T.; Hsei, I.J.; Chen, C. Bioog. Med. Chem. 2006, 14, 6106–6119. doi: 10.1016/j.bmc.2006.05.007
- (a) Horton, D.A.; Bourne, G.T.; Smythe, M.L. Chem. Rev. 2003, 103, 893; (b) Weekes, A.; Westwell, A. Curr. Med. Chem. 2009, 16, 2430.
- (a) Porcari, A.R.; Devivar, R.V.; Kucera, L.S.; Drach, J.C.; Townsend, L.B. J. Med. Chem. 1998, 41, 1252; (b) Song, X.; Vig, B.S.; Lorenzi, P.L.; Drach, J.C.; Townsend, L.B.; Amidon, G.L. J. Med. Chem. 2005, 48, 1274.
- Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Eur. J. Med. Chem. 2004, 39, 291–298. doi: 10.1016/j.ejmech.2003.11.014
- (a) Kumar, D.; Jacob, M.R.; Reynolds, M.B.; Kerwin, S.M. Bioorg. Med. Chem. 2002, 10, 3997; (b) Huang, S.T.; Hsei, I.J.; Chen, C. Bioorg. Med. Chem. 2006, 14, 6106.
- Zasshi, Y. J. Pharmaceut. Soc. Jpn. 1992, 112, 81.
- Benazzouz, A.; Boraud, T.; Dubédat, P.; Boireau, A.; Stutzmann, J.M.; Gross, C. Eur. J. Pharmacol. 1995, 284, 299–307. doi: 10.1016/0014-2999(95)00362-O
- Mader, M.; de Dios, A.; Shih, C.; Bonjouklian, R.; Li, T.; White, W.; de Uralde, B.L.; Sánchez-Martinez, C.; del Prado, M.; Jaramillo, C.; de Diego, E.; Martín Cabrejas, L.M.; Dominguez, C.; Montero, C.; Shepherd, T.; Dally, R.; Toth, J.E.; Chatterjee, A.; Pleite, S.; Blanco-Urgoiti, J.; Perez, L.; Barberis, M.; Lorite, M.J.; Jambrina, E.; Nevill, C.R.; Lee, P.A.; Schultz, R.C.; Wolos, J.A.; Li, L.C.; Campbell, R.M.; Anderson, B.D. Bioorg. Med. Chem. Lett. 2008, 18, 179–183. doi: 10.1016/j.bmcl.2007.10.106
- (a) Mavrova, A.T.; Anichina, K.K.; Vuchev, D.I.; Tsenov, J.A.; Denkova, P.S.; Kondeva, M.S.;Micheva, M.K. Eur. J. Med. Chem. 2006, 41, 1412; (b) Kohara, Y.; Kubo, K.; Imamiya, E.; Wada, T.; Inada, Y.; Naka, T. J. Med. Chem. 1996, 39, 5228; (c) Elmer, G.I.; Pieper, J.O.; Goldberg, S.R.; George, F.R. Psychopharmacology 1995, 117, 23.
- Liu, J.; Liu, Q.; Xu, W.; Wang, W. Chin. J. Chem. 2011, 29, 1739–1744. doi: 10.1002/cjoc.201180310
- Villemin, D.; Hammadi, M.; Martin, B. Synth. Commun. 1996, 26, 2895–2899. doi: 10.1080/00397919608005224
- Doise, M.; Dennin, F.; Blondeau, D.; Sliwa, H. Tetrahedron Lett. 1990, 31, 1155–1156. doi: 10.1016/S0040-4039(00)88750-X
- Hein, D.W.; Alheim, R.J.; Leavitt, J.J. J. Am. Chem. Soc. 1957, 79, 427–429 doi: 10.1021/ja01559a053
- Salehi, P.; Dabiri, M.; Zolfigol, M.A.; Otokesh, S.; Baghbanzadeh, M. Tetrahedron Lett. 2006, 47, 2557–2560. doi: 10.1016/j.tetlet.2006.02.049
- Mohammadi, M.; Bardajee, G.R.; Pesyan, N.N. RSC Adv. 2014, 4, 62888–62894. doi: 10.1039/C4RA11877D
- So, Y.H.; Heeschen, J.P. J. Org. Chem. 1997, 62, 3552–3561. doi: 10.1021/jo960441u
- Terashima, M.; Ishii, M.; Kanaoka, Y. Synthesis 1982, 6, 484–485. doi: 10.1055/s-1982-29847
- Chakraborti, A.K.; Rudrawar, S.; Kaur, G.; Sharma, L. Synlett. 2004, 9, 1533–1536. doi: 10.1055/s-2004-829089
- Chen, W.H.; Pang, Y. Tetrahedron Lett. 2009, 50, 6680–6683. doi: 10.1016/j.tetlet.2009.09.084
- Shen, W.; Kohn, T.; Fu, Z.; Jiao, X.; Lai, S.; Schmitt, M. Tetrahedron Lett. 2008, 49, 7284–7286. doi: 10.1016/j.tetlet.2008.10.030
- Heravi, M.M.; Sadjadi, S.; Oskooie, H.A.; Shoar, R.H.; Bamoharram, F.F. Catal. Commun. 2008, 9, 504–507. doi: 10.1016/j.catcom.2007.03.011
- Trivedi, R.; De, S.K.; Gibbs, R.A. J. Mol. Catal. A: Chem. 2006, 245, 8–11. doi: 10.1016/j.molcata.2005.09.025
- Inamdar, S.M.; More, V.K.; Mandal, S.K. Tetrahedron Lett. 2013, 54, 579–583. doi: 10.1016/j.tetlet.2012.11.091
- Bala, M.; Verma, P.K.; Sharma, U.; Kumar, N.; Singh, B. Green Chem. 2013, 15, 1687. doi: 10.1039/c3gc40137e
- Shelkar, R.; Sarode, S.; Nagarkar, J. Tetrahedron Lett. 2013, 54, 6986–6990. doi: 10.1016/j.tetlet.2013.09.092
- Teimouri, A.; Chermahini, A.N.; Salavati, H.; Ghorbanian, L. J. Mol. Catal. A: Chem. 2013, 373, 38–45. doi: 10.1016/j.molcata.2013.02.030
- Yang, D.; Zhu, X.; Wei, W.; Sun, N.; Yuan, L.; Jiang, M.; You, J.; Wang, H. RSC Adv. 2014, 4, 17832–17839. doi: 10.1039/C4RA00559G
- Yu, J.; Xia, Y.; Lu, M. Synth. Commun. 2014, 44, 3019–3026. doi: 10.1080/00397911.2014.914221
- Yu, J.; Lu, M. J. Chin. Chem. Soc. 2014, 61, 578–582. doi: 10.1002/jccs.201300577
- Bardajee, G.R.; Mohammadi, M.; Yari, H.; Ghaedi, A. Chin. Chem. Lett. 2016, 27, 265–270. doi: 10.1016/j.cclet.2015.10.011
- Ouasri, A.; Rhandour, A.; Saadi, M.; Ammari, L.El. Acta Crystallogr. Sect. E 2014, 70, 174. doi: 10.1107/S1600536814001068
- Ouasri, A.; Rhandour, A.; Saadi, M.; Ammari, L.El. Acta Crystallogr. Sect. E 2013, 70, 92–93. doi: 10.1107/S1600536813034144
- Ouasri, A.; Elyoubi, M.S.; Guedira, T.; Rhandour, A.; Mhiri, T.; Daoud, A. Spectrochim. Acta Part A. 2001, 57, 2593–2598. doi: 10.1016/S1386-1425(01)00431-0
- Elyoubi, M.; Ouasri, A.; Jeghnou, H.; Rhandour, A.; Dhamelincourt, M.-C.; Dhamelincourt, P.; Mazzah, A. J. Raman Spectrosc. 2004, 35, 1056–1062. doi: 10.1002/jrs.1254
- Jeghnou, H.; Ouasri, A.; Elyoubi, M.; Rhandour, A.; Dhamelincourt, M.-C.; Dhamelincourt, P.; Mazzah, A. J. Raman Spectrosc. 2004, 35, 261–265. doi: 10.1002/jrs.1145
- Jeghnou, H.; Ouasri, A.; Rhandour, A.; Dhamelincourt, M.-C.; Dhamelincourt, P.; Mazzah, A. J. Raman spectrosc. 2003, 34, 126–130. doi: 10.1002/jrs.964
- Ouasri, A.; Rhandour, A.; Dhamelincourt, M.-C.; Dhamelincourt, P.; Mazzah, A.; Taibi, M. Phase Transitions 2003, 76, 701–709. doi: 10.1080/0141159021000037240
- Ouasri, A.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A. Spectrochim. Acta Part A. 2003, 59, 357–362. doi: 10.1016/S1386-1425(02)00165-8
- Ouasri, A.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A.; Taibi, M. J. Raman Spectrosc. 2002, 33, 715–719. doi: 10.1002/jrs.902
- Banerjee, S.; Payra, S.; Saha, A.; Sereda, G. Tetrahedron Lett. 2014, 55, 5515–5520. doi: 10.1016/j.tetlet.2014.07.123
- (a) Riadi, Y.; Mamouni, R.; Azzalou, R.; Haddad, M.E.; Routier, S.; Guillaumet, G.; Lazar, S. Tetrahedron Lett. 2011, 52, 3492; (b) Padalkar, V.S.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, N.; Sekar, P.G. Green Chem. Lett. Rev. 2012, 5, 139; (c) Patil, S.S.; Bobade, V.D. Synth. Commun. 2010, 40, 206; (d) Riadi, Y.; Mamouni, R.; Azzalou, R.; Haddad, M.E.; Routier, S.; Guillaumet, G.; Laza, S. Tetrahedron Lett. 2011, 52, 3492; (e) Praveen, C.; Nandakumar, A.; Dheenkumar, P.; Muralidharan, D.; Perumal, P.T. J. Chem. Sci. 2012, 124, 609; (f) Inamdar, S.M.; More, V.K.; Mandal, S.K. Tetrahedron Lett. 2013, 54, 579; (g) Shelkar, R.; Sarode, S.; Nagarkar, J. Tetrahedron Lett. 2013, 54, 6986; (h) Azizi, N.; Amiri, A.K.; Baghi, R.; Bolourtchian, M.; Hashemi, M.H. Monatsh. Chem. 2009, 140, 1471; (i) Chen, G.F.; Jia, H.M.; Zhang, L.Y.; Chen, B.-H.; Li, J.T. Ultrason Sonochem. 2013, 20, 627.