ABSTRACT
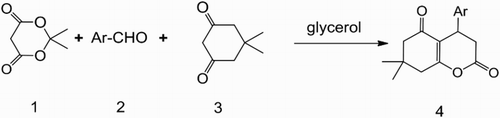
Glycerol is a new green organic solvent. It not only acts as a phase transfer catalyst but also as a recyclable solvent by significantly enhancing the intramolecular cyclization. A series of 4-aryl-7,7 -dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin derivatives were synthesized by one-pot multicomponent reaction of Meldrum’s acid with benzaldehyde, and cyclohexanedione in glycerol. Compared to classic methods, this method has the advantages of milder reaction conditions, good yields, easy processing and environmental benignity.
GRAPHICAL ABSTRACT
Introduction
As an organic reaction medium, the exploration of glycerol is still very limited, but because of its physical and chemical properties of low toxicity, non-corrosivity, non-combustibility, involatility and wide liquid range, glycerol rightly meets most requirements of ideal green solvents ( Citation1). It was shown that glycerol, as reaction medium, can not only increase the reaction rate, but also improve the reaction selectivity ( Citation2, Citation3). Because of glycerol’s lower solubility in nonpolar organic solvents and miscibility with water, the separation of the products can be realized by an organic solvent extraction method or water washing.
Coumarins are important compounds found in different plant species in nature ( Citation4). The specialty of coumarins in inhibiting cancer cells, strengthening immunity, inducing apoptosis and nonlinear optics has drawn extra attention in the fields of laser dye and anti-neoplasm ( Citation5–7). Experimental studies have found that the multi-biological activities of coumarins, such as anti-HIV, anti-tumor, anti-oxidation, anti-inflammatory and other pharmacological activities, are widely applied to anti-coagulation and lymphatic edema in clinic ( Citation8–11). In addition, coumarins are also widely used in spice, detergent and electroplating ( Citation12). Thus, the synthesis of various derivatives of coumarin is one of the hotspots in organic synthesis.
There have been a number of reports on the syntheses of 4-aryl-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin derivatives, in either organic solvent or water by catalysis ( Citation13–15), but these methods present the problem of pollution. Gao et al. ( Citation16) have have completed the synthesis in ethanol under the microwave-induced condition, but the workup was inconvenient to a certain extent. Du et al. ( Citation17) and Li et al. ( Citation18) have completed the synthesis in ionic liquid, but the cost and toxicity of ionic liquids have not been affirmed completely ( Citation19).
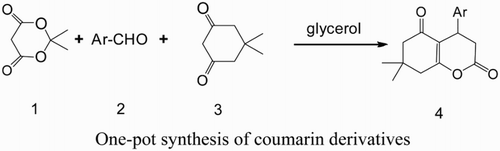
Recently, we have reported the synthesis of hexahydrocoumarin derivatives in PEG-400 media by one-pot reaction, and have got good results ( Citation20). In this communication, we report a simple and effective method for synthesis of a series of hexahydrocoumarin derivatives by one-pot multicomponent reaction of Meldrum’s acid (1) with benzaldehyde (2), 5,5-dimethyl-1,3-cyclohexanedione (3) in glycerol media. 4-aryl-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin derivatives (4) were obtained in high yields (Scheme 1).
4a:Ar=4-ClC6H4 4b:Ar=4-FC6H4 4c:Ar=4-CH3C6H4 4d:Ar=4-NO2C6H4 4e:Ar=4-CH3OC6H4 4f:Ar=2,4-Cl2C6H3 4g:Ar=3,4-(CH3)2C6H3 4h:Ar =3-CH3O-4-OHC6H3 4i:Ar=4-(CH3)2NC6H4
Results and discussion
We tried the synthesis of 4a with the reactive substrates 4-chlorobenzaldehyde, Meldrum’s acid and 5,5-dimethyl-1,3-cyclohexanedione at different reaction conditions. It indicated that the yield of 4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin (4a) was affected by the time and temperature of reaction. We concluded that the desired 4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin (4a) can be obtained at 84% yield in glycerol at 100°C for 5 h.
In order to demonstrate the efficiency and applicability of the present method, we performed the reaction of a variety of substituted benzaldehydes in glycerol at 100°C (). As shown in , a series of 4-aryl-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin derivatives 4 bearing electron-withdrawing groups (such as halide) or electron-donating groups (such as hydroxyl group and alkoxyl group) were synthesized by reaction of Meldrum’s acid with benzaldehyde and 5,5-dimethyl-1,3-cyclohexanedione to give the corresponding products 4 in good yields under the same reaction conditions. Therefore, we concluded that the electronic nature of the substituents has no significant effect on this reaction.
Table 1. Synthesis of hexahydrocoumarin derivatives 4 in glycerol.
In summary, this work describes an efficient and environmentally friendly approach for the one-pot multicomponent synthesis of 4-aryl-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin derivatives in glycerol. The present method offers very attractive features such as excellent yields, simple reaction conditions, environmental benignity and easy work up. Moreover, glycerol acts not only as a catalyst but also as a clean solvent by significantly enhancing the intramolecular cyclization.
Experimental section
All reagents were obtained commercially and used without further purification. Meldrum’s acid 1 was synthesized according to the literature methods ( Citation21). Melting points were measured on an XT-4 electrothermal micromelting-point apparatus, and the thermometer was uncorrected. C, H and N analyses were obtained by using an Elemental Vario-EL automatic elemental analysis instrument. FT-IR spectra were recorded using KBr pellets on a Digilab Merlin FT-IR spectrophotometer. 1H NMR spectra were recorded, on a Varian Mercury plus-400 instrument using dimethyl sulfoxide or CDCl3 as solvents and tetramethyl silane as internal standard.
A mixture of Meldrum’s acid 1 (2 mmol), benzaldehyde 2 (2 mmol), 5,5-dimethyl-1,3-cyclohexanedione 3 (2 mmol) and in glycerol (2 g) was stirred 4–5 h at 100°C. The progress of the reaction was monitored by thin layer chromatography. After completion of the reaction, the reaction mixture was filtered and the precipitate was washed with water. The crude products were purified by recrystallization from ethanol (95%) to give products 4a–4i.
4-(4-Chlorophenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4a.
Yield 4a (84%), m.p.161–163°C (lit ( Citation20) m.p.160–162°C); IR (KBr) ν: 1680, 1662, cm−1; 1H NMR (400 MHz, CDCl3, δ): 1.09 (s, 3H, CH3), 1.15 (s, 3H, CH3), 2.32 (s, 2H, CH2), 2.53 (s, 2H, COCH2), 2.87–2.94 (m, 2H, OCOCH2), 4.26 (t, J = 6.8 Hz, 1H, CH), 7.10 (d, J = 8.0 Hz, 2H, ArH), 7.39 (d, J = 8.0 Hz, 2H).
Anal. calcd for C17H17ClO3: C 67.00, H 5.62; found C 67.21, H 5.58.
4-(4-Fluorophenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4b.
Yield 82%, m.p.166–168°C (lit ( Citation22) m.p.167–168°C); IR (KBr): 1655, 1601, cm−1; 1H NMR (400 MHz, CDCl3, δ): 1.09 (s, 3H, CH3), 1.15 (s, 3H, CH3), 2.32 (s, 2H, CH2), 2153 (s, 2H, CH2), 2188–2197 (m, 2H, CH2), 4128–4129 (m, 1H, CH), 6195–7114 (m, 4H, ArH).
Anal. calcd for C17H17FO3: C 70.82, H 5.94; found C 71.03, H 5.91.
4-(4-Benzyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4c.
Yield 80%, m.p.105–107°C (lit ( Citation20) m.p.107–109°C); IR (KBr) ν: 1789, 1680 cm−1; 1H NMR (400 MHz, CDCl3, δ): 1.06 (s, 3H, CH3), 1.16 (s, 3H, CH3), 2.27 (s, 2H, CH2), 2.29 (s, 3H, CH3), 2.51 (s, 2H, COCH2), 2.83–2.93 (m, 2H, OCOCH2), 4.20–4.24 (m, 1H, CH), 7.03 (d, J = 8.0 Hz, 2H, ArH), 7.07 (d, J = 8.0 Hz, ArH).
Anal. calcd for C18H20O3: C 76.03, H 7.09; found C 75.81, H 7.02.
4-(4-Nitrophenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4d.
Yield 85%, m.p.133–134°C (lit ( Citation20) m.p.132–134°C); IR (KBr) ν: 1789, 1655 cm−1; 1H NMR (400 MHz, CDCl3, δ): 1.08 (s, 3H, CH3), 1.16 (s, 3H, CH3), 2.32 (s, 2H, CH2), 2.58 (s, 2H, COCH2), 2.90–3.03 (m, 2H, OCOCH2), 4.35–4.38 (m, 1H, CH), 7.30 (d, J = 8.0 Hz, 2H, ArH), 8.15 (d, J = 8.0 Hz, 2H, ArH).
Anal. calcd for C17H17NO5: C 64.75, H 5.43, N 4.44; found C 64.63, H 5.48, N 4.41.
4-(4-Methoxyphenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4e.
Yield 75%, m.p.130–131°C (lit (Citation22) m.p. 128–130°C); IR (KBr) ν: 1771, 1650 cm−1; 1H NMR (400 MHz, CDCl3, δ):1.13 (s, 3H, CH3), 1.17 (s, 3H, CH3), 2.37 (s, 2H, CH2), 2.56 (s, 2H, COCH2), 2.89–2.93 (m, 2H, OCOCH2),3.76 (s, 3H, OCH3), 4.24–4.28 (m, 1H, CH), 6.69–7.05 (m, 4H, ArH).
Anal. calcd for C18H20O4: C 71.98, H 6.71; found C 71.73, H 6.75.
4-(2,4-Dichlorophenyl) -7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4f.
Yield 84%, m.p.172–174°C (lit ( Citation23) m.p.173–174°C); IR (KBr) ν: 1659, 1472, cm−1; 1H NMR (400 MHz, CDCl3, δ): 1.18 (s, 6H, 2CH3), 2.34 (s, 2H, CH2), 2.60 (s,2H, CH2), 2.91–2.93 (m, 2H, CH2), 4.58–4.68 (m,1H,CH), 6.83–7.43 (m,3H, Ar H).
Anal. calcd for C17H16Cl2O3: C 60.19, H 4.75; found C 60.31, H 4.81.
4-(3,4-Dimethylphenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4g.
Yield 85%, m.p.121–122°C (lit ( Citation17) m.p.124–126°C); IR (KBr) ν: 1770, 1659, cm−1; 1H NMR (400 MHz, CDCl3, δ): 1.01 (s, 3H, CH3),1.10 (s, 3H, CH3), 1.12 (s, 3H, CH3), 1.16 (s, 3H, CH3), 2.32 (s, 2H, CH2), 2.54 (s, 2H, CH2), 2.90–2.92 (m, 2H, CH2), 4.24 (m, 1H, CH), 6.85–7.05 (m, 3H, ArH).
Anal. calcd for C19H22O3: C 76.48, H 7.43; found C 76.29, H 7.48.
4-(3-Methoxy-4-hydroxyphenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4h.
Yield 76%, m.p.179–182°C (lit ( Citation20) m.p.178–180°C); IR (KBr) ν: 1786, 1652, cm−1; 1H NMR (400 MHz, CDCl3, δ): 1.14 (s, 3H, CH3), 1.19 (s, 3H, CH3), 2.39 (s, 2H, CH2), 2.54 (s, 2H, COCH2), 2.90–2.94 (m, 2H, OCOCH2), 3.87 (s, 3H, OCH3), 4.22–4.27 (m, 1H, CH), 6.63–6.89 (m, 3H, ArH), 10.90 (s, 1H, OH).
Anal. calcd for C18H20O5: C 68.34, H 6.37; found C 68.41, H 6.31.
4-(4-(Dimethylamino)phenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro coumarin 4i.
Yield 73%, m.p.180–182°C (lit ( Citation20) m.p. 182–184°C); IR (KBr) ν: 1791, 1703 cm−1; 1H NMR (400 MHz, CDCl3, δ): 0.90 (s, 3H, CH3), 1.15 (s, 3H, CH3), 2.11 (s, 2H, CH2), 2.40 (s, 2H, COCH2), 2.67 (m, 2H, OCOCH2), 2.89 (s, 6H, 2NCH3), 4.10–4.35 (m, 1H, CH), 6.51–7.17 (m, 4H, ArH).
Anal. calcd for C19H23NO3: C 72.82, H 7.40, N 4.47; found C 72.95, H 7.46, N 4.51.
Conclusions
In conclusion, a series of 4-aryl-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydrocoumarin derivatives were synthesized by three-component one-step reaction in glycerol. The dual roles of glycerol were reaction solvent and catalyst, and glycerol can be recycled.
By contrast, the traditional two-component method has the advantages of milder reaction conditions, good yields, easy processing and environmental benignity. Compared with the reaction in organic solvent or in water by noxious catalyzer (such as hexadecyl trimethyl ammonium bromide), glycerol is more environment-friendly. Compared to the reaction in ionic liquids, ionic liquids are expensive and their toxicity needs further confirmation. In contrast to the microwave-induced reaction in ethanol, their yields are not high enough and the work-up is inconvenient to a certain extent. In comparison with the one-pot reaction in PEG-400, this method, which makes the product separation more convenient and the reaction solvent easily accessible, could significantly shorten the reaction time and improve the yield. We wish to report a simple and green but effective synthesis of hexahydrocoumarin derivatives by using glycerol as reaction medium and catalyst.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Xue-Mei Wang is an associate prof. of organic chemistry, School of Mechanical and Electronical Engineering, Lanzhou University of Technology, People's Republic of China. She has published papers in green organic synthesis fields, focusing on organic chemistry and textile chemistry.
Xi-Cun Wang is a professor of organic chemistry, College of Chemistry and Chemical Engineering, Northwest Normal University, People's Republic of China. He has published papers in fine organic synthesis and green organic synthesis fields, focusing on fine organic synthesis chemistry, green chemistry and heterocyclic chemistry.
Chen-Fei Wang is a postgraduate of textile chemistry and dyeing and finishing engineering, School of Mechanical and Electronical Engineering, Lan Zhou University of Technology, People's Republic of China. He has published papers in modified starch fields.
Liang Yang is an associate prof. of textile chemistry and dyeing and finishing engineering, School of Mechanical and Electronical Engineering, Lan Zhou University of Technology, People's Republic of China. He has published papers in natural biological macromolecule and synthesized polymer fields, focusing on polymer-modified, composite prepare and controlled-release drug delivery.
Additional information
Funding
References
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Nature. 1999, 399, 28–29. doi: 10.1038/19887
- Wolfson, A.; Dlugy, C.; Shotland, Y. Environ. Chem. Lett. 2007, 5, 67–71. doi: 10.1007/s10311-006-0080-z
- Gu, Y.L.; Barrault, J.; Jérôme, F. Adv. Synth. Catal. 2008, 350, 2007–2012. doi: 10.1002/adsc.200800328
- Kroschwitz, J.I.; Howe-Grant, M. Encyclopedia of Chemical Technology; John Wiley &. Sons Inc: New York, 1999; pp 650–654.
- Trkovnik, M.; Ivezić, Z. J. Heterocyclic Chem. 2000, 37, 137–141. doi: 10.1002/jhet.5570370123
- Sardari, S.; Mori, Y.; Horita, K.; Micetich, R.G.; Nishibe, S.; Daneshtalab, M. Bioorg. Med. Chem. 1999, 7, 1933–1940. doi: 10.1016/S0968-0896(99)00138-8
- Birau, M.M.; Yuan Wang, Z. Tetrahedron Lett. 2000, 41, 4025–4028. doi: 10.1016/S0040-4039(00)00576-1
- Nawrot-Modranka, J.; Nawrot, E.; Graczyk, J. Eur. J. Med. Chem. 2006, 41 (11), 1301–1309. doi: 10.1016/j.ejmech.2006.06.004
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Curr. Med. Chem. 2005, 12 (08), 887–916. doi: 10.2174/0929867053507315
- Tyagi, Y.K.; Kumar A.; Raj, H.G.; Vohra, P.; Gupta, G.; Kumari, R.; Kumar, P.; Gupta, R.K. Eur. J. Med. Chem. 2005, 40 (4), 413–420. doi: 10.1016/j.ejmech.2004.09.002
- Kong, L.L.; Hu, J.F.; Chen, N.H. Chin. Pharmacol. Bull. 2012, 28 (2), 165–169.
- Li, Y.; Chen, X.B. Beijing Agr. 2013, 30, 14–15 (in Chinese).
- Jin, T.S.; Wang, A.Q.; Cheng, Z.L.; Zhang, J.S.; Li, T.S. J. Chem. Res. 2004, 7, 457–459. doi: 10.3184/0308234042037275
- Bose, P.; Banerji, J. Indian J. ChemB. 1990, 29B, 422–424.
- Rong, L.C.; Chen, J.; Shi, D.Q.; Zhuang, Q.Y. J. Xuzhou Normal Univ. (Nat. Sci.Ed.). 2004, 22, 43–46 (in Chinese).
- Gao, Y.; Tu, S.J.; Lu, Z.S.; Niu, D.Z.; Sun, B.W. Chin. J. Org. Chem. 2001, 21, 599–602 (in Chinese).
- Du, B.X.; Li, Y.L.; Wang, X.S.; Shi, D.Q.; Tu, S.J. J. Xuzhou Normal Univ.(Nat. Sci. Ed.). 2008, 26, 60–62 (in Chinese).
- Li, Y.Z.; Su, Y.X.; Xue, W.X.; Fan, X.S.; Zhang, X.Y.; Qu, G.R. J. Henan Normal Univ. (Nat. Sci.). 2006, 34, 99–101 (in Chinese).
- Jastorff, B.; Störmann, R.; Ranke, J.; Mölter, K.; Stock, F.; Oberheitmann, B.; Hoffmann, W.; Hoffmann, J.; Nüchter, M.; Ondruschka, B.; Filser, J. Green Chem. 2003, 5, 136–142. doi: 10.1039/b211971d
- Wang, X.M.; Ye, H.L.; Qaun, Z.J.; Wang, X.C. Res. Chem. Intermed. 2013, 39, 2357–2367. doi: 10.1007/s11164-012-0762-z
- Davidson, D.; Bernhard, S.A. J. Am. Chem. Soc. 1948, 70, 3426–3428. doi: 10.1021/ja01190a060
- Xie, X.; Lu, J.; Chen, B.; Han, J.; She, X.; Pan, X. Tetrahedron Lett. 2004, 45, 809–811. doi: 10.1016/j.tetlet.2003.11.042
- Peng, J.; Deng, Y. Tetrahedron Lett. 2001, 42, 403–405. doi: 10.1016/S0040-4039(00)01974-2