ABSTRACT
A new series of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamides were designed based on molecular hybridization approach and synthesized by reaction of benzohydrazide derivatives with 9-phenylfuro[3,4-b]quinoline-1,3-diones in the presence of an Et3N catalyst. Simple reaction, excellent yield, simple separation process and eco-friendly approach by minimizing the chemical waste renders this protocol particularly attractive. The series 9a–g was evaluated for in vitro antibacterial activity against (+ve bacteria) Staphylococcus aureus ATCC 25923 and Enterococcus faecalis ATCC 29212, (−ve bacterium) Escherichia coli ATCC 25922. In vitro minimum inhibitory concentration (MIC) evaluations showed that the compound 9a was effective against E. coli (MIC: 0.25 mg/mL) S. aureus (MIC: 0.25 mg/mL) and E. faecalis (MIC: 0.5 mg/mL).
GRAPHICAL ABSTRACT
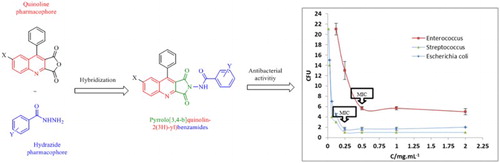
1. Introduction
With regard to the antibiotic resistance in different countries over the half-century, many studies have been focused on the development of new antibacterial agents (Citation1). Antibiotic misuse, self-medication and the failure of control measures to prevent the spread of resistant bacteria in the health care environment have led to an alarming increase in the number of infections caused by resistant bacteria, organisms that resist many currently available antibiotics (Citation2). Because of that, design and efficient synthesis of novel bioactive compounds are one of the main objectives of organic and medicinal chemistry. It is well established in the recent literature that the molecular hybridization approach could be a promising strategy to identify newer hybrid compounds with improved affinity and efficacy when compared with the parent drugs (Citation3).
Nitrogen-containing heterocycles play an important role in designing a new class of structural entities for medicinal applications. The quinoline and pyrrole nucleus are two of the most prevalent N-heterocyclic scaffolds and are found in several bioactive natural products (Citation4–10). In this perspective, we have chosen the quinoline skeleton for the design of new bioactive molecules.
On the other hand, it has been well established that the synthetic compounds with quinoline and pyrrol scaffold in their moieties exhibit a wide range of biological activities such as psychotropic (Citation11), antiallergic (Citation12), anti-inflammatory (Citation13) and estrogenic activity (Citation14). Pyrrolo[3,4-b]quinoline-based molecules are an important hybrids of these compounds (), and can be found as naturally occurring compounds such as camptothecin (Citation15), mappicine (Citation16) and quinocitrinines A and B (Citation17). These compounds are associated with a wide variety of pharmacological properties including antiviral (Citation15, Citation16), cytotoxic (Citation18), antimicrobial (Citation19), anticonvulsant (Citation20) and antitumor activities (Citation21–23). These observations gave us an additional motivation to search for potential pharmacologically active leads carrying Pyrrolo[3,4-b]quinoline substituents.
To the best of our knowledge, there are no reports about the synthesis and evaluation of biological activities of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives in the literature. Pertaining to these observations and with regard to our research interest within the area of multi-substituted quinoline derivatives (Citation24), herein we describe the synthesis of some new pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives (9a–g) and evaluation of their experimental antibacterial activity in vitro.
Our reaction consistence with some principles of green chemistry protocol such as atom economic, lack of side product, minimum waste and work up product with simple filtration instead of using high-cost chromatography method (which include using a large amount of solvent). These features of our reaction make it eco-friendly.
2. Results and discussion
The synthetic route for pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives (9a–g) is represented in Scheme 1. Esterification of different carboxylic acids lead to the correspondent ethyl ester (2a–e) and further hydrazineloysed yielded the hydrazides (3a–e). 2,3,4-trisubstituted quinolines (6a–d) were synthesized from different aromatic amino ketones 4a,b and dialkylacetylenedicarboxylate 5a,b in ethanol as reported (Citation25). The dicarboxyquinoline derivatives 7a,b were prepared from the corresponding quinolinedicarboxylates 6a–d by alkaline hydrolysis, which was acidified with 1:1 HCl at low temperature (Citation26). The 4-phenylquinoline-2,3-dicarboxylic acids (7a,b) were then converted to the corresponding anhydrides (8a,b) using a procedure adapted from Fehnel et al (Citation27). The target compounds 9a–g were synthesized in good yield by refluxing an equimolar mixture of benzohydrazide derivatives (3a–e) and respective 9-phenylfuro[3,4-b]quinoline-1,3-diones (8a,b) in the presence of catalytic amount of Et3N using toluene as solvent (). The reaction afforded very pure products after filtration and no need further separation with chromatography. All the synthesized compounds were confirmed by IR, 1H NMR, 13C NMR and elemental analyses.
Scheme 1. Synthetic route for the synthesis of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives.
![Scheme 1. Synthetic route for the synthesis of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives.](/cms/asset/9094963a-8502-4c23-819d-e5d58224de4b/tgcl_a_1380233_f0004_b.gif)
Table 1. Synthesis of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamides 9a–g.
2.1. Antibacterial studies
Agar dilution involves the incorporation of varying concentrations of the antimicrobial agent into a standard agar medium, usually using serial twofold dilutions, followed by the application of a defined bacterial inoculum to the agar surface of the plate. To perform the test, a series of tubes or plates were prepared with the Mueller–Hinton agar (MHA) medium to which various concentrations of antimicrobial agents were added. The tubes or plates are then inoculated with a standardized suspension of the test organism. After incubation at 35 ± 2°C, the tests were examined and the minimum inhibitory concentration (MIC) was determined. These results are often considered as the most reliable for the determination of an MIC for the test bacterium/antimicrobial combination (Citation28).
Antibacterial activities of dimethyl sulfoxide (DMSO) solution of 9a–g were carried out using disc diffusion method. Petri plates were prepared with 15 mL of sterile MHA. The test cultures were swabbed on the top of the solidified media. A sterile 6 mm filter paper disk was immersed in each solution for 10 s and placed on the surface of an MHA plate. Negative control was prepared using DMSO. Streptomycin (10 µg per disc) and penicillin (10 µg per disc) were used as a positive control against bacteria. The plates were incubated at 37°C for 24 h. Zones of inhibition were recorded in mm and experiment was repeated twice ().
Table 2. Antibacterial activity of 9a–g determined by measuring the diameter of inhibition zone.
It was noteworthy to see that among the tested pyrrolo[3,4-b]quinolin-benzamides, compound 9a, showed considerable activity against all the bacterial strains. In order to quantify the bioactivity of the compound, the minimum inhibition concentration was measured using Agar dilution method. Since this compound was precipitated in aqua solution, the agar dilution method using Muller–Hinton medium was carried out. Sterile 6 well plates containing twofold serial dilution of the compound in the medium were prepared and labeled under aseptic conditions ().
Figure 2. The experimental methodology to perform twofold serial dilution with solid modification. The procedure involves preparing twofold dilutions of the antimicrobial agent (e.g. 0.125, 0.25, 0.5, 1 and 2 mg/mL) in a MHA dispensed in 6-well plate (step 1 and 2). Then, each well is inoculated with a microbial inoculum prepared adjusted to 2 McFarland scale (step 3). After well-mixing, the inoculated 6-well plate are incubated at ∼37°C for 24 h.
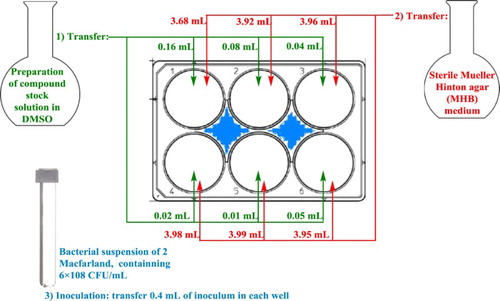
3.84 mL of MHA and 0.16 mL of compound 9a were separately pipetted into the first well and shake vigorously to obtain 2 mg/mL concentration. This process was repeated for the remaining five well with twofold dilution method to obtain intended concentration. Finally, 0.4 mL of Macfarland standardized suspension of tested bacteria (6 × 108 CFU/mL) was added to each well and mixed gently. The plates were incubated at ∼37°C for 24 h. Every cell in the population would divide and form a visible and countable colony to the naked eye. In theory, all the cells in a colony are derived from a single bacterium initially deposited on the plate and thus represent the number of cells that were present in the sample. The growth was calculated by plate count method. The experiments were carried out in triplicate. The data revealed the average of triplicate tests. The obtained results are presented in .
Figure 3. Growth inhibition effect of compound 9a. The experiments were carried out in triplicate and error bars indicate standard error of the mean.
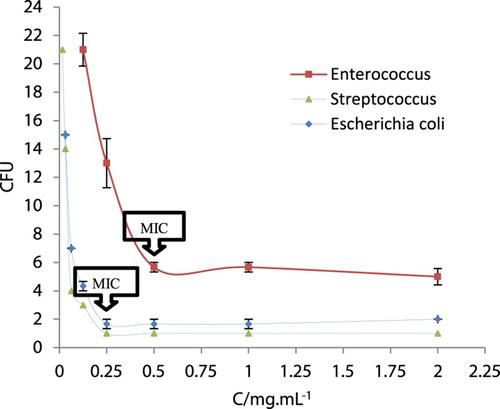
MIC results of 9a exhibited antibacterial activity against the gram-negative (E. coli, MIC: 0.25 mg/mL) and gram-positive (S. aureus, MIC: 0.25 mg/mL and E. faecalis, MIC: 0.5 mg/mL) bacteria.
A previous report has revealed that non β-lactam antibacterial compounds like quinazolines targeting penicillin binding protein (PBP) which led to bacterial growth inhibition (Citation29). The synthesized compounds have a quinoline moiety that may act like quinazoline in targeting of PBP. However, the improvement of this idea needs more investigation.
3. Conclusion
In conclusion, we have synthesized a series of new pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives. The antibacterial activities of compounds 9a–e have been evaluated and demonstrated potent inhibition against the gram-negative (E. coli) and gram-positive bacteria (Streptococcus and Enterococcus) tested bacteria. Amongst all the compounds screened, 9a showed very good activity against tested bacteria. Simple reaction procedure, excellent yields, importantly the purification of compounds by a non-chromatography method just by simple filtration made this approach eco-friendly.
4. Experimental
4.1. General
For the preparation of benzohydrazides, the literature procedures were employed (Citation26). All acetylene dicarboxylates were commercially available and used as received without further purification. The progress of the reactions was followed by TLC using silica gel SILIG/UV 254 plates. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker 400 MHz instrument. FT-IR spectra were recorded on a Perkin-Elmer RX-1 instrument. Elemental analysis for C, H and N were performed using a Heraeus CHN-O-Rapid analyzer. The melting points were determined in open capillaries with a Stuart Melting Point Apparatus and are uncorrected.
4.2. General procedure for the synthesis of N-(1,3-dioxo-9-phenyl-1H-pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamides (9a–g).
A mixture of 9-phenylfuro[3,4-b]quinoline-1,3-dione (1 mmol) and benzohydrazides (1 mmol) was refluxed in toluene (5 mL) in the presence of a catalytic amount of triethylamine (Et3N). The progress of reaction was monitored by TLC. At the end of the reaction, the precipitate was collected by filtration. The solid was dried in air to give 9a–g.
N-(1,3-dioxo-9-phenyl-1,3-dihydro-2H-pyrrolo[3,4-b]quinolin-2-yl)-4-methylbenzamide (9a): White solid (0.374 g, 95% yield); m.p. 276–278°C;1H NMR (400 MHz, CDCl3): δ 8.50 (s, N-H, 1H), 8.43–8.46 (m, 1H), 7.92–7.95 (m, 2H), 7.74 (d, J = 8.1 Hz, 2H), 7.67–7.70 (m, 1H), 7.53–7.57 (m, 3H), 7.47–7.49 (m, 2H), 7.20 (d, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 164.5, 162.5, 161.6, 149.9, 148.4, 147.3, 142.5, 131.7, 130.7, 130.5, 128.7, 128.4, 127.8, 127.3, 127.2, 126.7, 126.6, 20.6; IR (KBr) νmax/cm−1: 3252 (NH), 3060 and 3021 (Csp2-H), 1799 and 1748 (C=O); Anal. Calcd. for C25H17N3O3: C, 73.70; H, 4.21; N, 10.31. Found: C, 73.82; H, 4.36; N, 10.51. m/z 407 (M)+.
Supplement_Material.docx
Download MS Word (7.1 MB)Acknowledgments
The authors are grateful to the University of Mohaghegh Ardabili for the financial support and the laboratories of Tehran, Tabriz and Atatürk Universities for analyzing the products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributor
Tahere Hosseyni Largani is a Ph. D student of organic chemistry, University of Mohaghegh Ardabili.
Gholamhassan Imanzadeh is a Professor of organic chemistry, University of Mohaghegh Ardabili.
Saber Zahri is a Professor of biology, University of Mohaghegh Ardabili.
Nader Noroozi Pesyan is a Professor of organic chemistry, Urmia University.
Ertan Şahin is a Professor of inorganic chemistry, Atatürk University.
References
- Sangani, C.B.; Makawana, J.A.; Zhang, X.; Teraiya, S.B.; Lin, L.; Zhu, H.-L. Design, Synthesis and Molecular Modeling of Pyrazole–Quinoline–Pyridine Hybrids as a New Class of Antimicrobial and Anticancer Agents. Eur. J. Med. Chem. 2014, 76, 549–557. doi: 10.1016/j.ejmech.2014.01.018
- Fernández, J.; Bert, F.; Nicolas-Chanoine, M.-H. The Challenges of Multi-drug-resistance in Hepatology. J. Hepatol. 2016, 65 (5), 1043–1054. doi: 10.1016/j.jhep.2016.08.006
- Viegas-Junior, C.; Danuello, A.; Bolzani, V.D.; Barreir, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14 (17), 1829–1852. doi: 10.2174/092986707781058805
- Keri, R.S.; Patil, S.A. Quinoline: A Promising Antitubercular Target. Biomed. Pharmacother. 2014, 68 (8), 1161–1175. doi: 10.1016/j.biopha.2014.10.007
- Simoes, J.B.; de Fatima, A.; Sabino, A.A.; Almeida Barbosa, L.C.; Fernandes, S.A. Efficient Synthesis of 2,4-Disubstituted Quinolines: Calix[n]arene-catalyzed Povarov-hydrogen-transfer Reaction Cascade. RSC Adv. 2014, 4 (36), 18612–18615. doi: 10.1039/C4RA02036G
- Li, H.; Wang, C.; Huang, H.; Xu, X.; Li, Y. Silver-catalyzed Cascade Reaction of o-aminoaryl Compounds with Alkynes: An Aniline Mediated Synthesis of 2-substituted Quinolines. Tetrahedron Lett. 2011, 52 (10), 1108–1111. doi: 10.1016/j.tetlet.2010.12.102
- Walsh, C.T.; Garneau-Tsodikova, S.; Howard-Jones, A.R. Biological Formation of Pyrroles: Nature’s Logic and Enzymatic Machinery. Nat. Prod. Rep. 2006, 23 (4), 517–531. doi: 10.1039/b605245m
- Young, I.S.; Thornton, P.D.; Thompson, A. Synthesis of Natural Products Containing the Pyrrolic Ring. Nat. Prod. Rep. 2010, 27 (12), 1801–1839. doi: 10.1039/c0np00014k
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.-F. Lamellarins and Related Pyrrole-derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108 (1), 264–287. doi: 10.1021/cr078199m
- Fukuda, T.; Ishibashi, F.; Iwao, M. Synthesis and Biological Activity of Lamellarin Alkaloids: An Overview. Heterocycles. 2011, 83 (3), 491–529. doi: 10.3987/REV-10-686
- Nesterova, I.N.; Alekseeva, L.M.; Andreeva, N.I.; Golovina, S.M.; Granik, V.G. Synthesis and Study the Pharmacological Activity of Derivatives of 5-dimethylaminopyrano[3,2-c]quinolin-2-ones. Pharm. Chem. J. 1995, 29 (2), 111–114. doi: 10.1007/BF02226521
- Yamada, N.; Kadowaki, S.; Takahashi, K.; Umezu, K. MY-1250, a Major Metabolite of the Anti-allergic Drug Repirinast, Induces Phosphorylation of a 78-kDa Protein in Rat Mast Cells. Biochem. Pharmacol. 1992, 44 (6), 1211–1213. doi: 10.1016/0006-2952(92)90387-X
- Faber, K.; StÚckler, H.; Kappe, T. Non-steroidal Antiinflammatory Agents. 1. Synthesis of 4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl Alkanoic Acids by the Wittig Reaction of Quinisatines. J. Heterocyclic Chem. 1984, 21 (4), 1177–1181. doi: 10.1002/jhet.5570210450
- Akhmed Khodzhaeva, K.S.; Bessonova, I.A. Dokl. Akad. Nauk Uzh. SSR1982, 34–36 (Russ);Chem. Abstr. 1983, 98, 83727q. Dokl. Akad. Nauk Uzh. SSR1982, 34–36.
- Fortunak, J.M.D.; Mastrocola, A.R.; Mellinger, M.; Sisti, N.J.; Wood, J.L.; Zhuang, Z.-P. Novel Syntheses of Camptothecin Alkaloids, Part I. Intramolecular [4+2] Cycloadditions of N-arylimidates and 4H-3,1-benzoxazin-4-ones as 2-aza-1,3-dienes. Tetrahedron Lett. 1996, 37 (32), 5679–5682. doi: 10.1016/0040-4039(96)01204-X
- Yadav, J.S.; Sarkar, S.; Chandrasekhar, S. A Convergent Total Synthesis of Mappicine Ketone: A Leading Antiviral Compound. Tetrahedron. 1999, 55 (17), 5449–5456. doi: 10.1016/S0040-4020(99)00191-X
- Machtey, V.; Gottlieb, H.E.; Byk, G. Total Synthesis of Structures Proposed for Quinocitrinines A and B and their Analogs. Microwave Energy as Efficient Tool for Generating Heterocycles. Arkivoc. 2011,U308–U479.
- Wang, H.; Ganesan, A. Total Synthesis of the Cytotoxic Alkaloid Luotonin A. Tetrahedron Lett. 1998, 39 (49), 9097–9098. doi: 10.1016/S0040-4039(98)02004-8
- Van Es, T.; Staskun, B.; Van Vuuren, S. Sulphur-substituted Pyrrolo[3, 4-b]Quinolines: Synthesis, Chemistry and Antimicrobial Activity. S. Afr. J. Chem. 2005, 58, 74–81.
- Sorokina, I.K.; Alekseeva, L.M.; Parshin, V.A.; Granik, V.G. Functional Derivatives of Tetramic Acid: New Approaches to the Synthesis of Heterotricyclic Compounds. Pharm. Chem. J. 2007, 41 (10), 543–548. doi: 10.1007/s11094-008-0009-x
- Ramaprasad, G.C.; Kalluraya, B.; Sunil Kumar, B.; Mallya, S. Synthesis of New Oxadiazole Derivatives as Anti-inflammatory, Analgesic, and Antimicrobial Agents. Med. Chem. Res. 2013, 22 (11), 5381–5389. doi: 10.1007/s00044-012-0298-1
- Nagarapu, L.; Gaikwad, H.K.; Bantu, R.; Manikonda, S.R.; Ganesh Kumar, C.; Pombala, S. Lewis Acid-assisted Olefin Cross-metathesis Reaction: An Efficient Approach for the Synthesis of Glycosidic-pyrroloquinolinone Based Novel Building Blocks of Camptothecin and Evaluation of their Antitumor Activity. Tetrahedron Lett. 2012, 53 (10), 1287–1291. doi: 10.1016/j.tetlet.2012.01.001
- Nagarapu, L.; Gaikwad, H.K.; Bantu, R.; Manikonda, S.R. Chemoenzymatic Synthesis with Lipase Catalyzed Resolution and Evaluation of Antitumor Activity of (R/S)-2-[2-hydroxy-3-(4-phenylpiperazin-1-yl)propyl]-1H-pyrrolo[3,4-b]quinolin-3(2H)-one. Eur. J. Med. Chem. 2011, 46 (6),2152–2156. doi: 10.1016/j.ejmech.2011.02.069
- Mamaghani, M.; Larghani, T.H. Ultrasound Promoted One-pot Three-component Synthesis of Novel 7-aryl-8H-benzo[h]indeno[1,2-b]quinolin-8-ones Under Solvent-free Conditions. J. Chem. Res. 2012, 36 (4),235–237. doi: 10.3184/174751912X13319177859559
- Patil, D.R.; Deshmukh, M.B.; Salunkhe, S.M.; Anbhule, P.V. A Simple Synthesis of Trisubstituted Quinolines Through Transesterification. J. Heterocyclic Chem. 2011, 48 (6),1342–1346. doi: 10.1002/jhet.711
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry;5th ed.;Longman Scientific and Technical: Harlow, 1989.
- Fehnel, E.A.; Deyrup, J.A.; Davidson, M.B. Quinoline Analogs of Podophyllotoxin. II. Friedländer Reactions with Tetronic Acid: A General Method for the Preparation of 2-Hydroxymethyl-3-quinolinecarboxylic Acid Lactones1. J. Org. Chem. 1958, 23 (12), 1996–2001. doi: 10.1021/jo01106a051
- Schmith, K.; Reymann, F.E. Experimentelle og kliniske undersogelser over gonococcers folsomhed overfor sulfapyridin. Nord. Med. 1940, 8, 2493–2499.
- Bouley, R.; Ding, D.; Peng, Z.; Bastian, M.; Lastochkin, E.; Song, W.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Mobashery, S.; Chang, M. Structure–Activity Relationship for the 4(3H)-Quinazolinone Antibacterials. J. Med. Chem. 2016, 59 (10), 5011–5021. doi: 10.1021/acs.jmedchem.6b00372

![Figure 1. Some important pyrrolo[3,4-b]quinoline-containing drugs.](/cms/asset/9a8bc0c5-6a64-4b68-b7c6-526bc218d54c/tgcl_a_1380233_f0001_b.gif)