ABSTRACT
An expired pharmaceutical product, Carvedilol, was studied as save drug to prevent carbon steel (CS) corrosion in acidic environments by using weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. The inhibition efficiency increased with increasing drug dose and decreased with increasing temperature, reaching a maximum value of 98.94% at 25°C and 1.6 × 10−4 M. Polarization data showed that the drug is of a mixed type. The inhibition of CS corrosion by Carvedilol can be attributed to the adsorption ability of drug molecules onto the reactive sites of the metal surface. The adsorption process follows the Langmuir isotherm via physical adsorption. The surface morphologies of CS were examined by atomic force spectroscopy (AFM). The results obtained from different techniques are in good agreement.
GRAPHICAL ABSTRACT
1. Introduction
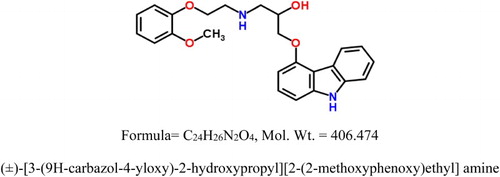
Carbon steel (CS) is the most worldwide used material for industrial and domestic applications, because of its good mechanical properties, availability and relatively reasonable cost. Hydrochloric acid is widely used in various technological processes in industry (in pickling baths, in the extraction and processing of oil and gas) and in other chemical and petrochemical industries. The corrosion behavior of CS in acidic media is an area of great interest due to its importance in applications. It has been found that one of the best methods of protecting metals against corrosion involves the use of synthetic organic compounds as corrosion inhibitors (Citation1–3) which are substances that slow down the rate of corrosion (Citation4,Citation5). But many of these compounds unfortunately are highly toxic to both human beings and the environment (Citation6). Due to the toxicity of some corrosion inhibitors, researches are concentrated toward the introduction of new, safe, non-toxic and environmentally benign natural products such as pharmaceutical compounds, leaves, fruits or seeds extracts, which can be used as corrosion inhibitors. Recently, several studies have been carried out on the use of drugs as potential candidate for the inhibition of metal corrosion because, through their functional groups, they form complexes with metal ions on metal surfaces. Antibiotics fall under the category of eco-friendly compounds and hence claimed as green correction inhibitors which are completely soluble in water and obtainable with high purity and relatively cheap. These properties would validate their use as corrosion inhibitors in various media. Likewise, the use of expired drugs as non-toxic corrosion inhibitors for CS and Al in acidic media was reported before (Citation7–11). The use of expired drugs as corrosion inhibitors can be traced back to 2009's and 2011's when Abd El-Hameed (Citation7) used expired ranitidine as corrosion inhibitor for carbon steel (Citation8) and for mild steel (Citation8) in HCl corrosive medium, respectively. The expired Carvedilol drug is big enough (molecular mass = 406.474, mol. formula = C24H26N2O4) and likely to effectively cover more surface area (due to adsorption) of the CS. Furthermore, Carvedilol drug is very cheap, easily available, environmentally friendly and non-toxic. Due to these properties, Carvedilol drug was chosen for the corrosion studies. The following table gives a comparison of %IE with different investigated drugs:
The main objective here is to investigate the corrosion process of CS in 1 M HCl solution in the absence and presence of different doses of expired Carvedilol drug and to test the various electrochemical studies towards the corrosion of CS. Also, our study was focused on the usage of expired drugs because most of the pharmaceutically active substances are more expensive than the organic inhibitors currently used. Also, the use of expired drugs will solve two problems: (a) economical which reduce the disposal costs of expired drugs and (b) environmental which limits the environmental pollution with pharmaceutically active compounds.
Carvedilol drug is the commercial name of ((±)-[3-(9H-carbazol-4-yloxy)-2-hydroxypropyl][2-(2-methoxyphenoxy)ethyl]amine) as shown in .
2. Experimental methods
2.1. Material
The composition of the CS specimen (in weight %) is C 0.2, Mn 0.6, P 0.04, Si 0.003 and Fe rest.
2.2. Solutions
All solutions and reagents were prepared from analytical reagent-grade compounds in bi-distilled water. The aggressive solutions of 1 M HCl were prepared by dilution of analytical grade 37% HCl with bi-distilled water.
2.3. Inhibitor
Carvedilol drug was kindly supplied by Boehringer Mannheim GmbH. A stock solution of 2.5 × 10−2 M Carvedilol was prepared by dissolving an accurate weight of the drug in ethanol and stored in the dark at 4°C. Dilute solutions of the drug were then prepared daily just before use in dark polyethylene bottles. The standard stock solutions were protected from light during the experimental procedures to prevent any possible degradation of the drug before use.
2.4. Weight loss measurements
Coupons cut into 2 × 2 × 0.2 cm dimensions are used for weight loss (WL) measurements. Prior to all measurements, the exposed area was mechanically abraded with 180, 320, 800 and 1200 grades of emery papers. The specimens were washed thoroughly with bi-distilled water, degreased and dried with ethanol. The solution volume is 100 mL, the immersion time for the WL is 30, 60, 90, 120 and 180 min at 25°C, 35°C, 45°C and 55°C. In order to get good reproducibility, parallel triplicate experiments were performed and the average WL value of three parallel CS sheets was obtained.
2.5. Electrochemical measurements
The electrochemical tests were conducted in a classical three-electrode cell with platinum as counter electrode, saturated calomel as the reference electrode, and CS specimen as the working electrode. Before starting the experiment, the working electrode with an area of 1 cm2 was treated as in WL experiments and then immersed in a test solution for 30 min until a steady-state open circuit potential (OCP) was attained. The Stern-Geary method (Citation21) used for the determination of corrosion current is performed by extrapolation of anodic and cathodic Tafel lines to a point which gives log icorr and the corresponding corrosion potential (Ecorr) for inhibitor free acid and for each dose of drug. All experiments were carried out three times for reproducibility and all measurements were conducted at 25°C.ll measurements were conducted at 25C
EIS was carried out at OCP in the frequency range 105–10−1 Hz, and the AC signal was 0.005 V in amplitude. EIS diagrams are given in the Nyquist and Bode representations.
PP was made by changing the electrode potential automatically from –800 to –200 mV versus OCP at a scan rate of 0.1 mV s−1.
EFM were conducted using two frequencies of 0.2 and 0.5 Hz with amplitude of 20 mV RMS. EFM were conducted using two frequencies of 02 and 05 Hz with amplitude of 20 mV RMS The base frequency was 0.1 Hz, so the waveform repeats after 1 s. The higher frequency must be at least two times the lower one and must also be sufficiently slow that the charging of the double layer does not contribute to the current response. Often, 10 Hz is a reasonable limit. The large peaks were used to calculate the corrosion current density (icorr), the Tafel slopes (βa and βc) and the causality factors CF-2 and CF-3 (Citation22). The electrode potential was allowed to stabilize 30 min before starting the measurements.
All electrochemical experiments were carried out using Gamry instrument PCI300/4 Potentiostat/Galvanostat/Zra analyzer, DC105 Corrosion software, EIS300 software for EIS tests, EFM140 software for EFM tests and Echem Analyst 5.5 for results plotting, graphing, data fitting and calculating.
2.6. Surface analysis studies
Atomic force microscope (AFM) was used for surface morphology studies. The polished metal specimens were immersed in acid solutions in the absence and presence of 1.6 × 10−6 M drug. After a few hours, the specimens were taken out, washed with bi-distilled water and dried. The protective film was examined with AFM using Nano Surf Easyscan 2 Flex AFM instruments (Nanotechnology Center, Mansoura University). The topology of the studied samples for a scanned area of 4 or 8 μm is evaluated for a set point of 20 nN and a scan speed of 10 mm s−1. The three dimensional topology of surface films gave various roughness parameters of the film, i.e. square mean value.
3. Results and discussion
3.1. WL measurements
The WL results of CS in 1 M HCl in the absence and presence of different doses of Carvedilol after 30, 60, 90, 120 and 180 min of hold time immersion at temperatures ranging of 25–55°C was studied and from the WL results, the %IE of the drug and θ were calculated using the following equation (Citation23):(1) where Winh and Wfree are the WL for CS in the existence and nonexistence of the drug in HCl solution, respectively.
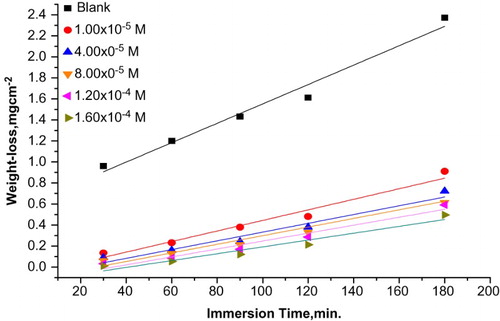
The data of revealed that the values of the %IE increased with an increase in the Carvedilol doses. This behavior could be attributed to the increase in the CS surface area covered by the adsorbed molecules of Carvedilol with the increase in its dose which makes a barrier for mass transfer and prevents further corrosion. On the other hand, WL of CS decreases with an increase in Carvedilol doses, this means that Carvedilol acts as an inhibitor. The maximum %IE for Carvedilol 98.94% was achieved at 1.6 × 10−4 M at 25°C after immersion time 30 min.
Table 1. Variation of %IE with dose of Carvedilol in 1 M HCl solution at 25°C after immersion time 30 min.
In order to elucidate the influence of immersion time on the corrosion rate of CS, the data as shown in show the WL time plots for CS corrosion with and without different doses of the extract. The WL of CS increases with immersion time and by increasing the dose of Carvedilol, the WLs of CS samples are decreased indicating that Carvedilol is adsorbed on the CS surface in HCl (Citation24).
3.2. Effect of temperature on %IE
Temperature has a great effect on the corrosion phenomenon. The effect of temperature on the %IE of the Carvedilol drug was determined by the WL method as shown in . The data in revealed that the %IE decreased by increasing the temperature, this is due to increased rate of the dissolution process of CS and partial desorption of the drug from the metal surface with temperature (Citation25). This indicates that the Carvedilol drug is physically adsorbed on the metal surface (Citation26).
Table 2. The effect of temperature on the %IE of the Carvedilol drug.
3.3. Thermodynamic activation parameters of the corrosion process
Thermodynamic parameters are important and useful tool for clarifying the adsorption behavior of inhibitors. The thermodynamic parameters such as activation energy (), enthalpy (
) and entropy (
) of activation for the corrosion of CS in HCl solution were calculated. These parameters were computed with and without various doses of Carvedilol drug by applying Arrhenius equation (2) and transition-state equation (3) respectively (Citation27):
(2)
(3) where CR is the corrosion rate, R is the universal gas constant, A is Arrhenius pre exponential factor, T is the absolute temperature, h is Planck’s constant and N is Avogadro’s number. shows the Arrhenius plot (log CR vs. 1/T). This plot gives straight lines and slope equal to
/2.303R from which the values of
for the inhibited corrosion reaction of CS have been calculated and recorded in . It was observed, for the HCl solution the
has a value 28.87 kJ mol−1 but in the presence of various doses of drug it had values from 52.89 to 65.73 kJ mol−1 revealing the retardation of the corrosion reaction. This change in
may be either precipitation in the electrode or may change the potential difference of the metal solution interface by adsorption. shows the transition-state plot (log CR/T vs. 1/T). This plot gives straight lines with a slope equal to –(
)/2.303R) and intercept (log R/2.303R + (
)/2.303R), from which
and
values were calculated and listed in . The positive sign of
reveals that the formation of the activated complex is endothermic. The negative values of
indicate that the inhibitor molecules were adsorbed in an orderly manner onto the CS surface causing the entropy to decrease (Citation28).
Figure 3. Arrhenius plots (log CR vs. 1000/T) for CS in 1 M HCl with and without different doses of Carvedilol drug.
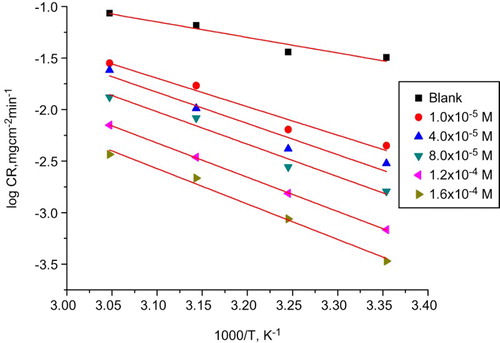
Figure 4. Arrhenius plots (log CR/T vs. 1000/T) for carbon steel in 1 M HCl with and without different doses of Carvedilol drug.
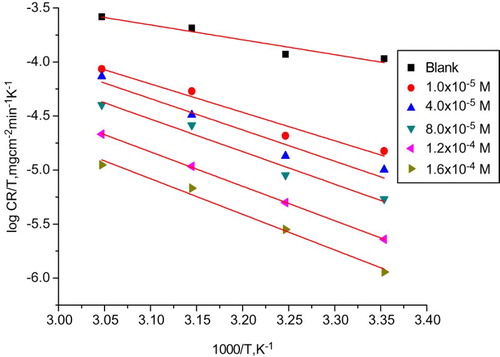
Table 3. Activation parameters for dissolution of CS in absence and presence of different doses of Carvedilol drug in 1 M HCl.
3.4. Adsorption consideration
The adsorption isotherm is necessary in studying the mechanism of adsorption. In order to gain more information about the mode of adsorption (physisorption, chemisorption or comprehensive adsorption) of the drug on the surface of CS, the experimental data have been tested with several adsorption isotherms (Langmuir, Freundlich, Temkin, kinetic-thermodynamic model and Flory–Huggins isotherm). A very good fit is observed with a regression coefficient (R2 = 0.9994) for the Langmuir adsorption isotherm which confirms the validity of this approach (Citation29). The Langmuir adsorption isotherm is based on the view that every adsorption site is identical and energetically equivalent (Citation30). The Langmuir isotherm is expressed as in the following equation:(4) where Kads is the adsorption equilibrium constant. shows the Langmuir adsorption isotherm for Carvedilol drug on the CS surface. The data derived from this isotherm are recorded in . The slight deviation of the slopes from unity is attributed to the molecular interaction among the adsorbed drug species, a factor which was not taken into consideration during the derivation of the Langmuir equation.
Figure 5. Langmuir adsorption isotherm plotted as C/Θ vs. C of Carvedilol drug for the corrosion of CS in 1 M HCl at 25°C.
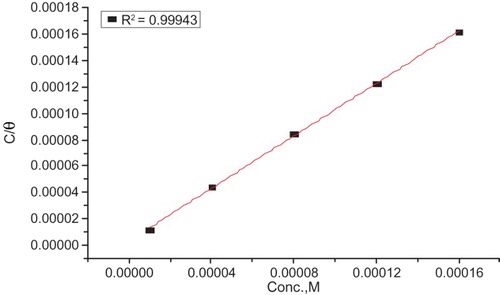
Table 4. Equilibrium constant (Kads) and standard free energy ( ) of adsorption of the drug on CS in 1 M HCl at different temperatures.
) of adsorption of the drug on CS in 1 M HCl at different temperatures.
The standard free energy of adsorption (ΔG°ads) was calculated by the following equation (Citation31):(5) where 55.5 is the molar dose of water in the solution in mol L−1. The negative value of
indicates the stability of the adsorbed layer on the CS surface (Citation32). It is well known that
values up to −20 kJ mol−1 are consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption) while those more negative than −40 kJ mol−1 involve sharing or transfer of electron from the drug molecule to the metal surface to form a coordinate bond (chemisorption) (Citation33). The values of
obtained are in the range of −40 kJ mol−1 indicating that the adsorption of Carvedilol on the CS surface in 1 M HCl solutions was of mixed type (chemisorption and physisorption).
3.5. Tafel polarization measurements
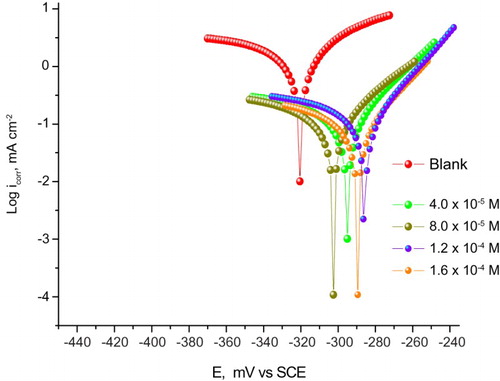
demonstrates the Tafel polarization curves for CS in 1 M HCl with and without the addition of different doses of the expired drug at 25°C. The obtained kinetic parameters such as corrosion current density (icorr), cathodic Tafel slope (bc), anodic Tafel slope (ba), corrosion potential (Ecorr) and %IE found from these curves are given in . The data in revealed that icorr decreased in the presence of drug while %IE increased. Also, it is noticed that ba and bc were not affected and the Tafel lines are parallel, denoting that the inhibiting effect of this drug has arisen by merely blocking of the available surface area via adsorption, i.e. the tested drug reduced the anodic dissolution and hydrogen evolution without changing the reaction mechanism (Citation34). The shift of the Ecorr values is not more than 85 mV (34 mV); hence, the studied drug may be classified as a mixed-type inhibitor.
Figure 6. Potentiodynamic anodic and cathodic polarization curves recorded for CS in 1 M HCl solutions without and with various doses of Carvedilol at 25°C.
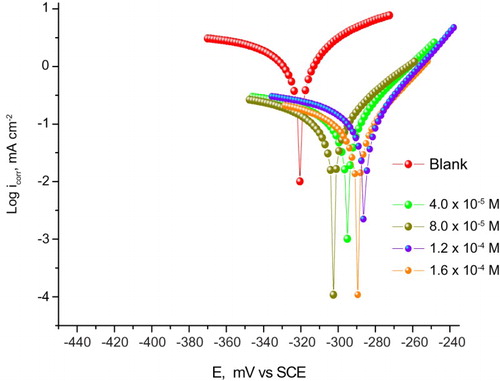
Table 5. Electrochemical parameters obtained from polarization curves for CS in 1 M HCl without and with various doses of Carvedilol at 25°C.
3.6. EIS measurements
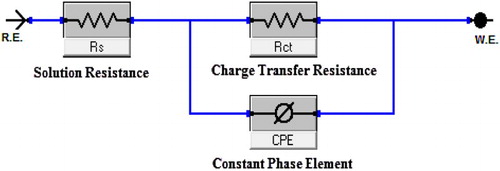
The experimental results obtained from EIS measurements for the corrosion of CS in the presence of Carvedilol drug in 1 M HCl at room temperature are summarized in . displays the Nyquist (a) and Bode (b) plots for CS in 1 M HCl in the absence and presence of various doses of expired Carvedilol drug. Clearly, the impedance spectra exhibit a single semicircle. The semicircle indicates that the corrosion of CS in 1 M HCl is mainly controlled by a charge transfer process (Citation35) and usually related to the charge transfer of the corrosion process and double-layer behavior. The diameter of the capacitive loop in the presence of the drug is bigger than in the absence of the drug (blank solution) and increases with inhibitor dose. This indicates that the impedance of inhibited substrate increases with the inhibitor dose. Noticeably, these impedance spectra are not perfect semicircles which can be attributed to the frequency dispersion effect. This anomalous behavior is generally attributed to the roughness and inhomogeneity of the metal surface (Citation36). The impedance spectra exhibit a single semicircle corresponding to one time constant in the Bode plots ((b)). It can be concluded that the charge transfer resistance Rct value increased with increase in the dose of the drug, whereas the values of double-layer capacitance (Cdl) decreased, with an increase in the inhibitor dose, which is most probably due to the decrease in the local dielectric constant and/or increase in the thickness of the electrical double layer (Citation37). The equivalent circuit model shown in was used to analyze the Nyquist curves and it consists of Rs and CPE (constant-phase element) parallel to the Rct.
Figure 7. Nyquist (a) and Bode (b) plots for CS corrosion in 1 M HCl solution with and without different doses of Carvedilol at 25°C.
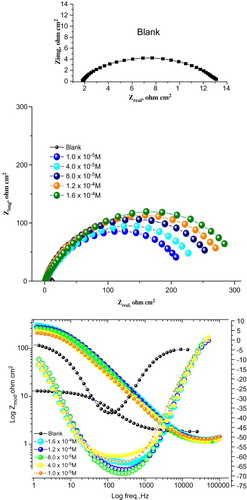
Table 6. Impedance parameters and inhibition efficiency for CS corrosion in1.0 M HCl solution in the absence and presence of different doses of expired Carvedilol drug at 25°C.
3.7. EFM method
The EFM is a non-destructive corrosion measurement technique that can directly give values of the corrosion current without prior knowledge of Tafel constants (Citation37). (a,b) shows the EFM spectrum obtained for CS corrosion in 1 M HCl solution in the absence and presence of 1 × 10−4 M of the investigated Carvedilol at 25°C, respectively. The larger peaks were used to calculate the corrosion current. Each spectrum is a current response as a function of frequency. shows the corrosion kinetic parameters such as inhibition efficiency (%IE), corrosion current density icorr, Tafel constants (ba, bc) and causality factors (CF-2, CF-3) as a function of drug dose in the absence and presence of the expired Carvedilol drug. It is obvious from that corrosion current density (icorr) values decrease, while those of inhibition efficiency (%IE) increase with an increase in Carvedilol drug dose. The great strength of the EFM is the causality factor, which serves as an internal check on the validity of the EFM measurement (Citation38). The standard values for CF-2 and CF-3 are 2.0 and 3.0, respectively. The deviation of causality factors from their ideal values might be due to that the perturbation amplitude was too small or that the resolution of the frequency spectrum is not high enough. The results obtained from EFM showed good agreement with those obtained from WL, polarization and EIS methods.
Figure 9. EFM recorded for CS corrosion in 1 M HCl solution (a) in the absence and (b) in the presence of 1.6 × 10−4 M expired Carvedilol drug at 25°C.
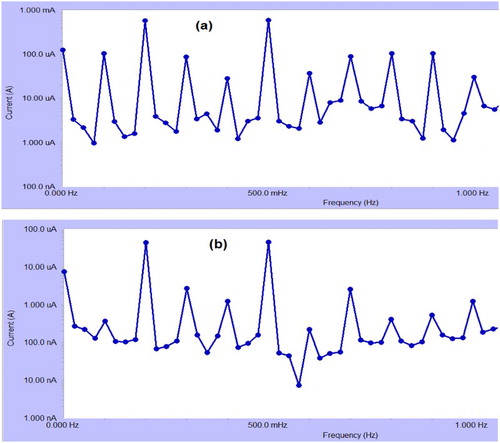
Table 7. EFM spectra of CS corrosion in 1 M HCl solution with and without different doses of Carvedilol at 25°C.
3.8. Surface studies (AFM characterization)
AFM is a powerful technique to investigate the surface morphology at nano-to-micro-scale and has become a new choice to study the influence of the inhibitor on the generation and the progress of the corrosion at the metal/solution interface (Citation39,Citation40). Analysis of the images allowed quantification of surface roughness over an area scale of 8 μm. The 3D AFM images of CS surface without and with inhibitor are shown in (a–c). The surface roughness of the CS surface in 1 M HCl is up to 392.6 nm while in the presence of drug ((b)), the roughness decreases to 75.9 nm ((c)). It shows that the CS surface in the presence of the drug is more compact and uniform compared with (a,b); so it can efficiently protect the CS surface from corrosion. Also, AFM images give the root-mean-square roughness and maximum peak to peak height values. confirms that the height values are less in the inhibited solution compared with the uninhibited one. These parameters confirm that the inhibited surface is smoother than the uninhibited one. This is due to the formation of a protective film on the metal surface.
Figure 10. AFM 3D images of CS (a) free specimen (b) in 1 M HCl for 12 h (c) in 1 M HCl containing 1.6 × 10−4 M for 12 h expired Carvedilol drug at 25°C.
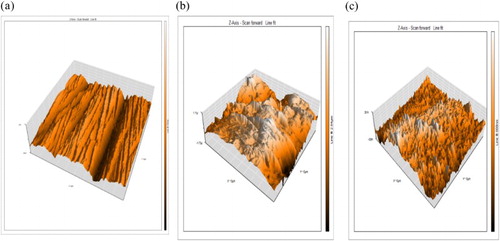
Table 8. AFM parameters for CS: (a) free specimen (b) in 1 M HCl for 12 h (c) in 1 M HCl containing 1.6 × 10−4 M expired Carvedilol drug at 25°C for 12 h immersion.
3.9. Mechanism of inhibition
It is generally assumed that the adsorption of the drugs at the metal/solution interface is the first step in the mechanism of inhibition in aggressive media. Also, it is accepted that the first step during the adsorption of an organic drug on a metal surface usually involves replacement of one or more water molecules absorbed at the metal surface (Citation41,Citation42). The adsorption is influenced by the nature and charge of the metal, chemical structure of inhibitor and type of aggressive electrolyte. The inhibitory action of Carvedilol drug may be due to the following mechanism; in acidic solutions, this compound could be protonated easily in the HCl solution, . The CS surface aquires positive charge (Citation43,Citation44). Accordingly, chloride ions are first adsorbed on the CS surface and consequently the CS surface becomes negatively charged, then the protonated molecules get adsorbed. Both molecular and protonated species can adsorb on the CS surface. The high %IE may be due to the larger molecular size of the drug which covers large areas from the CS surface.
4. Conclusions
The expired Carvedilol drug acts as good and efficient corrosion inhibitor for the corrosion of CS in 1 M HCl solution. The IE decreases with rise in temperature, but increases with an increase in the dose of the drug. The inhibition of CS corrosion by Carvedilol can be attributed to the adsorption ability of drug molecules onto the reactive sites of the metal surface. The adsorption of the drug on CS obeys the Langmuir adsorption isotherm. Polarization data indicate that the drug acts as a mixed-type inhibitor. AFM reveals the formation of a smooth uniform surface on CS in the presence of the drug which indicates the formation of a good protective layer on the metal surface. The results obtained from different measurements gave consistent results.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
A.S. Fouda received his B.Sc. Chemistry, Cairo University, Egypt, 1972, M.Sc. in Physical and Inorganic Chemistry, from El-Mansoura University, Egypt, 1975 and Ph.D. in Physical Chemistry, El- Mansoura University, Egypt, 1979, 1988, Prof. of Physical Chemistry, B.Sc. in Chemistry 2013. He is a member of Egyptian corrosion Bulletin, Member of the editorial many Journals. He granted the Chemical Engineering Department and Material Science, Minnesota University, USA 1982–1983 and Institute, Bonn University Germany 1989. He had prizes from National Award for Chemistry from Academy of Scientific Research and Technology in Cairo, 1982, first class State Medal of Sciences and Arts, 1984 and Prize of El-Nasr Company for Fertilizers, Talkha, 1981. He is an international reviewer for a huge number of thesis, journals and projects. He had published more than 200 papers in international journals. His main research interests are in the field of Physical Chemistry, Especially electrochemistry (Corrosion and corrosion inhibition in all media for metals and alloys, Fuel cells, Electrode position of metals and oxides).
Dr. M.A. El Morsi got his B.Sc. in Chemistry 1973, M.Sc. in physical Chemistry 1977, Ph.D. in physical chemistry 1981. He pointed as lecturer in Tanta University 1981 to 1987, and as associate professor at Tanta University from 1987 to 1999 and full professor from 1999 till now. He published several papers in electrochemistry in international journals.
T. El Mogy is a student studying Ph.D. He got his B.Sc. 2000 and his M.Sc. 2013 in Electroanalytical chemistry, he registered in Ph.D. degree since 2013 in physical chemistry until now.
References
- Ahmad, I.; Prasad, R.; Quraishi, M.A. Thermodynamic, Electrochemical and Quantum Chemical Investigation of Some Schiff Bases as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solutions. Corros. Sci. 2010, 52, 933–942. doi: 10.1016/j.corsci.2009.11.016
- Yurt, A.; Aykin, O. Thermodynamic, Electrochemical and Quantum Chemical Investigation of Some Schiff Bases as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solutions. Corros. Sci. 2011, 53, 3725–3732. doi: 10.1016/j.corsci.2011.07.018
- Chetouani, A.; Hammouti, B.; Benhadda, T.; Daoudi, M. Inhibitive Action of Bipyrazolic Type Organic Compounds Towards Corrosion of Pure Iron in Acidic Media. Appl. Surf. Sci. 2005, 249, 375–385. doi: 10.1016/j.apsusc.2004.12.034
- Quraishi, M.A.; Sardar, R. Aromatic Triazoles as Corrosion Inhibitors for Mild Steel in Acidic Environments. Corrosion 2002, 58, 748–755. doi: 10.5006/1.3277657
- Juttner, K. Electrochemical Impedance Spectroscopy (EIS) of Corrosion Processes on Inhomogeneous Surfaces. Electrochim. Acta. 1990, 35, 1501–5108. doi: 10.1016/0013-4686(90)80004-8
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion Inhibition of Mild Steel in 1 M HCl Solution by Henna Extract: A Comparative Study of the Inhibition by Henna and its Constituents (Lawsone, Gallic Acid, α-d-Glucose and Tannic Acid). Corros. Sci. 2009, 51, 1935–1949. doi: 10.1016/j.corsci.2009.05.024
- Abdel Hameed, R.S. Expired Ranitidine Drugs as Corrosion Inhibitor for Aluminum in 1M HCl, Al-Azhar. Bull. Sci. 2009, 20, 151–163.
- Abdel Hameed, R.S. Ranitidine Drugs as Non-Toxic Corrosion Inhibitors for Mild Steel in HCl Medium, Port. Electrochim. Acta. 2011, 29, 273–285. doi: 10.4152/pea.201104273
- Al-Shafey, H.I.; Abdel Hameed, R.S.; Ali, F.A.; Aboul-Magd, A.S.; Salah, M. Effect of Expired Drugs as Corrosion Inhibitors for Carbon Steel in 1M HCL Solution. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 146–152.
- Abdel Hameed, R.S. Review Expired Drugs as Corrosion Inhibitors for Metals and Alloys. Phys. Chem. Ind. J. 2013, 8 (4), 146–149.
- Fouda, A.S.; Mahmoud, W.M.; Abdul Mageed, H.A. Evaluation of an Expired Nontoxic Amlodipine Besylate Drug as a Corrosion Inhibitor for Low-CS in Hydrochloric Acid Solutions. J. Bio. Tribo Corros. 2016, 2 (7), 1–11.
- Eddy, N.O.; Odoemelam, S.A.; Ekwumemgbo, P. Inhibition of the Corrosion of Mild Steel in H2SO4 by Penicillin G. Sci. Res. Essays 2009, 4, 033–038.
- Eddy, N.O.; Odoemelam, S.A. Inhibition of the Corrosion of Mild Steel in Acidic Medium by Penicillin V Potassium. Adv. Nat. Appl. Sci. 2008, 2, 225–232.
- Singh, A.K.; Quraishi, M.A. Effect of Cefazolin on the Corrosion of Mild Steel in HCl Solution. Corros. Sci. 2010, 52, 152–160. doi: 10.1016/j.corsci.2009.08.050
- Shukla, S.K.; Quraishi, M.A. Ceftriaxone: A Novel Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. J. Appl. Electrochem. 2009, 39, 1517–1523. doi: 10.1007/s10800-009-9834-1
- Shukla, S.K.; Quraishi, M.A. Cefotaxime Sodium: A New and Efficient Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2009, 51, 1007–1011. doi: 10.1016/j.corsci.2009.02.024
- Naqvi, I.; Saleemi, A.R.; Naveed, S. Cefixime: A Drug as Efficient Corrosion Inhibitor for Mild Steel in Acidic Media: Electrochemical and Thermodynamic Studies. Int. J. Electrochem. Sci. 2011, 6, 146–161.
- Singh, A.K.; Quraishi, M.A. Adsorption Properties and Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by Ceftobiprole. J. Appl. Electrochem. 2011, 41, 7–18. doi: 10.1007/s10800-010-0202-y
- Acharya, S.; Upadhyay, S.N. The Inhibition of Corrosion of Mild Steel by Some Fluoroquinolones in Sodium Chloride Solution. Trans. Indian Inst. Met. 2004, 57, 297–306.
- Ebenso, E.E.; Eddy, N.O.; Odiongenyi, A.O. Corrosion Inhibition and Adsorption Properties of Methocarbamol on Mild Steel in Acidic Medium. Port. Electrochim. Acta 2009, 27, 13–22. doi: 10.4152/pea.200901013
- Mu, G.; Li, X.; Liu, G. Synergistic Inhibition Between Tween 60 and NaCl on the Corrosion of Cold Rolled Steel in 0.5M Sulfuric Acid. Corros. Sci. 2005, 47 (8), 1932–1940. doi: 10.1016/j.corsci.2004.09.020
- de Souza, F.S.; Spinelli, A. Caffeic Acid as a Green Corrosion Inhibitor for Mild Steel. Corros. Sci. 2009, 51, 642–649. doi: 10.1016/j.corsci.2008.12.013
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M. AC and DC Study of the Temperature Effect on Mild Steel Corrosion in Acid Media in the Presence of Benzimidazole Derivatives. Corros. Sci. 2003, 45, 33–58. doi: 10.1016/S0010-938X(02)00072-0
- Odoemelam, S.A.; Eddy, N.O. Sparfloxacin and Norfloxacin as Corrosion Drugs for Mild Steel: Kinetics, Thermodynamics and Adsorption Consideration. J. Mater. Sci. 2008, 4 (1), 1–8.
- Blaedel, W.J.; Meloche, V.W. Elementary Quantitative Analysis; Theory and Practice, 2nd ed., Harper & Row, New York, 1963; pp 684–698.
- Abboud, Y.; Abourriche, A.; Saffaj, T.; Berrada, M.; Charrouf, M.; Bennamara, A.; Hannache, H. A Novel Azo Dye 8-Quinolinol-5-Azoantipyrine as Corrosion Drug for Mild Steel in Acidic Media. Desalination 2009, 237, 175–189. doi: 10.1016/j.desal.2007.12.031
- Bockris, J.O.M.; Reddy, A.K.N. Modern Electrochemistry, vol. 2;Plenum Press: New York, 1977.
- Elachouri, M.; Hajji, M.S.; Salem, M.; Kertit, S.; Aride, J.; Coudert, R.; Essassi, E. Some Nonionic Surfactants as Drugs of the Corrosion of Iron in Acid Chloride. Corrosion 1996, 52, 103. doi: 10.5006/1.3292100
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic Characterization of Metal Dissolution and Drug Adsorption Processes in Mild Steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric Acid System. Corros. Sci. 2005, 47, 2915–293. doi: 10.1016/j.corsci.2005.05.034
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. doi: 10.1021/ja02268a002
- Schorr, M.; Yahalom, J. The Significance of the Energy of Activation for the Dissolution Reaction of Metal in Acids. Corros. Sci. 1972, 12 (11), 867–868. doi: 10.1016/S0010-938X(72)80015-5
- Ekanem, U.F.; Umoren, S.A. Inhibition of Mild Steel Corrosion in H2SO4 using Exudate Gum from Pachylobus edulis and Synergistic Potassium Halides Additives. Chem. Eng. Comm. 2010, 197, 1339–1356. doi: 10.1080/00986441003626086
- Bensajjay, E.; Alehyen, S.; Achouri, M.; Kertit, S. Corrosion Inhibition of Steel by 1-phenyl 5-mercapto 1,2,3,4-tetrazole in Acidic Environments (0.5 M H2SO4 and 1/3 M H3PO4). Anti-Corros Meth Mater. 2003, 50, 402–409. doi: 10.1108/00035590310501558
- Gopi, D.; Govindaraju, K.M.; Kavitha, L. Investigation of Triazole Derived Schiff Bases as Corrosion Drugs for Mild Steel in Hydrochloric Acid Medium. J. Appl. Electrochem. 2010, 40, 1349–1356. doi: 10.1007/s10800-010-0092-z
- Essaghouani, A.L.; Elmsellem, H.; Boulhaoua, M.; Ellouz, M.; El Hafi, M.; Sebbar, N.K.; Essassi, E.M.; Bouabdellaoui, M.; Aouniti, A.; Hammouti, B. Adsorption Proprieties and Inhibition of Mild Steel Corrosion in HCl Solution by 1-Benzyl-4-phenyl-2,3-dihydro-1H-1,5-benzodiazep in-2-one. Der Pharma Chemica. 2016, 8 (2), 347–355.
- Singh, A.K.; Quraishi, M.A. Investigation of the Effect of Disulfiram on Corrosion of Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2011, 53, 1288–1297. doi: 10.1016/j.corsci.2011.01.002
- Issaadi, T.; Douadi, A.; Zouaoui, S.; Chafaa, M.; Khan, A.; Bouet, G. Novel Thiophene Symmetrical Chiff Base Compounds as Corrosion Drug for Mild Steel in Acidic Media. Corros. Sci. 2011, 53, 1484–1488. doi: 10.1016/j.corsci.2011.01.022
- Mohammed, A.A.; Ahmed, M.A.; Arida, H.A.; Arslan, T.; Saracoglu, M.; Kandemirli, F. Monitoring Corrosion and Corrosion Control of Iron in HCl by Non-ionic Surfactants of the TRITON-X Series – Part II. Temperature Effect, Activation Energies and Thermodynamics of Adsorption. Corros. Sci. 2011, 53 (2), 540–548. doi: 10.1016/j.corsci.2010.09.019
- Bosch, R.W.; Bogaerts, W.F. Instantaneous Corrosion Rate Measurement with Small-amplitude Potential Intermodulation Techniques. Corrosion 1996, 52, 204–212. doi: 10.5006/1.3292115
- Singh, A.K.; Quraishi, M.A. Investigation of the Effect of Disulfiram on Corrosion of Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2011, 53, 1288–1297. doi: 10.1016/j.corsci.2011.01.002
- Wang, B.; Du, M.; Zhang, J.; Gao, C.J. Electrochemical and Surface Analysis Studies on Corrosion Inhibition of Q235 Steel by Imidazoline Derivative Against CO2 Corrosion. Corros. Sci. 2011, 53, 353–361. doi: 10.1016/j.corsci.2010.09.042
- Oguzie, E.E.; Onuoha, G.N.; Onuchukwu, A.I. Inhibitory Mechanism of Mild Steel Corrosion in 2 M Sulphuric Acid Solution by Methylene Blue Dye. Mater. Chem. Phys. 2005, 89, 305–311. doi: 10.1016/j.matchemphys.2004.09.004
- El-Azhar, M.; Mernari, B.; Traisnel, M.; Bentiss, F.; Lagrenee, M. Corrosion Inhibition of Mild Steel by the New Class of Inhibitors [2,5-bis(n-pyridyl)-1,3,4-thiadiazoles] in Acidic Media. Corros. Sci. 2011, 43, 2229–2238. doi: 10.1016/S0010-938X(01)00034-8
- Li, W.H.; He, Q.; Zhang, S.T.; Pei, C.L.; Hou, B.R.J. Some New Triazole Derivatives as Drugs for Mild Steel Corrosion in Acidic Medium0. Appl. Electrochem. 2008, 38, 289–295. doi: 10.1007/s10800-007-9437-7