ABSTRACT
The synthesis of linear alkylbenzenes (LABs) from biomass-derived furan and linear alkenes via Diels−Alder cycloaddition followed by acid-catalyzed dehydration is presented. Furan was reacted with 1-dodecene, the alkene model compound, over the niobic acid catalyst in a laboratory-scale batch reactor. The effects of reaction temperature, catalyst loading, and initial reactant ratio were systematically investigated. Our experiments reveal that 1-alkene isomerization, furan alkylation to produce dodecylfuran, and self Diels−Alder cycloaddition of furan to produce benzofuran are the major side reaction pathways competing with LAB production. The highest conversion of 1-dodecene to 1-phenyldecane, our target LAB, was found to be at approximately 12 mmol/mol. The main reasons for this low LAB yields include high furan activity and volatility, coke formation, and mass transfer limitations. Despite low LAB yields, our work demonstrates for the first time that it is possible to synthesize LABs from biomass-derived furan and linear alkenes over solid acid catalysts.
GRAPHICAL ABSTRACT
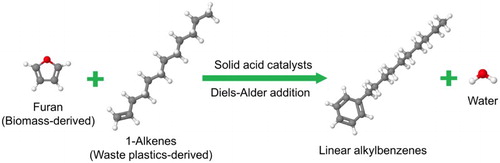
1. Introduction
Linear alkybenzenes (LABs) are the precursors of linear alkylbenzene sulfonates (LASs), the world’s largest commodity biodegradable surfactant commonly used in cleaners, detergents, and laundry powders (Citation1). The global market for LABs was estimated at $6.74 billion in 2011, and it is projected to be over $8 billion in 2018 (Citation2). Commercially, LABs are produced from Friedel−Crafts alkylation between benzene and linear C10−C14 alpha olefins (i.e. 1-alkenes) using hydrogen fluoride (HF) or aluminum chloride (AlCl3) as a catalyst () (Citation3). The reaction mechanism proceeds with a two-step process: catalytic alkene protonation followed by benzene alkylation (Citation4). Since all but the two terminal carbon atoms can be protonated, a mixture of LAB isomers with different chain lengths of R1 and R2 are produced.
Scheme 1. Current commercial production of linear alkylbenzenes (LABs) from benzene and linear alpha olefins using HF or AlCl3 homogeneous acid catalyst (Citation3).
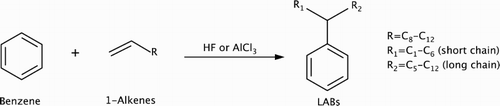
Two major challenges exist for LAB production via this reaction scheme. First, HF and AlCl3 are extremely corrosive and difficult to handle. The potential accidental release of HF or AlCl3 has raised environmental safety concerns. Even though maximum safety measures have been taken for LAB production, replacing HF and AlCl3 with a safer catalyst is still viewed as a long-term solution. Second, among the LABs produced, 2-phenylalkanes (i.e. R1 is CH3) are the most desirable, since their structure is the most linear, enabling them to possess the best surfactant properties and biodegradability (Citation5). Reaction pathways leading to other isomers, however, cannot be completely eliminated, and 2-phenylalkane yields can only be as high as about 20% (Citation6). To overcome the above shortcomings, recent research efforts in the literature have focused on developing new environmentally benign catalysts with improved 2-phenylalkane yields (Citation5–17). To date, only UOP’s proprietary DA−114, a solid silica-alumina catalyst used in the Detal™ and Detal-Plus™ processes, is successfully commercialized (Citation18). Although DA−114 is not toxic or corrosive, it still requires the toxic HF to be a starting material for its synthesis.
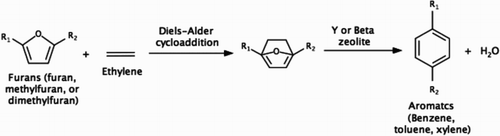
Recent discovery of aromatic hydrocarbon (i.e. benzene, toluene, and xylene) synthesis by reacting biomass-derived furans (i.e. furan, methylfuran, and dimethylfuran (DMF)) with ethylene over solid acid catalysts (Citation19–28) offers an opportunity of breakthrough in LAB production. The reaction proceeds with Diel−Alder addition followed by dehydration over solid catalysts, such as HY, H-BEA, and niobic acid (). As high as 90% xylene yields from DMF and 50% benzene yields from furan have been reported (Citation20).
Scheme 2. Production of aromatic hydrocarbons from biomass-derived furans and ethylene (Citation19–28).
Based on , a new reaction pathway leading to LABs by reacting furan with linear alkenes over acid catalysts () is hypothesized and studied in this work. This new reaction pathway provides three major benefits. First, solid Brønsted acid catalysts are used for the production of LABs, instead of the corrosive and harmful HF or AlCl3 catalysts used in the traditional LAB production processes. Second, only 1-phenylalkanes are produced, providing the best biodegradability compared to the LABs produced from existing commercial processes (2-, 3-, 4-phenylalkanes, etc.). To our knowledge, this type of highly linear LABs was only successfully synthesized by Galdi et al. (Citation29) with good selectivity via catalytic olefin polyinsertion using inexpensive ethylene, styrene, and hydrogen as feedstocks. Third, furan is biomass-derived, providing a renewable source to synthesize LABs instead of petroleum-derived benzene. Since as high as 20% molar yields of linear alkenes with sizes between C12 and C16 were observed during polyethylene pyrolysis (Citation30), the linear 1-alkenes used in this reaction scheme could also be obtained from waste polyethylene, potentially allowing the entire feedstock to be obtained from solid waste. Note that one needs to carefully consider the tradeoff between utilizing environmentally friendly feedstocks versus their availability and the cost to recover and purify these compounds.
Scheme 3. The new LAB production pathway from furan and linear alpha olefins over solid acid catalysts studied in this work.
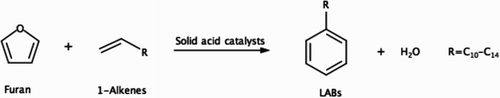
To test the above hypothetical reaction scheme, batch reactions of furan and 1-dodecene over niobic acid catalyst were conducted in this work. The effects of reaction temperature, catalyst loading, and reactant ratio on the formation of 1-phenyldecane, the target LAB, were investigated. The experimental methods, results, and possible reaction pathways leading to the main and side products are discussed in the following sections.
2. Experimental
2.1. Reactor design
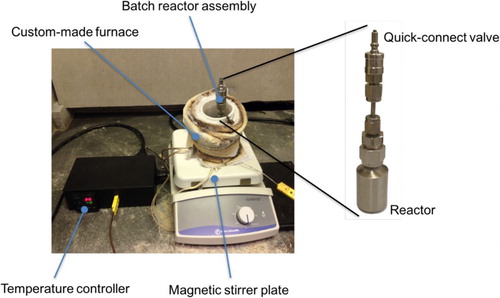
A batch reactor assembly () made of stainless steel parts was used for conducting the experiments in this work. The reactor assembly consists of a 10-mL reactor (Swagelok, SS-4CS-TW-10) and a quick-connect double-end shutoff valve (Swagelok, MS-CRD-QC2) that enables the reactor to connect to a vacuum line for removing unwanted air in the system. A laboratory-scale furnace was custom-made using an alumina cylinder with a diameter of 4 cm (AdValue Technology, AL-2100). The furnace is surrounded by a high-temperature heating tape (McMaster-Carr, 4550) and wrapped with insulation materials (Flexible Ceramic Insulation by McMaster-Carr). The heating tape is connected to a PID controller (Omega, CN742), which controls the furnace temperature based on the readings of a thermocouple (Omega, KMQSS-062U-12) positioned in the center of the furnace.
2.2. Experimental procedure
The experiments started with loading the reactor with a clean magnetic stir bar and 3–5 mg of n-eicosane (C20H42, 99%, Alfa Aesar), which was used as the internal standard in our experiments. One to 15 mg of niobic acid catalyst (CBMM, HY-340, surface area = 118 m2/g, Brønsted acid site density = 51 µmol g−1) were then loaded, followed by adding approximately 250 mg of 1-dodecene (C12H24, 96%, Alfa Aesar). Niobic acid was selected as the catalyst in our experiments since it was found to provide higher p-xylene yields than several other types of acid catalysts, including Y, WOx-ZrO2, and SiO2/Al2O3, during Diels−Alder cycloaddition between DMF and ethylene (Citation26). Furan (C4H4O, 99%, Alfa Aesar) was loaded into the reactor the last before the reactor was immediately closed due to its high volatility. The weight of the reactor was measured after each reagent was added to obtain the accurate mass measurement of each compound before the reaction.
Note that the niobic acid catalyst was loaded into the reactor without pretreatment, as suggested by Wang et al. (Citation26). The physiochemical and catalytic properties of niobic acid are also reported in their work.
To remove air in the reactor for preventing the undesired oxidation reactions, the closed reactor was immersed into a liquid nitrogen bath for 4 min to freeze the reactants. This prevents the volatile reactants from evaporation during the subsequent vacuum process. The reactor was then attached to a vacuum system driven by a rotary pump (Edwards, E2M2 27746) using the quick-connect valve for air removal. A pressure transducer (HPS, 910 Duel Trans) connected to the vacuum line was used to monitor the progress of this process.
Once the air in the reactor was completely removed, the reactor was disconnected from the vacuum line and removed from the liquid nitrogen bath. The reactor was then thawed in a water bath back to the room temperature before being inserted into the furnace pre-set at the reaction temperature. The magnetic stir bar in the reactor was used for mixing during the reaction.
After the reaction, the reactor was removed from the furnace and immediately cooled in a water bath until the reactor reached room temperature. The weight of the reactor was measured to ensure mass balance closure and no leak (i.e. no escape of the volatile furan) during the reaction. Product mixtures were extracted with 5 mL heptane (C7H16, 99%, Alfa Aesar), filtered with a 0.2 µm nylon membrane filter (VWR International, 28145–487), and collected for subsequent gas chromatography (GC) analysis.
2.3. Analytical methods
Identification of the reaction products was achieved using a Shimadzu GC2010 Plus GC equipped with a mass spectrometer (MS). The samples were injected into the GC/MS system equipped with a Shimadzu SH-RXi-5Sil MS column (30 m). High-purity helium (Airgas) was used as a carrier gas in the column with a constant flow rate of 129.8 mL/min. The inlet temperature was set at 305°C. The programed temperature regime for the GC oven was: start at 35°C, hold for 7 min, ramp up to 185°C at 7.5°C/min, ramp up to 305°C at 20°C/min, and hold at 305°C for 12 min. The temperature of the MS detector was set at 280°C.
Quantification of the reaction products was achieved using a second analytical channel equipped with a flame ionization detector (FID) on the same GC. The samples were injected into the GC/FID system equipped with a Shimadzu Rxi-5ms column (15 m). The GC was programed with the following inlet operating parameters: high-purity helium carrier gas set at a constant flow pressure of 22.1 kPa, inlet temperature set at 285°C, and carrier gas with the split ratio of 1:150. The detector temperature was set at 285°C, with an air flow rate of 400 mL/min and a hydrogen gas flow rate of 40 mL/min. The GC oven was programed with the following temperature regime: start at 35°C, hold for 7 min, ramp up to 185°C at 7.5°C/min, ramp up to 285°C at 20°C/min, and hold at 285°C for 12 min.
Mixtures of n-eicosane and 1-phenyldecane (C16H26, 99%, Oakwood) in four different mass ratios, dissolved in 5 mL heptane, were used as standards to obtain the calibration curves for the GC/FID analysis. This calibration enables individual quantification of the reaction products by their peak sizes.
3. Results and discussion
3.1. Reaction products
shows a representative gas chromatogram of reaction mixtures after reacting furan with 1-dodecene over the niobic acid catalyst at 250°C for 6 h. A total of 11 compounds were identified by GC/MS. The chemical structures and formulae of these compounds are summarized in . Among the molecules in the chromatogram, peaks for 1-phenyldecane (Compound 8, the target LAB product), furan (Compound 1, the reactant), 1-dodecene (Compound 6, the reactant), and n-eicosane (Compound 11, the internal standard) were identified by injecting the same compounds purchased from the vendors. Compounds 3 (1-decene) and 7 (tetradecene isomers) were identified as the impurities of 1-dodecene. Among the reaction products, benzofuran (Compound 4) and dodecene isomers (Compounds 5) were identified by comparing their mass spectra with known spectra in the NIST library. Very small amount of furan oxide (Compound 2) was found, which could be resulted from oxidation of furan by water. For Compounds 9 and 10, their mass spectra both revealed a parent ion of 236 m/z, corresponding to a molecular formula of C16H26O. Based on the fragmentation patterns, Compound 9 is likely dodecylfuran, an alkyl furan produced from alkylation of furan by 1-dodecene. Compound 10 is likely hydrated 1-phenyldecane, an intermediate during LAB synthesis before the acid-catalyzed dehydration step. A detailed discussion of reaction pathways leading to LABs and side products is provided later in the text.
Figure 2. Representative gas chromatograph of reaction mixtures from Diels−Alder reaction between furan and 1-dodecene over niobic acid. The reaction temperature was 250°C, and the reaction time was 6 hours.
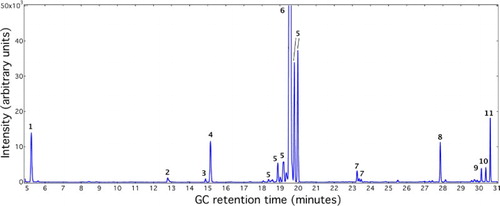
Table 1. Compounds identified by the GC/MS.
3.2. The effect of reaction temperature
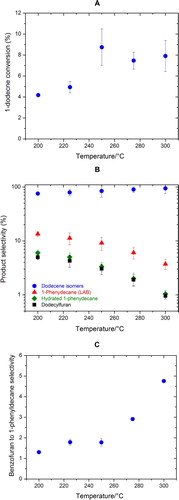
To understand the effect of reaction temperature, experiments at five different temperatures between 200°C and 300°C were conducted with a reaction time of 6 h. Each experiment was repeated three times to verify reproducibility. The standard deviations of the three experiments are shown as error bars in the figures. The initial molar ratio of furan to 1-dodecene was controlled between 6.5 and 8.5 in these experiments. Due to the volatile nature of furan, furan was selected as the excess reactant and 1-dodecene was selected as the limiting reactant. The conversion and selectivity of a given species were thus calculated based on 1-dodecene as:
Due to the difficulty to precisely quantify the amount of solid coke on the catalyst surface, the conversion and selectivity values reported in this work are solely based on liquid products. Since benzofuran is formed from self Diel−Alder addition of furan without the involvement of 1-dodecene, it was excluded in our conversion and selectivity analysis. Instead, the selectivity of benzofuran to 1-phenyldecane is calculated:
The catalyst loading in these experiments was set at 0.2 ± 0.02 g per gram of 1-dodecene, where 0.050 ± 0.002 g of catalysts and 0.25 ± 0.02 g of 1-dodecene were loaded in the reactor before the reactions.
As shown in (A), our experiments show that the conversion of 1-dodecene, the limiting reactant, to liquid phase products initially increases with reaction temperature and plateaus at approximately 9% when reaction temperatures are higher than 250°C. The major liquid phase products include 1-dodecene isomers, 1-phenyldecane (the target LAB), hydrated 1-phenyldecane (i.e. hydrated LAB), and dodecylfuran. The selectivity of 1-dodecene isomers is the highest, which increases with reaction temperatures ((B)). The selectivities of other products decrease with reaction temperatures, suggesting that higher reaction temperatures favor dodecene isomerization over LAB formation and furan alkylation. The selectivity of 1-phenyldecane, our target LAB, starts at approximately 13% at 200°C and drops to approximately 4% at 300°C. Hydrated 1-phenyldecane (hydrated LAB) and dodecylfuran have lower but similar selectivities, following the same formation trend of 1-phenyldecane. The selectivity of benzofuran to 1-phenyldecane as a function of reaction temperature ((C)) shows that self Diel−Alder addition of furan to produce benzofuran is also competing with LAB formation, particularly at higher reaction temperatures. Since benzofuran is believed to be a coke precursor (Citation21), this implies that high reaction temperatures could favor coke formation. The presence of coke on catalyst surfaces was observed by the change of colors of the membrane filters after the reaction. To balance 1-dodecene conversion and 1-phenyldecane selectivity, a reaction temperature of 250°C was selected to investigate other possible factors influencing reaction kinetics, described in the following sections.
3.3. The effect of catalyst loading
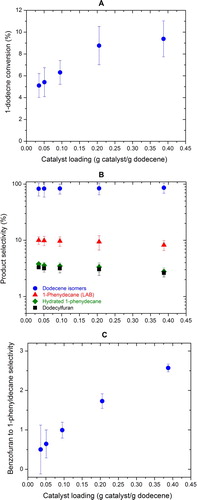
The effect of catalyst loading was studied at 250°C with a reaction time of 6 h. Five different catalyst to 1-dodecene ratios were investigated, with the absolute 1-dodecene mass kept unchanged. The molar ratio between furan and 1-dodecene was again controlled in between 6.5 and 8.5. As shown in (A), the conversion of 1-dodecene to liquid products increases slightly with higher catalyst loadings ((A), at approximately 10% at a catalyst loading of 0.4 g catalyst/g 1-dodecene). The selectivities of different products, however, are insensitive to different catalyst loadings ((B)). Higher catalyst loadings also lead to increased self Diel−Alder addition of furan to produce benzofuran ((C)), which could further lead to increased coke formation (Citation21). It is believed that coking on the catalyst surfaces leads to catalyst deactivation (Citation31–Citation32), which may explain why a catalyst loading higher than 0.2 g catalyst/g 1-dodecene does not further promote 1-dodecene conversion to 1-phenyldecane. For this reason, a catalyst loading of 0.2 g catalyst/g 1-dodecene was selected to study the effect of initial reactant ratio in our experiments.
3.4. The effect of initial furan to 1-dodecene ratio
It was found that the initial ratio between the reactants plays a critical role in the Friedel–Crafts alkylation of benzene by linear alkenes for LAB production (Citation14). To investigate if this effect is also present in our reaction system, furan to 1-dodecene ratio (F/D, in moles) was varied in our experiments. Higher furan concentrations could increase the intrinsic kinetics for LAB formation and also help pressurize the system, keeping more reactants in the condensed phase as possible. On the other hand, a high F/D ratio might promote the formation of benzofuran, leading to increased coke and catalyst deactivation. It is thus anticipated that an optimal F/D ratio exists to obtain a maximum LAB yield. Note that the ratio between furan and 1-dodecene cannot be controlled precisely at a given value in our experiments due to the volatile nature of furan, as shown by large error bars in this quantity in .
Figure 5. The effect of initial reactant ratio on (A) the conversion of 1-dodecene, (B) the selectivities of major products, and (C) benzofuran to 1-phenyldecane selectivity.
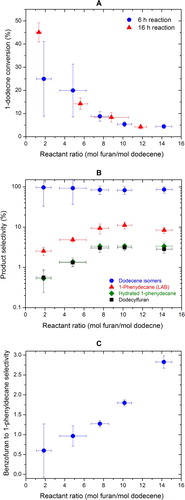
(A) shows the effect of initial F/D ratio on the conversion of 1-dodecene to liquid products. These results were obtained from a set of experiments at 250°C with reaction times of 6 and 16 h, respectively. The catalyst loadings were set at 0.2 ± 0.01 g catalyst per gram of 1-dodecene. The error bars in the figure represent the standard deviation of three repeated runs. Our experiments show that the conversion of 1-dodecene decreases with increased F/D ratio. A longer reaction time of 16 h does not result in significant difference in the trend. A closer analysis on the selectivities of the reaction products ((B)) suggests that higher dodecene conversion accompanies with the lower selectivity of 1-phenyldecane, our target LAB, where dodecene isomers had the highest selectivity. This suggests that isomerization of 1-dodecene is favored at low F/D ratios due to the lack of furan. When F/D ratios increases, the selectivity of 1-dodecene isomers decreases and the selectivity of 1-phenyldecane increases. However, when the F/D ratio is higher than 10, the selectivity of 1-phenyldecane starts to decline. This could be explained by the increased formation of benzofuran, a coke precursor, relatively to 1-phenyldecane ((C)), suggesting increased coke and catalyst poisoning at elevated F/D ratios. Hydrated 1-phenyldecane (hydrated LAB) and dodecylfuran again have similar selectivities and follow the same formation trend of 1-phenyldecane.
3.5. Possible reaction pathways
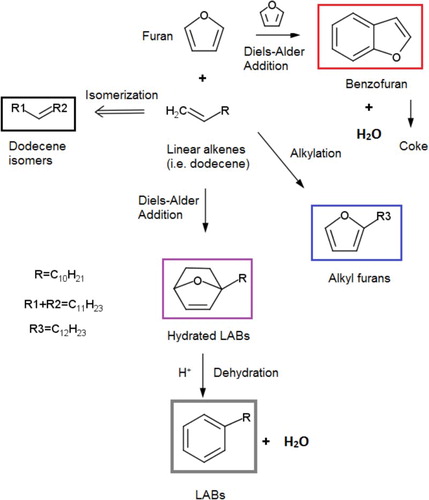
Based on our experimental findings, a reaction mechanism for LAB synthesis from furan and linear 1-alkenes over niobic catalysts is proposed in . The main reaction pathway proceeds with first forming hydrated LABs (in the purple box) via Diels−Alder cycloaddition between furan and 1-alkenes. Dehydration subsequently takes place over acid catalysts to form LABs (in the gray box). Our experimental results suggest that at least three competing reaction pathways exist. First, furan reacts with linear alkenes to form alkyl furans (in the blue box). Our experiments show that the selectivities of LABs, alkyl furans, and hydrated LABs follow the same trend, suggesting that the formation of these compounds follows similar kinetics, i.e. their formation rates are dependent on the concentrations of both furan and 1-alkenes. Second, self Diels−Alder cycloaddition of furan leads to the formation of benzofuran (in the red box). The reaction rate is likely only dependent on furan concentration, thus is more favorable at high furan to alkene ratios, which was observed in our experiments. Since benzofuran is likely a coke precursor (Citation21), this pathway could eventually lead to coke formation. Finally, 1-alkenes can undergo isomerization to form other alkene isomers. This reaction is likely only dependent on the concentrations of 1-alkenes, thus is more dominant at low furan to alkene ratios, confirmed by the high selectivity of dodecene isomers at low F/D ratios in our experiments. Some studies reported that adding heptane during Diels−Alder cycloaddition between DMF and ethylene helps reduce side reactions (Citation27). In our experiments, however, no obvious effects were found when heptane was added into the reactant mixtures (results not shown).
Note that the highest overall conversion of 1-dodecene to 1-phenyldecane (the target LAB) in our experiments was still very low, at approximately 12 mmol/mol. Three possible reasons could contribute to this low LAB yield. First, the lack of stabilization by the methyl groups in furan makes it highly active, compared to methylfuran and 2,5-DMF. Consequently, furan is prone to side reactions, which is also observed in the study by Wang et al. (Citation26), where benzene, toluene, and xylene (BTX) were synthesized from furan, methylfuran, and 2,5-DMF, respectively, via Diels−Alder addition. It was found in their work that the presence of methyl substituents has a significant impact on the selectivity and yields of BTX during Diels−Alder cycloaddition. For instance, a xylene yield of 80% was achieved when ethylene was reacted with 2,5−DMF, whereas only a benzene yield of 18% was achieved when ethylene was reacted with furan under the same reaction conditions. Second, the presence of coke could deactivate catalysts, leading to low LAB yields. Third, furan is highly volatile. Under typical reaction conditions, the majority of furan is likely present in the vapor phase, while the other reactant, 1-dodecene are present in the condensed phase along with the solid acid catalysts. Although a magnetic stir bar was used to promote mixing, actual reactions might have only taken place at the interface between the gas and the condensed phases, causing the reactions to be diffusion limited. Despite low LAB yields, our work demonstrates for the first time that it is possible to synthesize LABs from biomass-derived furan and waste plastics-derived linear 1-alkenes.
Several research directions should be further undertaken before commercial LAB production from furan and linear alkenes can be realized. First, understanding of coke formation chemistry in this reaction system is still limited. Development of a detailed kinetic mechanism for coke formation can elucidate coke formation pathways, potentially providing guidance to mitigate coke formation for higher LAB yields. Second, studies of mass transfer effects are needed. This can be achieved with experiments of controlled mixing between the reactants and the catalysts or with elevated pressure to keep more furan in the condensed phase so that the reaction is kinetic limited. Third, a process mathematical model with detailed kinetic parameters should be developed for further understanding the effects of feedstock composition and reaction conditions on product selectivity and yields. This process model, validated by experiments, can then be used to discover the optimal operating conditions for achieving the highest LAB yields.
4. Conclusions
Diels−Alder cycloaddition reaction between furan and 1-dodecene over niobic acid catalyst to synthesize LABs was studied using a batch reactor setup. The effects of reaction temperature, catalyst loading, and reactant molar ratio on reaction kinetics were investigated. Key findings from this study include:
The main reaction for LAB production proceeds with Diels−Alder cycloaddition between furan and 1-dodecene followed by acid-catalyzed dehydration. The major competing reaction pathways include isomerization of 1-alkenes, alkylation of furan by 1-alkenes to produce alkyl furans, and self Diels−Alder cycloaddition of furan to produce benzofuran.
Higher reaction temperature and catalyst loadings promote 1-dodecene conversion but also favor 1-dodecene isomerization, resulting in decreased selectivity of 1-phenyldecane, our target LAB. Higher initial furan to 1-dodecene (F/D) molar ratio, on the other hand, promotes LAB formation and increases the selectivity of 1-phenyldecane. At very high F/D ratio (>10), however, self Diels−Alder cycloaddition of furan to produce benzofuran becomes significant, leading to coke formation, catalyst poisoning, and reduced 1-phenyldecane selectivity.
The highest overall conversion of 1-dodecene to 1-phenyldecane, the target LAB, was still low in our experiments, at about 12 mmol/mol. The main reasons responsible for this low LAB yields include high activity and volatility of furan, coke formation, and mass transfer limitations.
Despite low LAB yields, our work demonstrates for the first time that it is possible to synthesize LABs from biomass-derived furan and linear 1-alkenes over solid acid catalysts. Possible future work includes changing the reaction operation conditions, such as system pressure, and using different mixing methods for increased mass transfer to improve LAB yields.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Wilbur Zuo was a master's student at the University of Massachusetts Lowell and received his degree in 2016.
Hsi-Wu Wong is Assistant Professor of Chemical Engineering at the University of Massachusetts Lowell. His research interests include chemical reaction engineering, catalysis, and green chemistry.
Additional information
Funding
References
- Wu, M.M. Alkylated Aromatics. In Synthetic Lubricants and High-performance Functional Fluids; Rudnick, L.R., Shubkin, R.L., Eds., 2nd ed.; Marcel Dekker: New York, 1999; pp 195–214.
- Research, T.M. Linear Alkyl Benzene (LAB) Market for Heavy-duty Laundry Liquids, Laundry Powders, Light-duty Dish Washing Liquids, Industrial Cleaners and Household Cleaners – Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2012–2018 2013 [Online]. http://www.transparencymarketresearch.com/linear-alkyl-benzene-market.html
- Kocal, J.A.; Vora, B.V.; Imai, T. Production of Linear Alkylbenzenes. Appl. Catal. A. 2001, 221, 295–301. doi: 10.1016/S0926-860X(01)00808-0
- Perego, C.; Ingallina, P. Recent Advances in the Industrial Alkylation of Aromatics: New Catalysts and New Processes. Catal. Today 2002, 73, 3–22. doi: 10.1016/S0920-5861(01)00511-9
- Aslam, W.; Siddiqui, M.A.B.; Jermy, B.R.; Aitani, A.; Cejka, J.; Al-Khattaf, S. Selective Synthesis of Linear Alkylbenzene by Alkylation of Benzene with 1-Dodecene Over Desilicated Zeolites. Catal. Today 2014, 227, 187–197. doi: 10.1016/j.cattod.2013.10.015
- Han, M.; Cui, Z.; Xu, C.; Chen, W.; Jin, Y. Synthesis of Linear Alkylbenzene Catalyzed by HBeta-zeolite. Appl. Catal. A. 2003, 238, 99–107. doi: 10.1016/S0926-860X(02)00334-4
- Aitani, A.; Wang, J.B.; Wang, I.; Al-Khattaf, S.; Tsai, T.C. Environmental Benign Catalysis for Linear Alkylbenzene Synthesis: A Review. Catal. Surv. Asia 2014, 18, 1–12. doi: 10.1007/s10563-013-9164-5
- Craciun, I.; Reyniers, M.F.; Marin, G.B. Effects of Acid Properties of Y Zeolites on the Liquid-phase Alkylation of Benzene with 1-Octene: A Reaction Path Analysis. J. Mol. Catal. A Chem. 2007, 277, 1–14. doi: 10.1016/j.molcata.2007.06.027
- Craciun, I.; Reyniers, M.F.; Marin, G.B. Liquid-phase Alkylation of Benzene with Octenes Over Y Zeolites: Kinetic Modeling Including Acidity Descriptors. J. Catal. 2012, 294, 136–150. doi: 10.1016/j.jcat.2012.07.014
- Faghihian, H.; Mohammadi, M.H. A Novel Catalyst for Alkylation of Benzene. C. R. Chim. 2012, 15, 962–968. doi: 10.1016/j.crci.2012.08.007
- Hernandez-Cortez, J.G.; Martinez, L.; Soto, L.; Lopez, A.; Navarrete, J.; Manriquez, M.; Lara, V.H.; Lopez-Salinas, E. Liquid Phase Alkylation of Benzene with Dec-1-ene Catalyzed on Supported 12-Tungstophosphoric Acid. Catal. Today 2010, 150, 346–352. doi: 10.1016/j.cattod.2009.12.003
- Hornacek, M.; Hudec, P.; Smieskova, A.; Jakubik, T. Alkylation of Benzene with 1-Alkenes Over Beta Zeolite in Liquid Phase. React. Kinet. Mech. Catal. 2010, 99, 431–437.
- Lin, J.S.; Wang, J.J.; Wang, J.; Wang, I.; Balasamy, R.J.; Aitani, A.; Al-khattaf, S.; Tsai, T.C. Catalysis of Alkaline-modified Mordenite for Benzene Alkylation of Diolefin-containing Dodecene for Linear Alkylbenzene Synthesis. J. Catal. 2013, 300, 81–90. doi: 10.1016/j.jcat.2012.12.031
- Meriaudeau, P.; Taarit, Y.B.; Thangaraj, A.; Almeida, J.L.G.; Naccache, C. Zeolite Based Catalysts for Linear Alkylbenzene Production: Dehydrogenation of Long Chain Alkanes and Benzene Alkylation. Catal. Today 1997, 38, 243–247. doi: 10.1016/S0920-5861(97)00073-4
- Cao, Y.; Kessas, R.; Naccache, C.; Taarit, Y.B. Alkylation of Benzene with Dodecene. The Activity and Selectivity of Zeolite Type Catalysts as a Function of the Porous Structure. Appl. Catal. A. 1999, 184, 231–238. doi: 10.1016/S0926-860X(99)00089-7
- deAlmeida, J.G.; Dufaux, M.; Taarit, Y.B.; Naccache, C. Effect of Pore Size and Aluminium Content on the Production of Linear Alkylbenzenes Over HY, H-ZSM-5 and H-ZSM-12 Zeolites: Alkylation of Benzene with 1-Dodecene. Appl. Catal. A. 1994, 114, 141–159. doi: 10.1016/0926-860X(94)85113-1
- Liang, W.; Jin, Y.; Yu, Z.; Wang, Z.; Han, B.; He, M.; Min, E. Alkylation of Benzene with Dodecene Over HY Zeolite: Deactivation, Regeneration, and Product Distribution. Zeolites 1996, 17, 297–303. doi: 10.1016/0144-2449(96)00034-6
- Kocal, J.A. Detergent Alkylation Process Using a Fluorided Silica-alumina. U.S. Patent 5,196, 574, 1993.
- Patet, R.E.; Nikbin, N.; Williams, C.L.; Green, S.K.; Chang, C.C.; Fan, W.; Caratzoulas, S.; Dauenhauer, P.J.; Vlachos, D.G. Kinetic Regime Change in the Tandem Dehydrative Aromatization of Furan Diels-Alder Products. ACS Catal. 2015, 5, 2367–2375. doi: 10.1021/cs5020783
- Chang, C.C.; Green, S.K.; Williams, C.L.; Dauenhauer, P.J.; Fan, W. Ultra-selective Cycloaddition of Dimethylfuran for Renewable p-Xylene with H-BEA. Green Chem. 2014, 16, 585–588. doi: 10.1039/C3GC40740C
- Cheng, Y.T.; Huber, G.W. Production of Targeted Aromatics by Using Diels-Alder Classes of Reactions with Furans and Olefins Over ZSM-5. Green Chem. 2012, 14, 3114–3125. doi: 10.1039/c2gc35767d
- Do, P.T.; McAtee, J.R.; Watson, D.A.; Lobo, R.F. Elucidation of Diels-Alder Reaction Network of 2,5-Dimethylfuran and Ethylene on HY Zeolite Catalyst. ACS Catal. 2013, 3, 41–46. doi: 10.1021/cs300673b
- Li, Y.P.; Head-Gordon, M.; Bell, A.T. Computational Study of p-Xylene Synthesis from Ethylene and 2,5-Dimethylfuran Catalyzed by H-BEA. J. Phys. Chem. C 2014, 118, 22090–22095. doi: 10.1021/jp506664c
- Nikbin, N.; Do, P.T.; Caratzoulas, S.; Lobo, R.F.; Dauenhauer, P.J.; Vlachos, D.G. A DFT Study of the Acid-catalyzed Conversion of 2,5-dimethylfuran and Ethylene to p-xylene. J. Catal. 2013, 297, 35–43. doi: 10.1016/j.jcat.2012.09.017
- Nikbin, N.; Feng, S.; Caratzoulas, S.; Vlachos, D.G. p-Xylene Formation by Dehydrative Aromatization of a Diels-Alder Product in Lewis and Bronsted Acidic Zeolites. J. Phys. Chem. C 2014, 118, 24415–24424. doi: 10.1021/jp506027f
- Wang, D.; Osmundsen, C.M.; Taarning, E.; Dumesic, J.A. Selective Production of Aromatics from Alkylfurans Over Solid Acid Catalysts. Chem. Cat. Chem. 2013, 5, 2044–2050.
- Williams, C.L.; Chang, C.C.; Do, P.; Nikbin, N.; Caratzoulas, S.; Vlachos, D.G.; Lobo, R.F.; Fan, W.; Dauenhauer, P.J. Cycloaddition of Biomass-derived Furans for Catalytic Production of Renewable p-xylene. ACS Catal. 2012, 2, 935–939. doi: 10.1021/cs300011a
- Xiong, R.; Sandler, S.I.; Vlachos, D.G.; Dauenhauer, P.J. Solvent-tuned Hydrophobicity for Faujasite-catalyzed Cycloaddition of Biomass-derived Dimethylfuran for Renewable p-xylene. Green Chem. 2014, 16, 4086–4091. doi: 10.1039/C4GC00727A
- Galdi, N.; Lamparelli, D.H.; Oliva, L. One Pot Synthesis of Linear 1-alkylbenzenes from Styrene, Ethylene and Hydrogen. J. Mol. Catal. A: Chem. 2016, 418–419, 154–157. doi: 10.1016/j.molcata.2016.03.040
- Levine, S.E.; Broadbelt, L.J. Detailed Mechanistic Modeling of High-density Polyethylene Pyrolysis: Low Molecular Weight Product Evolution. Polym. Degrad. Stabil. 2009, 94, 810–822. doi: 10.1016/j.polymdegradstab.2009.01.031
- Barbier, J. Deactivation of Reforming Catalysts by Coking - A Review. Appl. Catal. 1986, 23, 225–243. doi: 10.1016/S0166-9834(00)81294-4
- Guisnet, M.; Magnoux, P. Organic Chemistry of Coke Formation. Appl. Catal. A. 2001, 212, 83–96. doi: 10.1016/S0926-860X(00)00845-0