ABSTRACT
Toxicology and related concepts are being taught within chemistry education programs to enable students to (1) develop an awareness of toxicological principles and concepts that are otherwise absent from the chemistry curriculum for undergraduates and (2) design chemical products and processes that have reduced health and environmental hazards. This manuscript presents three model courses from different higher education institutions, presenting different approaches for introducing toxicology concepts to students. The models are presented not as prescriptive approaches, but as inspirational models for others to use to generate their own unique approaches to integrating and teaching toxicology concepts.
GRAPHICAL ABSTRACT
1. Introduction
As the enterprise and industrial expansion of chemistry developed from the mid-1800s to the mid-1900s, relatively little attention was paid to environmental impact, since chemical practitioners functioned within parameters that ignored the disposition of chemicals in their manufacture and use. Beginning in the 1960s, the environmental movement began to recognize the more complete impact of chemicals on the environment. As scientists were prompted to consider the ethical and moral implications of their work, they began to adopt strategies towards minimizing human health and environmental exposures to hazardous chemicals. During the 1990s, the EPA moved towards prevention as the best means for reducing these chemical exposures. One of the key strategies for preventing pollution and chemical exposures, green chemistry, has emerged over the past 20 years. Green chemistry is an essential piece to eliminating human health and environmental exposures to hazardous and toxic chemicals.
Chemists are molecular designers and work with the fundamental building blocks that make up our industrial society. As chemists work towards greener, safer means for creating these building blocks, our society can realize tremendous benefits through a reduction in the use and generation of hazardous substances. As molecular designers, chemists require knowledge about how chemical structures and properties impact toxicity and environmental impact. Currently, this knowledge is absent from the education of a chemist. By giving chemistry students a better understanding of how chemicals impact human health and the environment, these students can be better prepared to design greener, safer chemical products and processes.
1.1 Green chemistry principles
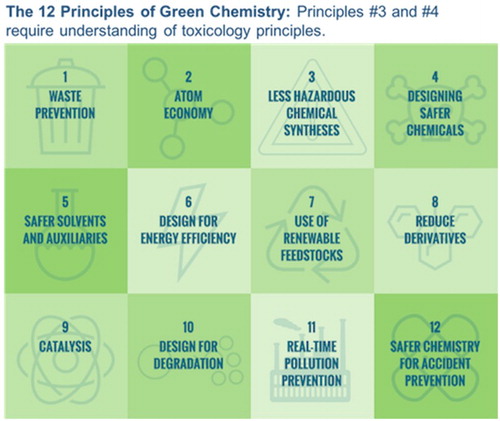
Green Chemistry is the design of chemical products or processes that reduce or eliminate the use or generation of hazardous substances (Citation1). Green Chemistry principles have been widely adopted by industry as a research framework and by academia as a teaching tool by which to design and teach more sustainable methods for chemical products and processes. The principles provide guidelines for practicing chemists and many of the principles have a significant number of resources to guide the practicing chemist towards viable, more sustainable options. For example, the pharmaceutical industry sector has devoted significant attention and resources towards principle #5, safer solvents and auxiliaries through the development of safer solvent guides (Citation2Citation3 Citation4–Citation5). These guides are widely available and have been useful towards decreasing the use of hazardous solvents across sectors and within research laboratories. There is also a focus on the use of Renewable Feedstocks (principle #7) as chemists shift towards bio-based polymers and materials (Citation6,Citation7). Atom economy (principle #2), and many more green chemistry metrics, are now used extensively to measure the efficiency of a synthetic reaction in addition to yield (Citation8). Catalysis (principle #9) has been of great interest for chemists to design more efficient reactions while minimizing the use of stoichiometric reagents (Citation9). Perhaps the most challenging of the 12 Principles of Green Chemistry are Principles #3 and #4 (), each of which mentions the toxicity of chemical products and substances.
Table 1. Green chemistry principles #3 and #4.
These two principles present a challenge to chemists, who traditionally have not had toxicology or environmental impact as part of their education (Citation10). Despite the challenges, these two principles also present the greatest opportunity for chemists, as molecular designers, to bring toxicology concepts and tools into their design processes and therefore to better design chemical products that have reduced hazard on humans and the environment.
2. Teaching green chemistry and toxicology
The teaching of green chemistry within academia has been on the rise since the 1990s due to significant work by green chemistry educators. Much of the early focus within academia has been within organic chemistry, where numerous resources are available for greener, more sustainable approaches to organic chemistry laboratory experiments (Citation11Citation12–Citation13). Green chemistry is now making its way into the other disciplines of chemistry and there is also currently a movement towards teaching toxicology concepts to chemistry students so that they have a mechanistic understanding of how chemicals impact humans and the environment (Citation10).
The Green Chemistry Commitment, a program of Beyond Benign, a non-profit dedicated to green chemistry education, specifically calls for the integration of toxicology concepts and tools into chemistry courses and programs through the adoption of green chemistry student learning objectives within chemistry programs (Citation14). Many signers of the Green Chemistry Commitment are adopting unique courses and embracing new ways of integrating these concepts throughout their curriculum. The different approaches reflect the diverse nature of academia and provide different models for incorporating these concepts into an already jam-packed curriculum.
Three unique models are presented within this manuscript from three different institutions: Simmons College, South Dakota State University (SDSU), and Wittenberg University. Simmons College and SDSU each offer a stand-alone course for science majors, while Wittenberg University provides students with a holistic approach through their laboratory safety program, while also offering students a stand-alone course.
2.1 Simmons college: CHEM 342: mechanistic toxicology
Simmons College offers a mechanistic toxicology course to chemistry and related science majors, co-taught by Dr. John Warner and Dr. Amy Cannon. The course has a pre-requisite of organic chemistry, with a suggested pre-requisite of biochemistry. It provides students with a grounding in green chemistry, followed by an understanding of the concepts of toxicology. The course develops the students’ understanding of fundamental toxicological mechanisms and tools for reducing hazard through molecular design, while also discussing ecological toxicity and how chemicals impact ecosystems and the environment. Topics discussed during the Mechanistic Toxicology course are outlined in .
Table 2. Simmons College CHEM 342: mechanistic toxicology course topics.
The course also brings in outside speakers as content experts to discuss relevant topics, such as Dr. Laura Vandenberg (University of Massachusetts, Amherst) on the topic of the Endocrine System, Endocrine Disruption and Low Dose Effects, and Dr. Richard Clapp (Boston University) and Molly Jacobs (University of Massachusetts Lowell) on the topic of Epidemiology through an engaging epidemiological case study (Citation15). Mary Butow from the University of Massachusetts Lowell’s Toxics Use Reduction Institute also provides the students with information and resources on how to find toxicological and hazard data using an on-line database to inform decision making (Citation16).
As the outline of topics in suggests, the mechanistic toxicology course first provides students with a grounding in the principles of toxicology and the language of toxicology. The instructors then build on that grounding through toxicokinetics and toxicodynamics, giving students more skills to help them connect the chemical and physical properties of a chemical to the behavior of that chemical within the body (i.e. how it will be absorbed, distributed, metabolized, and excreted (ADME)). A lecture from an outside expert, Dr. Laura Vandenberg, helped students to understand the emerging field of endocrine disruption and how scientists are working to identify endocrine disrupting chemicals early in the molecular design stage (Citation17). Epidemiology is discussed through a case-study to help students to understand how large sets of data in epidemiological studies can be used to determine toxicological effects, as well as understanding how the fields of toxicology and epidemiology are related. The effects of chemicals on the global environment is also discussed through ozone depletion and climate change chemistry. The students are introduced to design rules for biodegradability (Citation18), helping them to understand how chemical structure can be used to determine the persistence or biodegradability of chemicals. The course is then focused on structure-activity relationships (Citation19) and predictive tools that can be used to predict toxicological endpoints based on structure, giving students better means for designing chemicals with reduced human and environmental hazards.
2.2 SDSU: infusing toxicology into the curriculum.
SDSU has an optional upper division, “Chemical Toxicology” course. This course covers toxicodynamics and toxicokinetics, as well as aspects of environmental toxicology and mechanistic toxicology. After offering this course a few times, efforts were made to bring exposure to toxicology to all department majors (biochemistry, chemistry, and chemical education) early in the curriculum.
SDSU uses a modified 1–2–1 curriculum for departmental majors and honors students in their first two years. That is, the first semester covers introductory chemistry, followed by a year (second and third semesters) of predominantly organic chemistry and another semester of introductory chemistry. The goal was to provide students with a knowledge of the basics of toxicology that they would carry with them throughout the rest of their degree studies. The second-semester course (first semester of organic chemistry) was used to begin this effort. A three-pronged approach was taken to achieve this goal: (1) during classroom lectures principles of toxicology were introduced, (2) a two-week laboratory exercise reinforced lectures and introduced bioassays and toxicity testing, and (3) a survey measured potential changes in student attitudes toward topics of concern.
2.2.1 Toxicology in organic chemistry lectures
Toxicological principles were introduced as part of normal content in the first organic course (second-semester freshmen). This included three primary discussions. During lectures on stereochemistry, the drug thalidomide and its tragic consequences of severe birth defects when taken by women during pregnancy was used as an example. This led to a discussion on the concepts of exposure and dose/response. When halogenation was introduced, the non-stick polymer Teflon® was used as an example, with an extended discussion of perfluorooctanic acid exposure, remediation, and disposal hazards. Grilling food and the formation of polycyclic aromatic compounds provided the opportunity to discuss exposure routes, biological response, toxicokinetics, and toxicodynamics. Vocabulary and practical examples from the intersection of organic chemistry and toxicology were emphasized. The learning objectives in the course included that students would know (a) elementary toxicological principles, vocabulary, and methodologies and (b) resources to identify chemical hazards upon course completion.
2.2.2 Laboratory investigation of the toxicity of fat-soluble vitamins
To demonstrate the applicability of toxicology, a laboratory exercise was developed and implemented covering the toxicity of fat-soluble vitamins. Compounds such as caffeine and nicotine were considered. However, students are aware that these compounds have associated toxicity. On the other hand, they are bombarded with vitamin-fortified products with associated health-benefit claims. An examination of the toxicity of vitamins can demonstrate that “the dose makes the poison.” Students read a New York Times article on increasing incidence of vitamin toxicity due to overconsumption of supplements and fortified foodstuffs (Citation20). Then they consulted the U.S. EPA's EPI (Estimation Program Interface) SuiteTM Suite toxicological database (Citation21) for toxicological information on common vitamins (A, B12, C, D, E, and K) and calculated the toxic dosage of A, D, E, and K for their body weight. Additional prelab activities included a review article reading on vitamin metabolism and its effects on the liver and a series of questions related to vitamin toxicity.
During the first week in the laboratory, students prepared solutions of each fat-soluble vitamin and created a broth of healthy hepatic cells. They inoculated the hepatic-cell broth culture with vitamin solutions and incubated the cell cultures for one week. The following week, students performed a colorimetric assay to determine cell survival/death ratio and uploaded their data on the course website. Using the collective data from all students, they drew conclusions about the potentially lethal doses of fat-soluble vitamins and compared this with FDA guidelines (Citation22).
Using formal and informal feedback from the students, several conclusions were made. Regarding the laboratory experiment, results from the dose/response curve for vitamin K were inconclusive and closer examination of the number of trials, vitamin concentrations, and inclusion of water-soluble vitamins is needed. Student critical-thinking skills were improved as they were better able to critique articles (both from the peer-reviewed literature and the popular press) and question the methods used. Their use of toxicology terminology increased. The students seemed much more engaged during this laboratory exercise.
2.2.3 Student attitudes toward chemical toxicology
In addition to knowledge about chemical toxicology, SDSU was interested in how student attitudes toward toxicology-related issues were influenced by the knowledge learned in their coursework. Results are summarized in .
Table 3. Survey of student attitudes toward chemical toxicity
Students were surveyed with the questions in . The survey was administered to the same group of students at the beginning and end of the first organic course (second-semester freshmen) where the toxicology activities described were administered. The same students took the survey the following semester after their second semester of organic chemistry. A separate group of juniors and seniors took the survey following the Chemical Toxicology stand-alone course. In most cases, little, if any, change in the average score for these questions were observed as students matriculated. However, if the distribution of responses (as reflected by the standard deviation of the mean response) are considered, as students progressed through the curriculum, they showed an increased concern over the chemical safety to which they are exposed in their courses, increased their awareness of chemical hazards, and are more knowledgeable on how to avoid or minimize chemical exposure potential.
Other questions were more open-ended. As the students progress, they are more likely to use specific resources, like professional journals and established databases, to find toxicity information. They also increasingly felt that responsibility for evaluating the toxicity of chemicals used in consumer products should fall on the companies that create these products.
2.2.4 Future toxicology activities in the chemistry curriculum at SDSU
Following the freshman-level toxicology activities, students claimed to be more likely to take the upper-division Chemical Toxicology course. This has led to faculty conversations on how to further infuse toxicology topics into the curriculum. For example, the second organic course is considering the synthesis of a drug compound, accompanied by an EPI Suite review and brine shrimp assay. The analytical course routinely extracts and characterizes environmental toxins, and the use of assessing the green chemistry parameters during an analytical method is also considered.
2.3 Wittenberg University: integrating toxicology through laboratory safety
Most contributions to the undergraduate chemistry curriculum about chemical toxicology are represented by stand-alone courses as described elsewhere in this paper. These courses are usually based on the ADME perspective of chemical toxicology and require some working knowledge of organic structures and reactivity and biochemistry. This section describes another perspective of toxicology addressed through the lens of chemical health and safety.
Chemical health and safety addresses, in part, the topic of the various hazards chemicals present to chemists working in the laboratory. The main categories of hazards are flammability, reactivity, and health. A consideration of the last category begs the question, “How can this chemical harm me?” This raises questions about routes of entry into the body and the distinction between acute and chronic toxicants. These concerns, and how they can be addressed in the undergraduate curriculum, are the focus of this section of this paper.
At Wittenberg University, a wide variety of safety topics are introduced that are inserted into every laboratory course in our curriculum, as recommended by the ACS Committee on Professional Training in the 2015 Guidelines (Citation23). This is accomplished by the use of a textbook designed for this purpose (Citation24). shows the chapter titles in this textbook with titles in italics from which toxicology sub-topics are drawn. (The numbers in parentheses are the number of sections in each chapter.)
Table 4. Chapter titles from Laboratory Safety for Chemistry Students
This textbook is written in a “layered” fashion with various topics directed at either introductory, intermediate, or advanced students. The second digit of each section number in each chapter indicates the “level” of the topic. shows the list of sections in the book that address issues related to toxicology.
Table 5. Section titles addressing toxicology topics from Laboratory Safety for Chemistry Students.
2.3.1 Teaching across the curriculum
One advantage of teaching about chemical health and safety across the chemistry curriculum is that students are constantly reminded that chemical health and safety is part of all laboratory experiments. And since virtually all chemicals present some degree of acute and/or chronic toxicity, repeated exposure to various toxicological topics provides the same opportunity to address this in all lab courses, and with increasing degrees of “chemical sophistication” as the students progress from introductory to advanced courses.
Presented below is an overview of the other main topics taught in various courses. As an aside, it should be noted that in addition to the 12 topics listed in , about 60 other chemical health and safety topics are introduced throughout the various courses.
In Wittenberg’s general chemistry courses, “routes of entry” are introduced very early in the first course since this quickly leads to the discussion of personal protective equipment in the lab. Along with the four main routes of entry (inhalation, dermal/eyes, ingestion and injection) the basic concepts of dose as the duration of exposure is introduced. In addition to introducing the maxim of “the dose makes the poison,” this sets a context for subsequent discussions of acute and chronic effects.
The Globally Harmonized System of Classifying and Labelling Chemicals (GHS) and Safety Data Sheets (SDS) is also presented early in our program as well as the concepts of dose-response curves, factors affecting toxicity, and animal-to-human extrapolation of toxicity data. These topics raise important critical-thinking issues of “what’s a safe level?,” “how do we measure LD50?,” “how do we know a threshold level?” and other vexing questions that remove any veil of false simplicity about these important questions. Indeed, teaching about toxicology from the perspective of using data to make decisions about chemist’s lab behavior and the basis for governmental regulations is a rich area for teaching critical thinking.
Discussions are also held about defining and characterizing acute vs. chronic toxicity. As with LD50 for acute toxicity, a similar discussion is considered with regard to measuring and defining the chronic toxicity parameters of occupational exposure limits.
Finally, a brief discussion in general chemistry is held about assessing and managing risk which includes an overview of some common federal regulations (such as the Safe Drinking Water Act, the Toxic Substances Control Act, and the Delaney Clause of the Federal Food, Drug and Cosmetic Act) that illustrate the challenge of using scientific data to establish exposure limits. This includes a brief mention of the Precautionary Principle.
In organic chemistry, chronic toxicity and carcinogens are discussed in further detail. In other intermediate and advanced courses and in the seminar program where students have more chemical knowledge, a more thorough discussion of various laws and regulations is held, many of which dip into toxicology issues. There are also sections about bloodborne pathogens and the safety of nanomaterials.
Wittenberg University sees this perspective of toxicology introduced as a part of chemical health and safety as a partner to a more traditional toxicology ADME course also taught in the curriculum. Indeed, the hope is that the ideas introduced here along with the questions raised stimulate further exploration of chemical toxicology, including career choices in the field.
Conclusion
Presented herein are three models of courses in which toxicology and related concepts are being taught within chemistry programs. The models are meant to create a dialogue for faculty considering how to incorporate these concepts into an already jam-packed curriculum. What is it that we should teach as chemical educators within a crowded curriculum? This raises many questions and presents faculty with the challenge to consider what knowledge and skills are essential in the education of chemists in the twenty-first century. The inclusion of the concepts of toxicology and green chemistry speak to a growing moral obligation and ethical responsibility for chemists to understand more about the chemistry that we use and create. As molecular designers, chemists have tremendous power to create chemical products and processes in ways that reduce or eliminate the use of hazardous substances. Through greater knowledge of toxicology concepts and processes, students can be armed with the knowledge and skills to contribute towards this tremendous responsibility.
We welcome the development of other means by which chemical toxicology can be included in the chemistry curriculum and look forward to the day when key concepts in toxicology are included in the chemistry curriculum so that future chemists, and other scientists who take chemistry courses, have a working knowledge of this important field of chemistry so that the enterprise of chemistry and the work of chemists acknowledge the goals of sustainability.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Amy S. Cannon holds the world's first Ph.D. in Green Chemistry from the University of Massachusetts Boston where her research involved the environmentally benign synthesis of photoactive materials. She is the co-founder and executive director of Beyond Benign, a non-profit organization dedicated to green chemistry education. She received her M.S. in chemistry from the University of Massachusetts Boston and her undergraduate degree in Chemistry from Saint Anselm College in Manchester, NH. Amy worked as an Assistant Professor of Green Chemistry and Director of Outreach and Community Education at the Center for Green Chemistry at the University of Massachusetts Lowell until September of 2007 when she left to co-found Beyond Benign where she leads many educational initiatives to bring together multiple stakeholders around this common goal.
David C. Finster, Ph.D., is Professor of Chemistry at Wittenberg University, where he has served as chair of the Chemistry Department. He is the university's Chemical Hygiene Officer and a Certified Chemical Hygiene Officer (NRCC, 1999). He is a member of the American Chemical Society (ACS) Committee on Chemical Safety and chair of the Safety Committee in the Division of Chemical Education. He is on the Board of Editors of the Journal of Chemical Health and Safety. He has presented numerous talks and workshops on the application of intellectual development theory to learning science and chemistry and has been a volunteer firefighter and hazmat technician for many years. His recent professional interests focus on laboratory safety, implementing green chemistry in the curriculum, and the intersection of these areas. He is co-author of the textbook, Laboratory Safety for Chemistry Students.
Dr Doug Raynie is Department Head and Associate Professor in the Department of Chemistry and Biochemistry at South Dakota State University. Prior to joining SDSU, he was employed for eleven years as a Senior Scientist at Procter and Gamble's Corporate Research Division. He earned his Ph.D. at Brigham Young University under the direction of Dr. Milton L. Lee. His undergraduate degree is from Augustana (South Dakota) College,with majors in chemistry and biology. Dr. Raynie's broad research interests are in the field of sustainability and green chemistry. His two major areas of research are bioprocessing using supercritical fluids and analytical separations. Current research is centered on the development of deep eutectic solvents for novel applications and applications of high-resolution chromatography to food science applications. Analytical separations research includes high-resolution chromatography (high-temperature LC and SFC), chromatographic sample preparation (ASE, SFE, SPME, and SPE), chromatography theory, green analytical chemistry, and problem-based learning in analytical chemistry. He serves as Sample Preparation Perspectives columnist for LC/GC magazine.
John C. Warner is the recipient of the 2014 Perkin Medal, widely acknowledged as the highest honor in American Industrial Chemistry, and was named a 2016 AAAS-Lemelson Invention Ambassador. He received his BS in Chemistry from UMASS Boston, and his PhD in Chemistry from Princeton University. After working at the Polaroid Corporation for nearly a decade, he then served as tenured full professor at UMASS Boston and Lowell (Chemistry and Plastics Engineering). In 2007 he founded the Warner Babcock Institute for Green Chemistry, LLC (A research organization developing green chemistry technologies) where he serves as President and Chief Technology Officer, and Beyond Benign (a non-profit dedicated to sustainability and green chemistry education). He is one of the founders of the field of Green Chemistry, co-authoring the defining text Green Chemistry: Theory and Practice with Paul Anastas. He has published nearly 300 patents, papers and books and has won numerous awards for his pioneering work in green chemistry.
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998.
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A., Kleine, H.P., et al. Green Chemistry Tools to Influence a Medicinal Chemistry and Research Chemistry Based Organization. Green Chem. 2008, 10, 31–36. doi: 10.1039/B711717E
- Henderson, R.K.; Jimenez-Gonzalez, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s Solvent Selection Guide – Embedding Sustainability into Solvent Selection Starting at Medicinal Chemistry. Green Chemistry 2011, 13, 854–862. doi: 10.1039/c0gc00918k
- Denis Prat, D.; Pardigon, O.; Flemming, H.W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E., et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Organic Process Research & Development, 2013, 17, 1517–1525. doi: 10.1021/op4002565
- American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable Solvent Selection Guide, Version 2.0 Issued March 21, 2011. https://www.acs.org/content/dam/acsorg/greenchemistry/industriainnovation/roundtable/acs-gci-pr-solvent-selection-guide.pdf (accessed Feb 21, 2017).
- Clark, J.H.; Budarin, V.; Deswarte, F.E.; Hardy, J.J.E.; Kerton, F.M.; Hunt, A.J.; Luque, R., et al. Green Chemistry and the Biorefinery: A Partnership for a Sustainable Future. Green Chemistry 2006, 8, 853–860. doi: 10.1039/b604483m
- Guido, W. High-Performance Polymers from Nature: Catalytic Routes and Processes for Industry. ChemSusChem 2014, 7 (8), 2081–2088. doi: 10.1002/cssc.201402379
- Sheldon, R.A. The E Factor 25 Years on: The Rise of Green Chemistry and Sustainability. Green Chem 2017, 19 (1), 18–43. doi: 10.1039/C6GC02157C
- Delidovich, I.; Palkovits, R. Catalytic Versus Stoichiometric Reagents as a Key Concept for Green Chemistry. Green Chem 2016, 18 (3), 590–593. doi: 10.1039/C5GC90070K
- Cannon, A.S.; Levy, I.J. In ACS Symposium Series Promise of Chemical Education: Addressing Our Students’ Needs; Daus, K., Rigsby, R., Eds.; American Chemical Society: Washington, DC, 2015, Vol. 1193, pp. 115–125.
- Dicks, A.P. Green Organic Chemistry in Lecture and Laboratory; Taylor & Francis: Boca Raton, FL, 2012.
- Levy, I.J.; Haack, J.A.; Hutchison, J.E.; Kirchhoff, M.M. Going Green: Lecture Assignments and Lab Experiences for the College Curriculum. J. Chem. Educ. 2005, 82 (7), 974–976. doi: 10.1021/ed082p974
- Doxsee, K.M.; Hutchison, J.E. Green Organic Chemistry: Strategies, Tools, and Laboratory Experiments;Brooks/Cole: Pacific Grove, CA, 2004.
- The Green Chemistry Commitment, Green Chemistry Student Learning Objectives. http://www.greenchemistrycommitment.org/faculty/student-learning-objectives/ (accessed Feb 20, 2017).
- 25 Years Later, a Poisoned Town Can’t Come Clean, May 23, 2011, by Jared Bowen, WGBH, http://www.wgbh.org/articles/25-Years-Later-A-Poisoned-Town-Cant-Come-Clean-3057
- Toxics Use Reduction Institute, Environmental Health, and Safety Data Resources. http://guides.turi.org/beyondmsds (accessed Feb 21, 2017).
- Schug, T.T.; Abagyan, R.; Blumberg, B.; Collins, T.J.; Crews, D.; DeFur, P.L.; Dickerson, S.M., et al. Designing Endocrine Disruption Out of the Next Generation of Chemicals. Green Chem. 2013, 15 (1), 181–198. doi: 10.1039/C2GC35055F
- Boethling, R.S.; Sommer, E.; DiFiore, D. Designing Small Molecules for Biodegradability. Chem. Rev. 2007, 107, 2207–2227. doi: 10.1021/cr050952t
- Anastas, N.D.; Warner, J.C., In ACS Symposium Series Green Chemistry Education: Changing the Course of Chemistry, Anastas, P.T., Levy, I.J., Parent, K.E., Eds.; American Chemical Society: Washington, DC, 2009, Vol. 1011, pp. 117–136.
- Offit P.A. Don’t Take Your Vitamins, New York Times, June 8, 2013, http://www.nytimes.com/2013/06/09/opinion/sunday/dont-take-your-vitamins.html (accessed Feb 21, 2017).
- EPI SuiteTM – Estimation Program Interface, United States Environmental Protection Agency, https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed Feb 21, 2017).
- U.S. Food & Drug Administration, Fortify Your Knowledge About Vitamins. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm118079.htm#fat (accessed Feb 21, 2017).
- Undergraduate Professional Education in Chemistry, ACS Guidelines and Evaluation Procedures for Bachelor’s Degree Programs, Spring 2015, American Chemical Society, Committee on Professional Training. https://www.acs.org/content/dam/acsorg/about/governance/committees/training/2015-acs-guidelines-for-bachelors-degree-programs.pdf (accessed Feb 21, 2017).
- Hill, R.H.; Finster, D.C. Laboratory Safety for Chemistry Students, 2nd ed.; John Wiley & Sons: Hoboken, NJ, 2016.
