ABSTRACT
In this study, we investigated the synthesis of silver nanoparticles using Peganum harmala water extract at ambient temperature. The Ag nanoparticles (AgNPs) were characterized by ultraviolet–visible spectroscopy (UV–vis), Fourier transform infrared spectroscopy (FT–IR), powder X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), and transmission electron microscopy (TEM). The average particle size of the silver nanoparticles was about 23 nm. Inhibitory activity of the synthesized AgNPs was tested against human pathogens like Escherichia coli and Staphylococcus aureus. The results indicated that the AgNPs showed moderate inhibitory actions, demonstrating its antibacterial value against pathogenic diseases.
GRAPHICAL ABSTRACT
Introduction
The ability to create novel materials at the nanoscale size and innovations in measuring and modifying nanomaterials has led to the fast growth of nanotechnology (Citation1). Till now, noble metals such as Ag, Au, Pd, and Pt have been used for the synthesis of metallic nanoparticles (Citation2) and are mostly applied in products that come in direct contact with the human body. Therefore, there is a growing concern to develop green, eco-friendly processes for nanoparticle synthesis instead of processes using toxic chemicals (Citation3, Citation4). Among the nanometal particles, silver nanoparticles (AgNPs) have become the focus of numerous studies due to their unique optical, electrical, and biological properties, which offer potential applications in various fields including catalysis, electronics, and biology (Citation2).
AgNPs can be synthesized using physical or chemical methods such as ultrasonic fields, ultraviolet and microwave radiation, photochemical reduction, and also photochemical and sonoelectrochemical methods. However, the use of toxic chemicals as reducing agents in many of these processes, which is potentially dangerous to the environment and biological systems, is of great concern (Citation5, Citation6). Compared with other methods, biological methods using microorganisms (Citation7, Citation8), enzymes (Citation9), and plants or plant extracts like Nothapodytes nimmoniana (Graham) Mabb. fruit extracts (Citation10), Alternanthera dentate aqueous extract, Acorus calamus and Boerhaavia diffusa plant extracts (Citation11), Allophylus serratus leaf and leaf-derived callus extracts (Citation12) are environmentally friendly and alternatives to chemical and physical methods for the synthesis of AgNPs (Citation13). Synthesizing nanoparticles using plant extracts can be advantageous over other biological processes by omitting the elaborate process of maintaining cell cultures (Citation14), complex extraction and purification processes (Citation15) and it also allows easy scale up to produce larger amounts of nanoparticles that are free of contamination and have well-defined size and morphology (Citation16).
Chemical methods for reducing silver ions may have incompatible effects in medical applications due to the attachment of hazardous chemicals, polar solvents and reducing compounds used for synthesis to the surface of AgNPs (Citation17).
Green synthesized AgNPs displaying antibacterial activity are known to have various advantageous in medical applications in medicine and biological fields (Citation18), for example, to reduce infections, prevent bacterial growth on prostheses, dental materials, vascular grafts, catheters, human skin, and stain-less steel materials (Citation19) or in designing newer drugs (Citation20). Therefore, biological and green approaches for the synthesis of nanoparticles are better than chemical and physical methods due to being clean, non-toxic, cost-effective, environmental friendly (Citation21, Citation22), low energy and time consumption, and non-toxic solvents and non-dangerous materials (Citation23).
Various plant seed or leaf extracts such as Euphorbia antiquorum L. (Citation24), Melia azedarach L. (Citation25), Averrhoa bilimbi (Citation26), Silybum marianum (Citation27), Calendula officinalis (Citation28, Citation29), Eucalyptus camaldulensis (Citation30), Ulva flexuosa (Citation31), and Withania somnifera (Citation32) have already been reported for the synthesis of AgNPs with different characteristics.
Peganum. harmala L. (Peganaceae) known as Syrian Rue, usually called Esfand or Espand in Iran, is a plant of the Nitrariaceae family and is distributed in Central Asia, the Middle East, and North Africa. This perennial herbaceous and glabrous plant grows in different soils, including coastal and inland habitats. It is a highly branched plant standing 30–60 cm tall and with short creeping roots. It has narrow leaves arranged alternately on fleshy, bright green stiff stems. The flowers are isolated, small, pale yellow or white, and five-petaled. The fruits are capsules with three chambers and about 6–10 mm across. The capsules contain more than 50 small black-brown triangular seeds. The immature fruits are green and turn orange-brown when mature. It is applied as a traditional medicine due to its long-lasting antibacterial, fungicide, and herbicide effects. Many authors have reported the inhibitory effects of P. harmala on bacteria, parasites, viruses, and cancer cells (Citation33–36).
To summarize, in this paper, we report the green synthesis of AgNPs by P. harmala L. as a reducing agent at ambient temperature. The synthesized nanoparticles were characterized using different methods such as UV–Vis spectroscopy, FT–IR, XRD, scanning electron microscopy (SEM), Energy dispersive X-ray spectroscopy (EDS), and TEM. Moreover, the antibacterial activity of the synthesized AgNPs against Gram-positive and Gram-negative bacteria was also studied.
Materials and methods
Preparation of water extract
P. harmala seeds were collected from Tehran, Iran, in September 2016. AgNO3 (99.80%) was purchased from Merck. All aqueous solutions were prepared using double-distilled water. Dried seeds (5 g) were ground into a powder and extracted with double-distilled water (ratio 1:10), by boiling the mixture for 15 min in a water bath at 70°C. The mixture was then filtered with Whatman Paper (No. 14) and kept in a dark bottle in a refrigerator for further experiments until one week.
Synthesis and purification of silver nanoparticles
A solution containing 50 mL of AgNO3 (1 mM, 2 mM, and 3 mM) and 5, 10, and 15 mL of water extract of P. harmala was mixed at ambient temperature (25°C) for 24 h under vigorous stirring. Then, the solution was centrifuged at 6000 rpm for 30 min several times. The AgNPs were dried in an oven at 80°C for 8 h and were collected for further characterizations.
Characterization of Ag NPs
The morphology of the AgNPs was studied using XRD (X’ Pert Pro, Panalytical Company). The SEM analysis was carried out with Zeiss, Sigma VP-500 equipped with an EDS detector (Oxford Instrument). The TEM observations were carried out using a transmission electron microscope (Zeiss, Germany, EM 10C-100 kV) to observe the size, shape, and morphology of the synthesized nanoparticles. The UV–vis analysis was performed over the absorption wavelength of 200–800 nm with a UV Bio-TEK UV–vis spectrophotometer. The synthesized silver nanoparticles were studied by FT–IR (Perkin model, U.S.A, Elmer) for evaluating the interaction between the extract and silver nanoparticles.
Antibacterial screening
The antibacterial activity of the synthesized AgNPs was investigated against Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria by the Bauer-Kirby disk diffusion method. First, 100 mL of a fresh bacterial culture was gently spread on an agar surface. The bacterial concentration utilized was 1.5 × 108 CFU (colony forming unit)/mL. In order to make a Mack Farland solution, a suspension of a bacterial colony dissolved in 2 mL of sterile physiological serum was made. Then, the bacterial suspension was subjected to dense culturing on Mueller Hinton agar culture plates.
Next, a thick suspension of the AgNPs was prepared and three dilutions of 1/10, 1/100, and 1/1000 (5, 0.5 and 0.05 μg/mL) were made from it. With a sterile sampler, 5 λ of the AgNP suspension with a 2 cm space was poured on a culture plate. For the control, antibiotic disks were placed 2 cm away from the wall of the culture plate.
The plates were incubated at 37°C for 24 h, and the zone of inhibition of bacterial growth was used as a measure of susceptibility.
Results and discussion
UV–VIS spectroscopy
The UV–vis spectroscopy technique can be used to examine the size and shape control of nanoparticles in aqueous suspensions (Citation37).
First, 50 mL of a 0.03 mM solution of silver nitrate was prepared in an Erlenmeyer flask. Then, 5, 10, and 15 mL of plant extract was added separately to the silver nitrate solution, keeping its concentration constant. Silver nanoparticles were also synthesized by varying the concentration of AgNO3 (1–3 mM) and keeping the extract volume constant (15 mL). This setup was incubated in a dark chamber to minimize the photo-activation of the silver nitrate at room temperature.
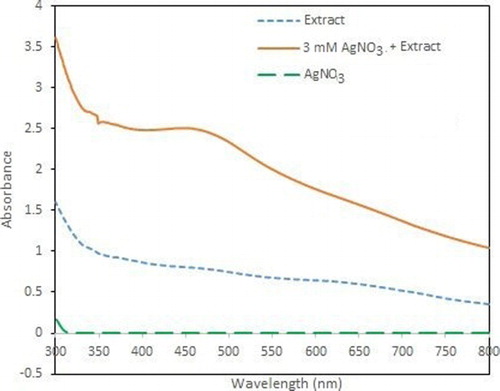
The color of the solution changed from yellow to red-brown due to the reaction of the silver salt and extract, indicating the formation of silver nanoparticles (Citation38–40). Its formation was also confirmed by UV–vis spectroscopy. The color change is caused by coherent excitation of the SPR in metallic nanoparticles (Citation41).
The surface plasmon resonance (SPR) of the silver nanoparticles remained between 400 and 450 nm, which shows the formation of silver nanoparticles (Citation42). The appearance of SPR at a wavelength range of 400–450 nm corresponds to AgNPs which absorb radiations intensely at a wavelength of 447 nm due to the transition of electrons.
The UV–vis spectra of the reaction medium recorded after 24 h of reaction with 0.03 mM of AgNO3 and also the plant extract are shown in . The spectra were recorded at a range of 300–800 nm and the maximum absorption wavelength was determined at 447 nm indicating the synthesis of AgNPs and with no absorption for plant extract in this region. If we increase the leaf extract concentration to 15 mL, the wavelength also increased, as presented in (a). The slight variations in the values of absorbance signify that the changes are because of the particle size (Citation43).
Figure 2. (A) UV–Vis spectra of different concentrations of plant extract (B) UV–Vis spectra of different concentrations of AgNO3.
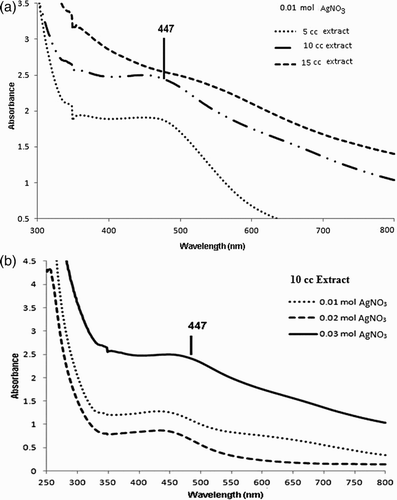
Parallel changes in color have been observed when different concentrations (1–3 mM) of silver nitrate were used and the plant extract (10 mL) was kept constant. It can also be observed from (b) that the intensity of the absorption peaks increases with an increase in the concentration of the silver nitrate salt and with an addition in AgNO3 concentration, the SPR peaks become sharper. All the results are in good agreement with those reported in literature, showing an absorbance at 447 nm for the silver nanoparticles.
X-ray diffraction analysis
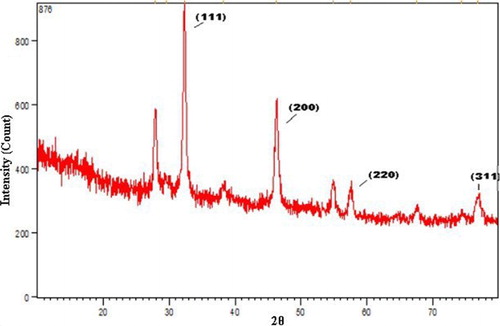
Further demonstration and confirmation of the presence of silver nanoparticles synthesized by using P. harmala extract in the reaction media were shown by XRD images. The characteristic peaks obtained in the XRD pattern are shown in . The XRD pattern of the silver nanoparticles shows intense peaks at the 2θ angles of 38°, 44°, 64°, and 77°, which correspond to the 111, 200, 220, and 311 planes, respectively (Citation44–46).
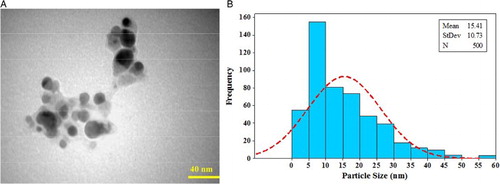
The size of the AgNPs was calculated by the Debye–Scherrer equation as follows:where S is the crystallite size of the AgNPs, λ is the wavelength of the X-ray source (1.54056 Å), β0.5 is the full width at half maximum (FWHM) of the diffraction peak in radian, k is the Scherrer constant that varies from 0.9 to 1, and θ is the Bragg angle in radian (Citation47). In the present XRD pattern, the average size of the nanoparticles was 23 nm.
Scanning electron microscopy
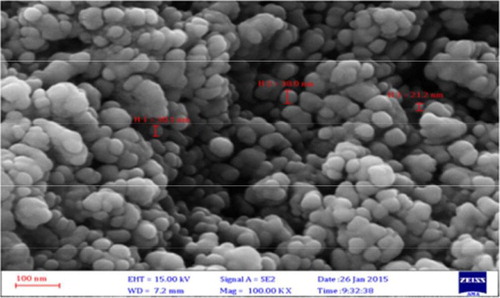
SEM images () show the morphological character of the AgNPs synthesized by the extract of P. harmala. The images reveal that the sizes of the nanoparticles are between 12.73 and 35.61 nm with spherical shapes.
Energy dispersive X-ray spectroscopy
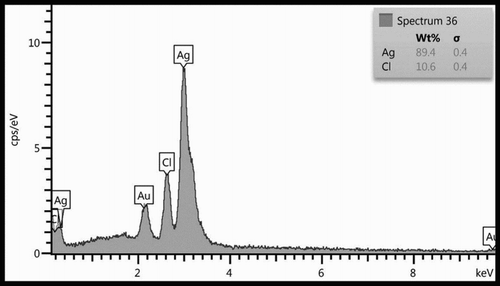
EDS was used for the chemical analysis of the synthesized silver nanoparticles (). A strong signal was obtained at the energy level of 3.4 keV for silver; some weak signals from Cl and Au were also observed. The major emission energy at 3.4 keV indicates that the silver has been correctly identified.
FT–IR analysis
FT–IR measurement reveals the functional groups of the silver nanoparticles synthesized using P. harmala extract, as shown in .
Figure 6. (A) FT–IR spectra of P. harmala extract, (B) Silver nanoparticle synthesized using P. harmala extract.
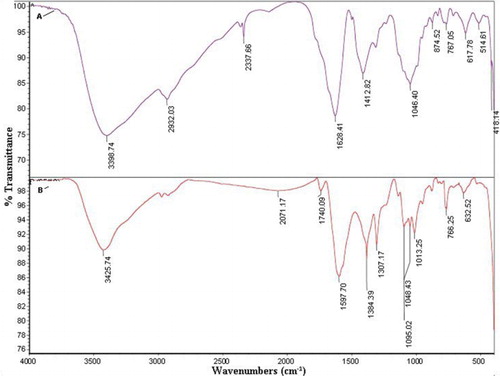
The FT–IR spectra of the dried aqueous extract and the synthesized AgNPs present similar peaks with a small shift in both spectra, which reveals that the synthesized AgNPs contain natural compounds from the extract. The stability of the AgNPs can be attributed to the presence of these compounds in the shell of the nanoparticles (Citation48, Citation49).
The bands at 3398.74 cm−1 (for the P. harmala extract spectrum) and at 3425.74 cm−1 (for the AgNP-extract spectrum) are assigned to OH-stretching of hydroxyl groups, alcohols, phenols, or the NH group of amines or amides. The absorption band 2932.03 cm−1 in the spectrum of the extract is attributed to CH-stretching. The bands at 2337.66 cm−1 shift to 2071.17 cm−1 and are assigned to a terminal CN group. The bands at 1628.41 cm−1 for the extract and at 1597.70 cm−1 for the AgNP-extract are assigned to the C=O group of carboxylic acid. The peaks at 1412.82 cm−1 for the P. harmala extract and at 1384.39 cm−1 for the AgNP-extract are assigned to the C=C group of alkenes. The range of 1050–1200 cm−1 is assigned to the CO group of alcohols, ethers, and esters.
The phenolic groups involved act as reducing agents and the C-H and N-H groups correspond to proteins that act as stabilizing agents. The carbonyl groups of carboxylic acids have a high affinity for Ag, and therefore, reduced and stabilized them (Citation50–54).
Transmission electron microscopy
The TEM images reveal that the silver nanoparticles obtained by the reduction of Ag+ by the P. harmala extract were spherical in shape and roughly 15 nm in size.
The size and distribution of the AgNPs were determined by TEM images and are shown in (a,b), respectively.
Antibacterial analysis
The antibacterial activity of the AgNPs synthesized by using whole plant extracts of P. harmala showed positive results ( and ).
Table 1. Inhibition zone against E. coli bacteria.
Table 2. The results for antibacterial activity of thesynthesized AgNPs by using plant extract.
The synthesized nanoparticles showed antibacterial activity against E. coli ((a)) and S. aureus ((b)). The synthesized AgNPs exhibit a good antibacterial activity against both Gram-positive and Gram-negative bacteria. From the results, we observed that the synthesized nanoparticles of exhibited good zone inhibition in both cases. When the zone inhibition was compared to that of Ciprofloxacin and Gentamicin antibiotics, we found that the silver nanoparticles were as effective as these antibiotics.
Figure 8. (A) Antibacterial activity of synthesized AgNPs against E.coli (B) Antibacterial activity of synthesized AgNPs against S. aureus.
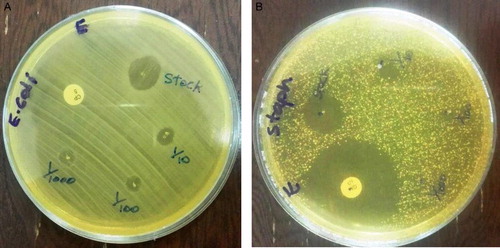
The antibacterial activity of AgNPs might be due to the release of silver ions in the cells (Citation55) and the attached bioactive constituents, such as flavonoids, in the plant (Citation56). Ag ions interact with bacterial cell membranes, leading to the plasmolysis and inhibition of cell membrane synthesis (Citation57). Likewise, AgNPs also can interfere with the thiol group of bacterial cells, causing seizure of the respiratory chain reaction, cell division, and death (Citation58).
Conclusion
The results demonstrated that silver nanoparticles were successfully synthesized using the seed extract of P. harmala with an average size of 23 nm and spherical shape at ambient temperature. The synthesis of the nanoparticles could also be confirmed by the color change of the extract from yellow to red-brown. Furthermore, the AgNPs were characterized by UV–vis, XRD, SEM, EDS, TEM, and FT–IR analyses. The use of plant extracts for making metallic nanoparticles is inexpensive, easily scaled-up, and environmentally benign. It is especially suited for making the nanoparticles that must be free of toxic contaminants, as required in therapeutic applications. Furthermore, the synthesized AgNPs showed antibacterial activity against E. coli and S. aureus, indicating its commercial viability in medicines.
Acknowledgements
We are grateful to the faculty members of both North Tehran Branch and Shahr-e-Qods Branch of the Islamic Azad University of Iran for their kind cooperation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Matin Azizi received M.Sc. degree in applied chemistry from Islamic Azad University-Shahre Ray, Yadegare Emam Branch, Tehran, Iran, in 2010. She is a PhD. Student in Applied Chemistry in Islamic Azad University-Tehran North Branch since 2014. Her main areas of interests are Nano technology, Green chemistry and water treatment.
Sajjad Sedaghat received the M.Sc. degree in organic chemistry from Shahid beheshti University, Tehran, Iran, in 2002. He received his PhD. in Applied chemistry from Islamic azad university, north Tehran branch in nano catalyst for fuel cells in 2006. Since 2006, he has been with Islamic azad university, Shahr-e-Qods branch, Tehran, Iran as a lecturer in the green chemistry group. His main areas interests are nano synthesis, nanocomposites and Green chemistry.
Kambiz Tahvildari received M.Sc. degree in applied chemistry from Islamic Azad University-Tehran North Branch, Tehran, Iran, in 1997. He received his PhD. in Applied Chemistry from same university in 2006. Since 2006 he is chemistry faculty member of this university and his main areas of interests are Biofuels, Nano technology, Green chemistry and water treatment.
Pirouz Derakhshi received M.Sc. degree in analytical chemistry from Islamic Azad University-Tehran North Branch, Tehran, Iran, in 1993. He received his PhD. in Applied Chemistry from same university in 2009. Since 2009 he is chemistry faculty member of this university and studied in Chemical Nano- Technology.
Ahad Ghaemi received the M.Sc. degree in chemical Engineering from Iran University of Science and Technology, Tehran, Iran, in 2003. He received his PhD. in Process Design, Modeling and simulation in Chemical Engineering from the same university in 2009. Since 2011, he has been with Iran University of Science and Technology, Tehran, Iran as a lecturer in the Process Design group. His main areas interests are Process Design; Modeling and simulation; Green chemistry.
ORCID
Ahad Ghaemi http://orcid.org/0000-0003-0390-4083
References
- Ju-Nam, Y.; Lead, J.R. Science Total Environ. 2008, 400 (1–3), 396–414. doi: 10.1016/j.scitotenv.2008.06.042
- Parveen, M.; Ahmad, F.; Malla, A.M.; Azaz, Sh. Appl. Nanosci. 2016, 6 (2), 267–276. doi: 10.1007/s13204-015-0433-7
- Murugan, A.; Shanmugasundaram, K.K. World J. Pharm. Pharm. Sci. 2014, 3 (10), 855–868.
- Song, J.Y.; Kim, B.S. Bioprocess Biosyst. Eng. 2009, 32, 79–84. doi: 10.1007/s00449-008-0224-6
- Sedaghat, S.; Esmaeili Agbolag, A.; Bagheriyan, S. J. Nanostructure Chem. 2016, 6 (1), 25–27. doi: 10.1007/s40097-015-0176-8
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Colloids Surf. B: Biointerfaces. 2014, 122, 767–777. doi: 10.1016/j.colsurfb.2014.08.018
- Sadowski, Z.; Maliszewska, I.H.; Grochowalska, B.; Polowczyk, I.; Koźlecki, T. Mater. Sci-Poland. 2008, 26 (2), 419–424.
- Tsibakhashvili, N.; Kalabegishvili, T.; Gabunia, V.; Gintury, E.; Kuchava, N.; Bagdavadze, N.; Pataraya, D.; Gurielidzse, M.; Gvarjaladze, D.; Lomidze, L. Nano Studies. 2010, 2, 179–182.
- Korbekandi, H.; Ashari, Z.; Iravani, S.; Abbasi, S. Iran. J. Pharm. Res. 2013, 12 (3), 289–298.
- Mahendran, G.; Ranjitha Kumari, B.D. Food Sci. Human Wellness. 2016, 5 (4), 207–218. doi: 10.1016/j.fshw.2016.10.001
- Ahmed, Sh.;Ahmad, M.; Swami, B.L.; Ikram, S. J. Adv. Res. 2016, 7 (1), 17–28. doi: 10.1016/j.jare.2015.02.007
- Jemal, K.; Sandeep, B.V.; Pola, S. J. Nanomat. 2017, 2017, 1–11. doi: 10.1155/2017/4213275
- Heydari, R.; Rashidipour, M. Int. J. Breast Cancer. 2015, 2015, 1–6. doi: 10.1155/2015/846743
- Bar, H.; Bhui, D.Kr.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Colloids Surf. A: Physicochem. Eng. Aspects. 2009, 339 (1–3), 134–139. doi: 10.1016/j.colsurfa.2009.02.008
- Ndikau, M.; Noah, N.M.; Andala, D.M.; Masika, E. Int. J. Anal. Chem. 2017, 2017, 1–9. doi: 10.1155/2017/8108504
- Mittal, A.K.; Chisti, Y.; Banerjee, U.Ch. Biotechnol. Adv. 2013, 31, 346–356. doi: 10.1016/j.biotechadv.2013.01.003
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Pawar, S.H. Mater. Lett. 2014, 116, 367–369. doi: 10.1016/j.matlet.2013.11.066
- Pethakamsetty, L.; Kothapenta, K.; Nammi, H.R.; Ruddaraju, L.K.; Kollu, P.; Yoon, S.G.; Pammi, S.V.N. J. Environ. Sci. 2017, 55, 157–163. doi: 10.1016/j.jes.2016.04.027
- Shameli, K.; Bin Ahmad, M.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Gharayebi, Y.; Sedaghat, S. Int. J. Nanomed. 2010, 5, 1067–1077. doi: 10.2147/IJN.S15033
- Rao, N.H.; Lakshmidevi, N.; Pammi, S.V.N.; Kollu, P.; Ganapaty, S.; Lakshmi, P. Mater. Sci. Eng.: C. 2016, 62, 553–557. doi: 10.1016/j.msec.2016.01.072
- Vijay Kumar, P.P.N.; Pammi, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameem, U. Ind. Crops Prod. 2014, 52, 562–566. doi: 10.1016/j.indcrop.2013.10.050
- Sedaghat, S. Curr. Nanosci. 2016, 12, 79–82. doi: 10.2174/1573413711666150812010229
- Afshar, P.; Sedaghat, S. Curr. Nanosci. 2016, 12, 90–93. doi: 10.2174/1573413711666150529202238
- Rajkuberan, Ch.; Prabukumar, S.; Sathishkumar, G.; Wilson, A.; Ravindran, K.; Sivaramakrishnan, S. Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J. Saudi Chem. Society. 2016, 21. In Press.
- Mehmood, A.; Murtaza, Ch.; Bhatti, T.M.; Kausar, R. Phyto-mediated synthesis of silver nanoparticles from Melia azedarach L. leaf extract: Characterization and antibacterial activity. Arab. J. Chem. 2017, 10, S3048–S3053. doi: 10.1016/j.arabjc.2013.11.046
- Rimal Issac, R.S.; Sakthivel, G.; Murthy, Ch. J. Nanotechnol. 2013, 2013, 1–7. doi: 10.1155/2013/906592
- Mohammadinejad, R.; Pourseyedi, Sh.; Baghizadeh, A.; Ranjbar, Sh.; Mansoori, G.A. Int. J. Nanosci. Nanotechnol. 2013, 9 (4), 221–226.
- Baghizadeh, A.; Ranjbar, Sh.; Kumar Gupta, V.; Asif, M.; Pourseyedi, Sh.; Karimi, M.J.; Mohammadinejad, R. J. Mol. Liq. 2015, 207, 159–163. doi: 10.1016/j.molliq.2015.03.029
- Fierascu, I.; Bunghez, I.R.; Fierascu, R.C.; Ion, R.M.; Dinu-Pîrvu, C.E.; Nuţă, D. Farmacia. 2014, 62 (1), 129–136.
- Bashir, T.; Qureshi, M.Z. J. Chil. Chem. Soc. 2015, 60 (1), 2861–2863. doi: 10.4067/S0717-97072015000100019
- Rahimi, Z.; Yousefzadi, M.; Noori, A.; Akbarzadeh, A. J. Persian Gulf. 2014, 5 (15), 9–15.
- Ahmed, Sh.; Annu ;Zafeer, I.; Ikram, S. J. Bionanosci. 2016, 10 (1), 47–53. doi: 10.1166/jbns.2016.1356
- Moussa, T.A.A.; Almaghrabi, O.A. Saudi J. Biol. Sci. 2016, 23 (3), 397–403. doi: 10.1016/j.sjbs.2015.04.013
- Hashemi Sheikh Shabani, S.; Seyed Hasan Tehrani, S.; Rabiei, Z.; Tahmasebi Enferadi, S.; Vannozzi, G.P. Biotechnol. Rep. 2015, 8, 138–143. doi: 10.1016/j.btre.2015.08.007
- Cheraghi Niroumand, M.; Farzaei, M.H.; Amin, Gh. J. Tradit. Chin. Med. 2015, 35 (1), 104–109. doi: 10.1016/S0254-6272(15)30016-9
- Aboualigalehdari, E.; Sadeghifard, N.; Taherikalani, M.; Zargoush, Z.; Tahmasebi, Z.; Badakhsh, B.; Rostamzad, A.; Ghafourian, S.; Pakzad, I. Osong Public Health Res. Perspect. 2016, 7 (2), 116–118. doi: 10.1016/j.phrp.2015.12.010
- Shrivastava, S.; Dash, D. J. Nanotechnol. 2009, 2009, 1–14. doi: 10.1155/2009/184702
- Shankar Sangaru, Sh.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Nat. Mater. 2004, 3 (7), 482–488. doi: 10.1038/nmat1152
- Korbekandi, H.; Asghari, Gh.; Jalayer, S.S.; Jalayer, M.S.; Bandegani, M. Jundishapur J. Nat. Pharm. Prod. 2013, 8 (1), 20–26. doi: 10.17795/jjnpp-7433
- Islam, N.U.; Jalil, K.; Shahid, M.; Rauf, N.; Muhammad, A.; Khan, A.; Shah, M.R.; Khan, M.A. Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arab. J. Chem. 2015. In Press.
- Kasithevar, M.; Saravanan, M.; Prakash, P.; Kumar, H.; Ovais, M.; Barabadi, H.; Shinwari, Z.Kh. J. Interdiscip. Nanomed. 2017, 2 (2), 131–141. doi: 10.1002/jin2.26
- Mevaa, F.E.; Segnou, M.L.; Ebongue, C.O.; Ntoumba, A.A.; Kedi, Ph.B.E.; Deli, V.; Etoh, M.A.; Mpondo, E.M. Rev. Bras. Farmacogn. (Braz. J. Pharmacog.). 2016, 26 (5), 640–646. doi: 10.1016/j.bjp.2016.06.002
- Ahmed, S; Saifullah, Sh.; Ahmad, M.; Swami, B.L.; Ikram, S. J. Radiat. Res. Appl. Sci. 2016, 9 (1), 1–7. doi: 10.1016/j.jrras.2015.06.006
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, Sh.; Bhatt, D.; Sreedhar, B. Mater. Sci. Eng.: C. 2016, 58, 36–43. doi: 10.1016/j.msec.2015.08.018
- Sheny, D.S.; Mathew, J.; Philip, D. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2011, 79 (1), 254–262. doi: 10.1016/j.saa.2011.02.051
- Ashokkumar, S.; Ravi, S.; Velmurugan, S. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2013, 115, 388–392. doi: 10.1016/j.saa.2013.06.050
- Raja, S.; Ramesh, V.; Thivaharan, V. Arab. J. Chem. 2017, 10 (2), 253–261. doi: 10.1016/j.arabjc.2015.06.023
- Ahmad, N.; Bhatnagar, Sh.; Ali, S.S.; Dutta, R. Int. J. Nanomed. 2015, 10, 7019–7030.
- Ramteke, Ch.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R.A. J. Chem. 2012, 2013, 1–7. doi: 10.1155/2013/278925
- Shaik, M.R.; Ali, Z.J.Q.; Khan, M.; Kuniyil, M.; Assal, M.E.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, M.; Adil, S.F. Molecules. 2017, 22 (1), 1–12. doi: 10.3390/molecules22010165
- Ajayi, E.; Afolayan, A. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2017, 8 (1), 1–8.
- Vanaja, M.; Gnanajobitha, G.; Paulkumar, K.; Rajeshkumar, Sh.; Malarkodi, Ch; Annadurai, G. J. Nanostruct. Chem. 2013, 3 (17), 1–8.
- Chidambaram, J.; Saritha, K.; Maheswari, R.; Muzammil, M.S. Chem. Sci. Trans. 2014, 3 (2), 773–777.
- Vilas, V.; Philip, D.; Mathew, J. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2014, 132, 743–750. doi: 10.1016/j.saa.2014.05.046
- Hsueh, Y.H.; Lin, K.S.; Ke, W.J.; Hsieh, Ch.T.; Chiang, Ch.L.; Tzou, D.Y.; Liu, Sh.T. Plos One. 2015, 10 (12), 1–17. doi: 10.1371/journal.pone.0144306
- Singh, K.; Panghal, M.; Kadyan, S.; Yadav, J.P. J. Nanomed. Nanotechnol. 2014, 5 (6), 1–7. doi: 10.4172/2157-7439.1000250
- Gahlawat, G.; Shikha, S.; Chaddha, B.S.; Chaudhuri, S.R.; Mayilraj, Sh.; Choudhury, A.R. Microb. Cell Fact. 2016, 15 (25), 2–14.
- Prasad, R.; Swamy, V.S. J. Nanopart. 2013, 2013, 1–6. doi: 10.1155/2013/431218