ABSTRACT
Biodiesel being one of the most promising renewable biofuels has seen rapid increase in production capacity due to high demand for diesel replacement; along with oversupply of its by-product, crude glycerol. Developing new industrial usage for glycerol is essential to defray the cost and sustainability of biodiesel industry and to promote the biodiesel industrialization. One of the approaches is by the transformation of glycerol into a liquid, referred as bio-oil through pyrolysis technology. Bio-oils produced by pyrolysis processes can be upgraded to produce transportation fuels or for power generation. However, current state of pyrolysis technologies are still major hurdles their development with respect to its commercial applications. Recently, microwave technology has attracted considerable attention as effective method for significantly reducing reaction time, improving the yields and selectivity of target products. Hence, this review strives extensively towards addressing the application of microwave-assisted technology applied to the pyrolysis process as a way of cost-effective and operationally feasible processes to directly utilize crude glycerol. The present review will focus on the pyrolyzed liquid product (bio-oil) derived by employing the microwave-assisted pyrolysis method. This review concludes that microwave-assisted glycerol conversion technology is a promising option as an alternative method to conventional glycerol conversion technology.
GRAPHICAL ABSTRACT
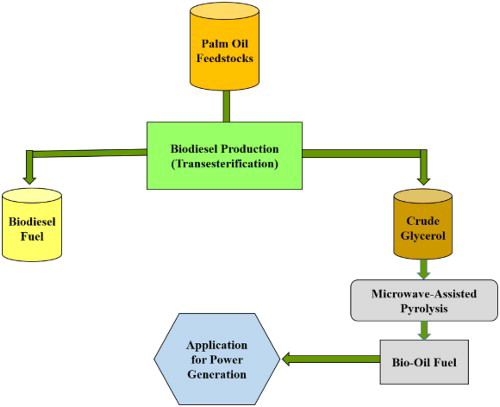
1. Introduction
Every aspect of modern society runs on energy. The conventional oil and natural gas resources are a valuable supply of energy; however, they are finite resources that contribute to greenhouse gas emissions as well as air pollution. Carbon dioxide (CO2) is the primary greenhouse gas emitted through human activities. Between 1990 and 2015, there has been an increase in CO2 emissions corresponding with increased energy use by an expanding economy and population, an overall growth in emissions from electricity generation, and increased demand for travel ( Citation1). Energy efficiency and conservation, as well as decarbonizing the energy sources, are a prerequisite for sustainable development. Reducing carbon emissions is very crucial to mitigate the significant climate-change risks posed by greenhouse gas emissions. The most effective way to reduce CO2 emissions is to reduce fossil fuel consumption. The first years of the twenty-first century have witnessed remarkably rapid growth in the production and consumption of renewable energy. Despite the significant growth in the use of renewable energy, reducing carbon emissions on the timescale is necessary to mitigate the significant climate-change risks ( Citation2). In developed societies, energy demands are however so high that renewables with limited supply potential such as geothermal or hydro will be able to meet only a small proportion of future global energy demands. The largest contribution will have to come from wind, ocean, and solar sources. However, wind, ocean, and solar projects have limited lifetimes and if applied globally, they might consume a remarkable share of construction materials. Although such renewables may reduce CO2 emissions’ footprint of the energy sector, on the other hand they may increase the CO2 footprint of the industrial sector, where the production of these construction materials creates additional CO2 emissions ( Citation3). In addition, nuclear is also a proven technology and can play an important role in a low-carbon strategy. Although the nuclear power plants do not release CO2 emissions, they generate highly radioactive spent nuclear fuel. The disposal of the generated radioactive waste, especially high-level ones, remains a major concern for society around the world ( Citation4). To overcome these challenges, a viable option is biofuels, which have been receiving increasing attention because of being a neutral carbon source ( Citation5). Biofuels have the ability to reduce about 41% CO2 emissions, have better engine performance due to complete combustion, require no modifications to diesel engine usage, and low price, which make them an environmentally friendly diesel replacement ( Citation6). Biodiesel is outstanding among the biofuels, and has become widely acceptable in the energy market owing to its unique features including higher cetane number, lack of sulfur, inherent lubricity, positive energy balance, higher flash point, non-toxicity, and high biodegradability ( Citation7,Citation8).
In Malaysia, to date, all of the established biodiesel production chains are using palm oil as primary feedstock. As a second largest producer and exporter of palm oil in the world, Malaysia accounts for 40% of total global palm oil produced annually. Palm oil has high potential because of its high yield per hectare and high oil content over other oils crops ( Citation9). Considering the comparative yields of various oil bearing crops, oil palm is clearly the most efficiently produced oil in the world today. When the world is looking at vegetable oils as renewable fuel, palm oil will undoubtedly stand out among other vegetable oils. In 2011, the total biodiesel production in Malaysia was 170,000 tons, which constitutes only 6% of the total capacity of 2.7 million tons from 23 biodiesel plants ( Citation10). Several issues in the Malaysian biodiesel industry have led to its underperformance such as lack of processing facilities. New facilities need to be installed in order to further develop biodiesel capacity and at present, the government is providing subsidies for the construction of additional facilities in existing refineries. The major hindrance to propping up the biodiesel production in Malaysia is the fuel subsidies given by the government for fossil fuels, which hamper the technology development and the market competitiveness of biodiesel. However, since 2012 the subsidies on petroleum fuel were gradually removed under the subsidy rationalization policy. This had somewhat created better price competitiveness for biodiesel. The Government’s latest commitment in its biodiesel mandates can be seen as an attractive investment venture for oil palm planters, which already have the main feedstock for the production of biodiesel. It may not be surprising to see a trend among cash-rich plantation companies lately to acquire “idle” biodiesel plants in Malaysia. These acquisitions will take place as plantation companies are beginning to realize that biodiesel can provide a safe avenue and act as a good buffer to support the crude palm oil price in case of oversupply. In spite of the attractiveness, the Malaysian palm oil biodiesel industry faces disadvantages regarding duties. Malaysian biodiesel and palm oil are subject to 30% export duties, whereas Indonesia imposes only a 2% export duty on biodiesel and 16.5% export duty on palm oil ( Citation10). This (export duty) disadvantage has contributed to Malaysia’s drop in ranking among other palm oil producers, such as Indonesia, Thailand, and Colombia, which are more competitive biodiesel producers. Other challenges include problems faced during pre-processes, and during post-processes (waste problem). Problems will arise when the feedstock is too expensive compared to the processing cost itself. As high as 75–90% of the total biodiesel production cost is actually used for purchasing the required raw materials alone. There will be significant challenges to the biodiesel industry when the demand for feedstock fluctuates and destabilizes the market. Another arising issue is the waste glycerol problem. It is estimated that approximately for every 100 kg of biodiesel produced, 10 kg of glycerol is generated as a by-product ( Citation11,Citation12). The abundance of waste glycerol generated from the biodiesel industry not only affects the cost of the biodiesel production but it also severely creates an environmental issue ( Citation13). A more sustainable biodiesel production can be achieved if the waste glycerol could be utilized in a more effective way such as conversion to a value-added product.
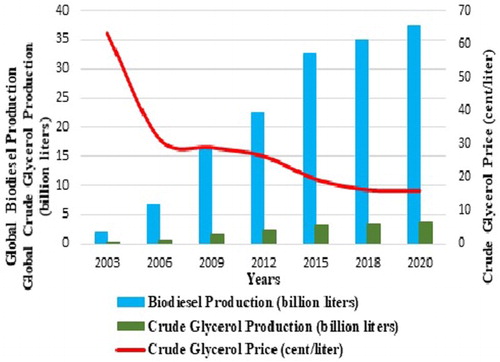
The world scenario of biodiesel production as shown in predicts that by 2020 the global production of glycerol will reach 4.0 billion liters. Thus, crude glycerol disposal and its utilization have become a serious issue and a financial and environmental liability for the biodiesel industry ( Citation14). Thus, waste glycerol abundance is a problematic issue in biodiesel production at present. The market value of pure glycerol was US cent 19.4–20.1 per liter in 2014 ( Citation15) and almost close to US cent 12.01–15.03 per liter of crude glycerol. The current and the projected market values of glycerol up to 2020 are expected to remain within this range ( Citation16). A marginal (2–5%) increase in the price in 2020 may be expected if there are new demands for vegetable oil-based glycerol as continuous research is being carried out to find new applications for crude glycerol. New applications for glycerol must be found to avoid the continuous fall of glycerol prices. Added to that is the fact that crude glycerol generated from biodiesel production is impure; purification of crude glycerol is needed to remove impurities in order to meet the requirements of the existing and emerging usage. The conventional techniques for purification of crude glycerol for reuse are cumbersome and too costly ( Citation17).
Figure 1. Global biodiesel production and crude glycerol price from 2003 to 2020, source from ( Citation14).
Over the past few years, the development of products from biomass through the pyrolysis technique has been intensively investigated. Pyrolysis technology has attracted more interest in producing liquid fuel product, known as bio-oils, because of its considerable advantages of being storable, transportable, and versatile in applications such as combustion engines, boilers, and turbines. However, it is still at an early stage of development and needs to overcome a number of technical and economic barriers to compete with traditional fossil fuel ( Citation18). The development of more advanced technologies is the next challenge for pyrolysis researchers to achieve this target. The ultimate goal of this technology is to produce high-quality bio-oil, which is able to compete with and eventually partially replace the non-renewable fossil fuels for direct use in micro gas turbines for power generation, and other sustainable products such as bioplastics and platform chemicals. Many researches are interested in the application of microwave irradiation, not only due to its ability to enhance the reaction rates and rapid heating, but also for its ability to revolutionize the chemical reactions bringing about products and reactions’ performance with unexpected results ( Citation19–21). Among the important benefits of microwave-assisted reactions are rapid heating, lower relative energy consumption, environmental friendliness, higher production yield, controllable processing, shorter processing time, and quality and properties’ improvement. Hence, the production of bio-oil is another promising use of glycerol since waste glycerol is difficult and costly to manage which also gives impetus to pyrolysis research. The aim of this review paper is to emphasize the principles of microwave-assisted pyrolysis and to show recent research on the application of this technology for waste glycerol.
2. New opportunity for the usage of crude glycerol
Crude glycerol from biodiesel plants has low economic value and is not pure enough for direct use in many applications, due to the presence of various impurities. The problem with crude glycerol from biodiesel production is that it has heavy contamination from toxic methanol and has a high salt and free fatty acids (FFA) content and a substantial color (yellow to dark brown). The impurities present in crude glycerol significantly affect its properties and thus its conversion to value-added products. Another major challenge for the utilization of crude glycerol is the inconsistency in its composition since it varies with the feedstock and production procedures ( Citation22). This makes crude glycerol unsuitable for most traditional glycerol markets. Many studies have reported various methods to purify crude glycerol; among them are vacuum distillation, ion exchange, membrane separation, and activated carbon adsorption whereby each of the purification techniques uses different properties of crude glycerol. The conventional techniques for purification of crude glycerol are energy extensive, too costly, cumbersome, and economically unavailable for the biodiesel producers ( Citation23–27). The crude glycerol must be purified before it can be used in food and pharmaceutical applications. Traditional Olechemicals refiners cannot use the crude glycerol from the biodiesel industry, even at 80% purity, because it would damage expensive pipes and storage equipment. Prior to use, it must be refined to an acceptable purity level at dedicated refineries and then sold at low price as “technical-grade” refined glycerol [Technical grade – used as a building block in chemicals, not for food or drugs] or further refined to USP grade [USP glycerol – suitable for food and pharmaceuticals]. However, most of the current methods of utilization of crude glycerol are only able to uptake small volumes of the waste glycerol. Coupled with huge amounts of crude glycerol generated in the last five years, its price is decreasing and this is one of the main reasons for extensive search to find out the direct usage of crude glycerol to avoid the purification step and at the same time to overcome the huge quantity of glycerol generated from the biodiesel industry. In the last 10 years, the world has seen a rise in usage of bio-glycerol as chemical ingenuity was rapidly opening the route to glycerol derivatives for use in fields as diverse as fuels, chemicals, pharmaceuticals, detergents, and the automotive and building industries. presents a summary of the main applications of crude glycerol.
Table 1. List of various applications of crude glycerol.
3. Utilization of biodiesel derived from waste glycerol for value-added products
Glycerol has a large number of applications in varied fields; however, the current market cannot accommodate this large volume of crude glycerol coming from biodiesel production, which needs further costly purification steps. For these commercial applications, the quality of glycerol must be improved until it has an acceptable purity (>98%) ( Citation38). The other viable solution for the residual glycerol from biodiesel production is for the synthesis of value-added chemicals or materials of industrial importance. From a technical standpoint, the multifunctional structure and properties of glycerol allow for its utilization by several different reaction pathways. Driven by the motivation to search for alternative and sustainable ways of utilizing crude glycerol, many researchers are looking at the production of valuable end products or intermediate feedstock for the production of new classes of renewable biofuels and important platform chemicals. The production of sustainable products, which can replace petroleum-based products, will ultimately extend the utilization of renewable resources for a sustainable environment. Generally, biomass can be transformed into many useful forms by thermochemical and biochemical conversion. In thermochemical conversion pathways, heat and chemical catalysts are used for the production of energy from biomass, while biochemical conversion pathways use biological organisms and biological catalysts for the transforming of biomass into energy and value-added products ( Citation24). For instance, combustion, gasification, and pyrolysis are known as the main thermochemical conversion pathways, while fermentation and anaerobic digestion are the most common biochemical conversion pathways. The crude glycerol utilization in microbial fermentation has been studied extensively and many excellent reviews have been published in the last 15 years, and can be referred to for further information ( Citation25–27,Citation60,Citation61).
3.1. Thermochemical conversion of crude glycerol: gasification and pyrolysis
Waste glycerol is abundantly available and can be utilized effectively by using appropriate technologies, for use as a renewable resource for energy and other chemicals’ production. Three different thermochemical conversion routes are found according to the oxygen content in the process: combustion (complete oxidation), gasification, (partial oxidation), and pyrolysis (thermal degradation without oxygen). Among them, combustion (also called incineration) is the most established route in industry but this is also associated with the generation of carbon oxides, sulfur, nitrogen, chlorine products (dioxins and furans), volatile organic compounds, polycyclic aromatic hydrocarbons, and dust. On the contrary, gasification and pyrolysis offer the potential for greater efficiencies in energy production and less pollution. Although pyrolysis is still under development in the waste industry, this process has received special attention, as not only a primary process of combustion and gasification, but also as an independent process leading to the production of energy-dense products with numerous uses.
Gasification refers to the thermochemical decomposition of organic material under high temperatures (>800°C) to produce a mixture of hydrogen, carbon dioxide, carbon monoxide, methane, and light hydrocarbon gases, termed as syngas, or producer gas. Crude glycerol has been frequently used as an additive in the gasification of biomass to improve gas yields and the hydrogen fraction of the produced gas. In a recent review, different biomass co-gasification processes were performed using crude glycerol with lignocellulosic biomass ( Citation62). Some of the studies done in the last 8 years on gasification of crude glycerol are summarized in . In this proven option for glycerol disposal and utilization, co-gasification of glycerol and other feedstock was able to improve the yield of syngas or hydrogen considerably.
Table 2. Studies on the gasification of crude glycerol to produce syngas.
Pyrolysis is another thermochemical conversion process similar to gasification. It generates gas, bio-oil, and char, three product streams, through the decomposition of biomass at high temperatures (>350°C) in the absence of oxygen. The difference between the two methods is that in gasification, higher temperatures are used, so the char produced in the pyrolysis stage is further converted to syngas. There are three basic categories of pyrolysis (slow pyrolysis, fast pyrolysis, and flash pyrolysis) based on the operating parameters. Each parameter resulted in varying product composition. shows the difference of the three common pyrolysis types ( Citation70).
Table 3. The three different types of pyrolysis processes.
Liquid bio-oil from pyrolysis can be a promising alternative energy source for fuel oil or diesel. The produced oil, however, needs to be upgraded by removing the residuals. The main industrial uses of pyrolysis oil are as a combustion fuel for heat generation; power generation; transportation fuel; wood flavors, wood preservatives, and liquid smoke; chemical and resin production; and in making adhesives.
Solid char from pyrolysis is a carbonaceous residue. It can be used as a solid fuel for the production of heat and electricity; feedstock for gasification processes; feedstock for activated carbon production, and feedstock for carbon nano filaments’ production, among other uses.
Syngas from pyrolysis; the gas produced during the pyrolysis process (light molecular weight gases) is another valuable by-product. A vast diversity of biomass materials can be converted to combustible gases (H2, CO, C2H2, CH4, and C2H4). These combustible gases can be used as the direct firing in boilers for heat production or in gas turbines or engines for electricity production.
Pyrolysis studies have been performed using crude glycerol as an auxiliary compound to pyrolyze different types of feedstock. The improvement in the bio-oil quality and quantity was observed in co-pyrolysis of glycerol with lignocellulosic biomass and manure in these studies as summarized in . Co-pyrolysis is undoubtedly a promising option; however, more research and detailed analysis are necessary to identify the mechanism for energy production enhancement. Researches have reported that water and methanol existing in crude glycerol acted as the catalysts in pyrolysis and decreased the activation energy of the decomposition of glycerol, demonstrating crude glycerol to be a very promising additive ( Citation71). Approximately, each 450 g of glycerol feedstock produce about 270 g of bio-oil, 90 g of char, and 90 g of gas when paralyzed. According to the literature, most of the pyrolysis processes have been conducted using several types of equipment heated by a conventional heating source (e.g. an electrical gas heater), namely melting vessels, blast furnaces, tubular or fixed bed reactors; these types of pyrolysis processes are termed generally as “conventional pyrolysis.” These types of equipment were used in a manner where the thermal energy is externally applied to the reactor and heats all the substances in the reactor including the evolved pyrolysis-volatiles, the surrounding gases, and the reactor chamber itself. In this case, energy is not fully targeted at the material being heated and this results in significant energy losses in terms of the energy efficiency of the whole process. Nevertheless, several of these processes have been developed into a pilot plant scale despite their limited energy efficiency ( Citation72,Citation73).
Table 4. Studies on the co-pyrolysis of crude glycerol with other biomass to produce bio-oil.
4. Microwave technology
In the pyrolysis process, the three components, which is solid (char), liquid (bio-oil), and syngas (gaseous), are produced and the yield would vary with the changing operational parameters. Generally, the bio-oil may be considered as the more valuable and useful. The bio-oil (also known as pyrolysis oil) can be upgraded into biofuel which can be readily stored and transported as a promising candidate to replace petroleum fuels for power generation, heat, or for extraction of valuable chemicals ( Citation79). Flash pyrolysis processes are so far the common technology for the production of a high liquid yield. It is achieved through rapid heating rates of 10 to >1000°C/s, short residence times of <2 s, temperatures of 400–650°C, and rapid quenching of the vapors in the absence of oxygen ( Citation80). However, the product is not readily available for consumption. Many issues negatively affect the ability of raw bio-oil’s usage as a source of fuel or for the usage in value-added chemical production. Its complex chemical composition is one of the main reasons it cannot be utilized directly in combustion systems. Bio-oil has a low heating value of 16–18 Mj/kg because of a 15–30 wt% of water and 35–50 wt% of oxygen; its high acidity of pH 2–3 also contributes to its undesirable qualities ( Citation81). There is a need for upgrading to improve the chemical and physical properties of the raw bio-oil/pyrolysis oil ( Citation82,Citation83). One of the main difficulties that occur during biomass pyrolysis is the selectivity of the desired compounds in the bio-oil. Different feedstock sources lead to different ratios in the chemical composition of the bio-oil/pyrolysis oil ( Citation84). Currently, many investigations are geared toward improving the bio-oil yield during the pyrolysis process as well as improving the selectivity of the desired compounds in the pyrolysis oil.
In the early 1980s, it was reported that microwave irradiation could promote organic chemical reactions and this has led to the surge in research involving microwave irradiation in various chemical reactions ( Citation85). The application of microwave technology for the thermal treatment of biomass saw a major increase around the mid-nineties. This technique not only reduces the energy consumption and processing time, but also enables the use of a unique internal heating phenomenon. It can also enhance the overall production quality ( Citation86). Microwave irradiation is an electromagnetic irradiation in the range of wavelengths from 0.01 to 1 m with the corresponding frequency range of 0.3–300 GHz. Most microwave reactors for chemical synthesis operate at 2.45 GHz frequency, which corresponds to a wavelength of 12.25 cm. Microwave technology can be very useful in chemical processing because products can be heated volumetrically instead of surface heating via convection and conduction. Microwaves cause molecular motion by migration of ionic species or rotation of dipolar species or both to generate heat because of the friction among molecules ( Citation87). Materials that absorb microwave irradiation are called microwave dielectrics. Microwave heating (also called as dielectric heating) transfers electromagnetic energy to thermal energy. Microwave energy can be delivered directly to the reacting or processing species by using their dielectric properties or by adding absorbers to materials, which allows more volumetric heating of materials. Researchers have reported two possible ways by which reactions are enhanced using the microwave irradiation technique ( Citation88,Citation89). The electric and magnetic field components are responsible for the microwave dielectric heating and magnetic loss heating, respectively. These two components of microwaves will interact differently with the material under different mechanisms. More details about the microwave heating mechanism can be found in some excellent reviews ( Citation88,Citation89). There are also claims that thermal effects alone are not sufficient to explain microwave reactions’ superiority such as reaction rate enhancement and have postulated the existence of a third mechanism called as the non-thermal microwave effects ( Citation90–92). More details on the non-thermal effect of microwave in enhancing the rate of reaction can be found in a recent review article ( Citation93). In terms of the characteristics of microwave heating and conventional heating, provides a summary of the features of both heating methods.
Table 5. Comparison between microwave heating and conventional heating ( Citation93).
4.1. Microwave-assisted pyrolysis process
A variety of conventional pyrolysis systems used have been covered in many research works. To date, however, only a few researches have investigated the microwave irradiation-induced pyrolysis process. Microwave pyrolysis technology has gained momentous attraction, which offers a number of merits over conventional pyrolysis. The main advantage is that microwave pyrolysis can be performed for large-sized particle feedstocks as the polar molecules that oscillate under the influence of an oscillating electric and magnetic field agitate the heating. Compared to fast pyrolysis like fluidized bed, pre-dried biomass is not required in microwave pyrolysis. The moisture in biomass needs to be removed for obtaining high heating rate in conventional fast pyrolysis. However, in microwave pyrolysis the moisture is a good adsorption material for irradiation that induces the pyrolysis. Microwave pyrolysis also produces clean products like bio-oil because the process does not have to use biomass powder and does not require agitation and fluidization ( Citation94). Typically, if the biomass that is being used is a poor candidate for absorbing microwave, then an additional material capable of absorbing microwave is required to carry out the thermochemical process. Microwave heating combined with the use of carbon material has been applied; carbon materials are good microwave absorbents that show high capacity to absorb and convert microwave energy into heat ( Citation86,Citation95). This type of pyrolysis, which involves the use of microwave radiation as heat source combined with the use of carbon materials as the microwave receptor to directly heat and pyrolyze the materials, is known to offer additional advantages over conventional pyrolysis techniques. It has been reported that a current traveling in phase with the electromagnetic field is induced within the carbon material when it is subjected to microwave radiation ( Citation86,Citation88). As a result, the π-electrons in the carbon material are displaced from their equilibrium positions and this leads to dielectric polarization. As the π-electrons repeatedly shift from one position to another under the influence of the alternating electric field, this leads to a charge buildup within the carbon material. The power generated by the forced flow of electrons and the accumulation of charge within the carbon material (which leads to field distortions and dielectric loss) result in energy being dissipated as heat and thus contributing to the heating effects ( Citation86,Citation88). The addition of a catalyst to the biomass improves the heating rate. This allows the quantity of the bio-oil to increase ( Citation86). Similarly, to facilitate the conversion process, a catalyst may also be used. Catalytic microwave-assisted pyrolysis has been at the center of many recent investigations and the aim was to improve selectivity and yield of bio-oil ( Citation96). Traditionally, zeolite catalysts have been employed, while the use of metal oxides as catalysts has been proven to be just as good and can be produced at a much cheaper price ( Citation97,Citation98). Among the advantages of using microwave pyrolysis are included reduction in residence time, rapid chemical reaction, and higher reaction temperature attained due to effective heat transfer profiles. It has been reported that microwave pyrolysis showed advantages in providing rapid heating and less power consumption when compared with conventional pyrolysis ( Citation94). Previous studies showed that the char yield decreases when the reaction temperature increases, while a longer residence time increases the char yield because of an increasing secondary reaction. In addition, higher heating rates favor the production of liquid and gaseous fractions ( Citation99). Many efforts have been made to estimate the energy recovery in microwave pyrolysis of waste materials. Studies have shown that the microwave pyrolysis process is capable of recovering pyrolysis products (e.g. hydrocarbon oils) whose calorific values are many times greater than the amount of electrical energy used in the operation of the process, showing both a positive energy ratio (energy content of hydrocarbon products/electrical energy supplied for microwave heating) and a high energy output ( Citation94).
In conventional pyrolysis, energy is transferred to the biomass sample through the processes of convection or conduction. There must be physical contact between the heating source and the biomass sample. This has proven to be very inefficient because of a large amount of energy that is needed to increase the temperature of the feedstock. The use of microwaves in pyrolysis has been proven to be more efficient because of the heating pattern; in conventional pyrolysis, heat is transferred from the outer layers of the sample to the interior. This results in uneven heating and the quantity and quality of the bio-oil produced is degraded. In microwave heating, the interior of the sample is heated first and it gradually spreads to the outer most regions of the sample. As a result, the heating is more uniform and this allows for greater yields of bio-oil. gives an extensive overview of microwave-assisted pyrolysis that has been applied to various lignocellulosic feedstocks. The relationships among heating, bio-oil yield, and feedstock particle size, moisture content, inorganics, and organics in microwave-assisted pyrolysis are given in detail in ( Citation100). An up-to-date summary of recent studies on advances on the microwave-assisted pyrolysis methods have been reviewed extensively ( Citation100). The review provides information that will aid in the development of a newer microwave-assisted pyrolysis system.
Table 6. Product yields of lignocellulosic biomass using microwave pyrolysis.
Table 7. Biomass feedstock properties and microwave-assisted pyrolysis ( Citation100).
4.2. Microwave-assisted pyrolysis of crude glycerol as sole biomass
There are already some studies on co-pyrolysis of crude glycerol with other biomass to produce bio-oil as shown previously in . However, research related to pyrolysis of waste glycerol (derived from biodiesel transesterification) as sole or major biomass with minimal or no co-substrate (5–20% of other biomass) addition to producing bio-oil is limited. In fact, studies on microwave-assisted pyrolysis of waste glycerol to produce bio-oil are even scarcer. Previous investigations of glycerol pyrolysis have mostly focused on the production of synthesis gas (syngas) ( Citation132,Citation133). In the work reported in 2008, 72 mol% of gaseous yield was obtained from glycerol at 800°C, in which the syngas contained 93.5 mol% of H2 and CO ( Citation133). Conventional furnace heating was used as the heat source ( Citation133). By varying the carrier gas flow rate and temperature, different gas yields and compositions can be achieved. Gas production was favored at a lower particle size of the packing materials. In another study, syngas was produced by pyrolysis of glycerol using the microwave-induced pyrolysis technique and high gas yield of 81 vol.%, consisting of 34.6 vol.% of H2 and 45.9 vol.% of CO were reported ( Citation134). Although microwave pyrolysis can provide various advantages over conventional pyrolysis, it still faces several challenges. The bio-oil yield (up to approximately 40 wt%) of microwave pyrolysis is much lower than that from fluidized bed pyrolysis, which indicates that the high bio-oil yield production remains a large challenge for microwave pyrolysis. The microwave pyrolysis method has shown to yield a higher conversion rate of glycerol into gaseous products compared to conventional heating processes in all cases, but the effects on solid and liquid products were not investigated ( Citation135). In a related study reported in 2015, the results obtained showed that microwave-assisted pyrolysis can be used to convert waste glycerol into hydrogen. In addition to hydrogen, there were also liquid products, such as methanol, allyl alcohol, acrolein, and some products that are not identified ( Citation136). The report also showed that activated carbon catalyst can produce more hydrogen than Ni/HZSM-5 catalyst ( Citation136). It is noteworthy that most waste glycerol to energy processing techniques focused on optimizing operating conditions (including effect of catalyst) to obtain synthesis gases with no or very minimal emphazising on the solid and liquid products.
In a recent study, it was reported that generally the presence of the catalyst during the microwave-assisted pyrolysis of crude glycerol resulted in an increase in liquid product and a decrease in gaseous product at both the temperatures of 300°C and 400°C ( Citation137). At 300°C, the use of the catalyst decreased the gaseous product by 4.2%, but increased the liquid product by 9.2%. The difference was more significant when the heating temperature was raised to 400°C and the gaseous product was reduced by 19%, while the liquid product increased by 22% ( Citation137). The same authors reported that the highest liquid (bio-oil) product yield was obtained at the heating temperature of 400°C. A further increase in the pyrolysis temperature resulted in a decrease of bio-oil product. At 700°C, the bio-oil product yield was 15.7%, while the gaseous product yield was 83%. The function of a catalyst is to increase heat transfer and assist in the thermal cracking process. Hence, a more complete cracking of the molecules with fewer solid products can be achieved. Studies have shown that adding a catalyst can increase the liquid yield and improve the quality of the liquid product ( Citation138,Citation139). The pyrolysis temperature is one of the main parameters that can affect product yields. Liquid bio-oil shows a maximum yield at an intermediate temperature but it decreases at higher temperatures, due to the thermal cracking of heavy compounds into small-chain products. Varying the carrier gas flow rate changes the residence time of the products during the pyrolysis process, as the nitrogen (N2) flow rate changes the distribution of carbon inside the reactor. A lower flow rate of carrier gases provides ample residence time for the product to undergo the chain-cracking process that leads to secondary reaction ( Citation134,Citation140). Furthermore, the higher N2 flow rate reduces the time available for gaseous products to undergo the condensation process. As a result, the pyrolysis product remains largely as gaseous product (approximately >80%). From the study published in 2017, researches have noted that during microwave-assisted pyrolysis of glycerol, the proportion of products’ phases is mainly dependent on the residence time inside the reactor, followed by the reaction temperature ( Citation141).
Generally, production of liquid product is more favored at lower pyrolysis temperature as more vapor is able to condense. At higher pyrolysis temperature (>600°C), more energy is available for the cracking of vapor, resulting in less liquid product ( Citation141). Not only is the quantity but also the quality of the pyrolysed liquid product is affected by pyrolysis temperature. When reaction temperature decreases, there is a tendency for the higher proportion of liquid product to form. However, for solid products, it is dependent on both decreasing temperature and carrier gas flow rate. In the same study, the authors have reported that for microwave pyrolysis of glycerol, the operating conditions of 400°C, 1000 mL/min of N2 gas flow rate, and residence time of around 30–50 seconds is the optimal setting where the liquid product exceeds that of the gaseous products ( Citation141). The report further mentions a third factor that affects the gaseous product portion. The activated carbon-based catalyst is a good microwave absorber. The porous structure of the activated carbon also prolongs the resistance time of volatiles. These factors allow the catalyst-assisted microwave pyrolysis to gain sufficient thermal energy to achieve temperatures exceeding 400°C. The higher selectivity of carbonaceous catalysts toward hydrogen gas formation will lead to greater H2/CO ratio. As the volume of H2 increases by proportion when a catalyst is used, the proportion of the overall gaseous product weight will decrease, as H2 has a lower molar mass ( Citation141). The activation energy and pre-exponential factor of microwave pyrolysis are much lower than conventional methods. Thus, the mechanism and kinetic of the reaction for the microwave pyrolysis are different from the conventional pyrolysis process ( Citation142). Finally, it can be envisaged that the conversion of waste into portable energy products through microwave pyrolysis makes crude glycerol a potential candidate for bioenergy production of bio-oil and syngas.
4.2.1. Properties of bio-oil obtained by microwave-assisted pyrolysis
The liquid or oil fraction of the biomass pyrolysis can be a promising alternative energy source for biofuel or a platform molecule for production of other products such as organic acids. Microwave pyrolysis offers the potential for greater efficiencies and less pollution for the production of reactive intermediate products from biomass in comparison with other techniques. The bio-oil or pyrolysis oil is usually a dark-brown organic liquid with the heating value of approximately half that of conventional fuel oil (due to the relatively high oxygen and water contents of bio-oil). Thus, further upgrading and refining processes are needed to increase its heating value. There have been intensive studies on bio-oil upgrading and various technologies have been developed for bio-oil upgrading, including hydro-treating, hydrocracking, supercritical fluids extraction, solvent addition/esterification, emulsification, steam reforming, and chemical extraction. Solvent addition (esterification) appears to be the most practical approach due to its simplicity, low cost of some solvents, and their beneficial effects on the oil properties. However, none of these bio-oil upgrading techniques has been commercialized due to low biofuel efficiency and their limitations and high up-grading cost of the overall pre-treatment process ( Citation79). It was reported that the properties of microwave pyrolysis bio-oil were slightly different from those of conventional pyrolysis bio-oil. The quality of the oil produced is also better because more of the chemical components in the feedstock sample are broken down into liquid products ( Citation143,Citation144). Hence, different feedstock sources lead to different ratios in the chemical composition of the bio-oil. The properties of bio-oils produced by microwave and conventional pyrolysis are listed in . The bio-oil from microwave pyrolysis have higher carbon content and higher heating value and lower oxygen content ( Citation139,Citation145,Citation146). Microwave pyrolysis of biomass has demonstrated the potential to produce a unique grade of products owing to the unique thermal gradients that exist during processing ( Citation123,Citation126,Citation147–151). A number of reviews and scientific articles have discussed the use of microwaves to induce the production of higher quality bio-oils, mostly from the perspective of optimizing the involved operational conditions (e.g. microwave power, temperature, residence time or concentration of different microwave susceptor additives). Furthermore, it was reported by a researcher in 2007 that the properties and stability of bio-oils can be improved by blending methanol or ethanol into the bio-oils ( Citation145).
Table 8. Properties of bio-oils produced by microwave and conventional pyrolysis ( Citation142).
4.2.2. Bio-oil derived from microwave pyrolysis as boiler fuel
The renewable liquid fuel, bio-oil derived from pyrolysis processes, has great potential to replace or substitute fossil fuel to generate heat, power, and/or chemicals. Boilers and furnaces (including power stations) can be fueled with bio-oil. Alternatively, the crude bio-oil could serve as a raw material for the production of adhesives and phenol-formaldehyde-type resins. Upgrading of the bio-oil to a transportation fuel is technically feasible but needs further development. Transportation fuels, such as methanol and Fischer-Tropsch liquids, can be derived from bio-oil as gasifier feedstock, instead of the solid biomass (can save transportation cost). Furthermore, there is a wide range of chemicals that can be derived from bio-oil. The quality of the combustion is directly comparable to the properties of the fuel. Pyrolysis bio-oil is completely different from fossil fuels oils; its properties can vary greatly depending on the feedstock and process used in production. When compared to fossil fuel oils, the differences in combustion properties are mainly due to the significant differences in chemical composition of these fuels. In combustion applications, the physical and chemical properties of the bio-oil are responsible for the negative impacts on atomization quality, ignition, droplet vaporization and burning rate, clogging, coking tendency, and emissions ( Citation152). The usage of bio-oil to completely replace fossil fuels has some limitations, since bio-oil has bad properties, such as high viscosity, water content, poor volatility, coking, and corrosiveness. These limitations cause the primary challenge in the combustion process and industrial applications ( Citation153).
The current method of utilizing bio-oils in the boiler requires preheating of the bio-oil prior to combustion and after use, the engine may need to be flushed by diesel or methanol to prevent corrosion and scale formation ( Citation146). In addition, modifications of the combustion boiler system and operating conditions are required in order to improve combustion of bio-oil with fossil fuels, since some problems might take place during the usage of standard equipment constructed for firing fossil fuels. For instance, the existing burner must be replaced with a modified oil burner or a newly designed bio-oil burner (such as a burner head configuration) for firing bio-oil. In addition, the pumping, piping, oil preheating, and valve systems must also be specially designed for bio-oil ( Citation153). The experiment conducted to investigate the spray combustion characteristics of bio-oil produced from rice husk found that after attaining steady-state combustion, the temperature in the center of the combustion chamber exceeded 1400°C ( Citation154). The carbon monoxide (CO) emission concentration dropped, indicating that complete combustion was improved under operating conditions with higher oxygen concentrations. Meanwhile, the NOx concentration was slightly increased due to the higher temperature and the increased O2 concentration. Furthermore, the measured SOx concentration was very low (smaller than 30 ppm), since bio-oils contain insignificant amounts of sulfur ( Citation154).
One possible non-laborious option is modifying the bio-oil to accommodate the boiler requirements through blending with other biofuels/additives ( Citation155). Hence, a low blend ratio of bio-oil to petroleum-derived diesel (low bio-oil content) has advantages as it can be easily combusted in the existing industrial boilers without modification of the burner and boiler sections ( Citation153). Since the bio-oil from microwave pyrolysis has higher carbon content and higher heating value as well as lower oxygen content, the bio-oil produced from microwave pyrolysis is an attractive candidate for blending with different petroleum-based oils, which eventually will enhance the performance and emissions characteristics on the boiler application. Standard test methods have been used to evaluate the fuel properties and specification for selection of suitable fuels for boilers ( Citation156). highlights the important pyrolysis oil properties required for boiler application ( Citation156). The specifications differentiate between two grades of pyrolysis oil, grade G and grade D. Grade G is intended for use in industrial burners and not suitable for residential heaters, small commercial boilers, engines, or marine applications. Grade D is for commercial/industrial burners requiring lower solids and ash content and only suitable in residential heaters, engines, or marine applications if they have been modified to handle these types of fuel. Based on the previous studies on microwave-derived pyrolysis oil properties, such as the one mentioned previously in , it is obvious that the bio-oil can be blended with diesel at a low percentage (up to 20%) for application as boiler oil.
Table 9. Standard test for pyrolysis oil for boiler fuel application by ASTM D 7544 ( Citation156).
5. Net energy analysis of the bio-oil produced from pyrolysis of glycerol
Acetaldehyde, acetone, methanol, ethanol, water, and acetic acid were the major liquid products obtained during the pyrolysis of glycerol as stated in . Acrolein and unreacted glycerol were also found in the liquid product for the pyrolysis runs at 400–500°C (
Citation157). Acetaldehyde, methanol, and acrolein could have been formed by the radical mechanism as reported by Büuhler et al. (
Citation157) and Antal et al. (
Citation158). Energy balance calculations for bio-oil production from the pyrolysis of glycerol are given as follows, which is based on the thermal cracking of glycerol reaction in the absence of water.
Table 10. Standard enthalpies of formation for major liquid components’ presence in the bio-oil during the pyrolysis of glycerol.
Specific heat capacity, the heat of formation, and standard enthalpy of formation of components such as glycerol, water, and acetaldehyde and others were obtained from Chemical Properties Handbook ( Citation159).
Feed at 25°C and Product at 400°C
Basis: 6 mol of glycerol in feed
Boiling temperature of glycerol is 290°C
The energy required to take liquid glycerol from 25°C boiling temperature (290°C):
Heat of vaporization of glycerol at 290°C is 66.13 kJ/mol
Energy required to vaporize the liquid glycerol at 290°C Energy required to take glycerol vapor from 290°C to 400°C
The total energy required for the feed at reaction temperature:
Calculation for the enthalpies of formation of the reaction (details are given in )
Table 11. The details of calculation for enthalpy of formation for the reactants and products.
By assuming the heat capacity for each product is constant from 25°C to 400°C,
Energy required to raise the product temperature from 25°C to 400°C, Q4 = 550.98 kJ ()
Total energy required for the reaction:
Energy output:
Taking the average heating value of bio-oil as 17 MJ/kg (
Citation142)
The molar mass of the bio-oil is assumed to be 350 g/mol (adopted from Järvik and Oja ( Citation160))
17,000 kJ/kg × 0.35 kg/mol = 5950 kJ/mol
By assuming 100% selectivity for bio-oil production and that the bio-oil product is equal to 1 mole, the total heating value of bio-oil = 5950 kJ/mol × 1 = 5950 kJ
The net energy gain is
6. Glycerol prices and production cost of bio-glycerol based polymer
Looking at the glycerol market worldwide, there has been a rapid increase in glycerol supply since 2003. The prices of both refined and crude glycerol have been on the decline due to a surplus of glycerol from biodiesel. The market surplus of glycerol from biodiesel is far from being tackled by new demand as platform chemical. Recent market analysis projects that demand glycerin by-product of oleochemicals and biodiesel production will expand at an annualized average rate of 7% during 2007–2021, with 6 million tons of overall production in 2025 ( Citation161). There are several studies on the techno-economic analysis of biomass fast pyrolysis for bio-oil production available in the literature. These studies have reported that bio-oil costs can range from US$0.62/gal to US$1.40/gal and the capital costs ranging from US$7.8 to US$143 million over a 240 MT/day to 1000 MT/day plant capacity ( Citation162). In terms of usage of glycerol for production of bio-based products, the versatile chemical acrolein is one of the most important. Acrolein is one of the very useful intermediates in the chemical industry due to its wide utilization of acrylic acid, superabsorbent polymer, 1,3-propanediol, and many more polymers or polyesters’ production ( Citation163). An analysis of the cost of production of acrolein from bio-glycerol (both pure and crude) will give a better understanding of the implication of utilization of waste glycerol for value-added products to the biodiesel industry. shows the several major components in the cost that differ greatly between propylene-based and bio-based methods for a 10,000-ton acrolein production. The quantities for steam only refer to what is used in the reaction. In addition, the energy listed in the table only refers to the part of the energy required to heat up the reactant(s) and required for the reaction (assuming the energy consumption of all the other processes are the same for both production methods). The quantities of the feedstocks required for both processes are calculated based on the stoichiometric relation under the assumption of 80 mol% acrolein yield, and the quantity calculated for crude glycerol was based on the glycerol purity of 80%.
Table 12. Comparison of propylene-based and bio-based acrolein production (10,000 ton/year) regarding feedstock and energy consumption.
In terms of the last two variations in the cost in , the bio-based method of producing acrolein using pure glycerol costs around USD 12.11–12.73 million (per 10,000 ton per year), which is about 27% less than the cost of acrolein production using the propylene-based method. Meanwhile, the bio-based method using crude glycerol for the production of acrolein costs around USD 7.88–8.52 million (per 10,000 tons per year), which is 47.0–50.8% lesser than that of the propylene-based method. Based on the calculation made, at the current developmental stage of gas-phase glycerol dehydration, the acrolein production price provided by the bio-based route using pure glycerol as the feedstock is much lower and significantly reduced (47.0–50.8% reduction) if the production begins with crude glycerol. The comparison of the cost provided for acrolein synthesis suggests that usage of crude glycerol could also bring about cost reduction and reduction in usage of petroleum-based raw materials. Even the production from pure glycerol may be worthwhile, because it is renewable and sustainable, and yet it does not increase the price much. The comparative cost analysis presented here will definitely be very valuable and meaningful in directing the industrial processes and future research thrusts involving bio-based product utilizing crude glycerol.
7. The state of the art in microwave-assisted pyrolysis
The overall prospect of pyrolysis technology is promising because it is already a proven concept. Pyrolysis offers more scope for recovering products from agricultural waste or biodiesel production waste than simply burning it. When agricultural or waste glycerol residues are burnt directly in a furnace/boiler, the only practical product is heat; however, when they are pyrolyzed first, bio-oil, syn-gases, and bio-char are produced. These pyrolysis products not only can be used as a fuel but also can be purified and used as a feedstock for petrochemicals and other applications. A significant number of researches have directed increasing activities on the fixed bed production technology for bio-oil from biomass. However, these systems are unlikely to give high liquid yields but are likely to give phase separated liquids ( Citation70). Microwave pyrolysis, which is a much newer process, has gained plenty of attention and its prospects are very optimistic. Hence, microwave-assisted pyrolysis can offer both technical and financial benefits. A number of works have been performed for economic evaluation of microwave pyrolysis of biomass to determine its viability. A commercial size bioenergy production was recently suggested, whereby a distributed bioenergy production strategy was proposed for the implementation on average-size farms to pyrolyze crop residues by exploiting the scalable fast microwave pyrolysis technology ( Citation169). The energy balance involved in the microwave pyrolysis of biomass is also an important issue. There are several reasons for the application of the use of microwave pyrolysis; first is the only little amount of energy needed to generate a high amount of heat and second is the efficiency of microwave heating during pyrolysis ( Citation70). There is little energy wasted and the majority of the dipole effect generated by the microwave is concentrated directly into the sample. Nevertheless, very few reports regarding energy input and output from microwave pyrolysis of waste material have been reported. One paper discussed the benefits of microwaves on pyrolysis of straw feedstock ( Citation170). The minimum microwave power was found to be about 0.371 kW (per kg straw) and the ratio of heat loss and conversion loss of electricity to microwave energy occupied in the total input energy was 42% (balancing against the energy content of the char, bio-oil, and gases). From the results presented in such a study, it can be derived that the energy efficiency of microwave pyrolysis of wheat straw is 79.8% (ratio between biofuel energy and the sum of energy required for pyrolysis plus energy content of biomass). In another report, the energy recovery from the raw biomass resulted in 91%, which is much higher compared to the energy recovery from similar biomass feedstock by the means of conventional flash pyrolysis technologies (35–39%) ( Citation171). Similarly, in a pyrolysis study at 500°C, using coffee hull as feed stock, the energy recovery from conventional pyrolysis was 84%, whereas it increased up to 99% using microwave technology ( Citation139). summarizes the state of the art in microwave-assisted pyrolysis in comparison with different reactor types.
Table 13. Advantages, disadvantages, and bio-oil yield from different pyrolysis reactors ( Citation100).
So far, there have been lesser research reported on the microwave pyrolysis of waste materials compared to microwave pyrolysis of other non-waste feedstocks. It has been reported that current microwave pyrolysis techniques offer a number of advantages and show excellent potential for treating waste materials. However, the current review still shows that there are gaps to be filled in order to fully exploit the advantages of using the microwave pyrolysis process in the treatment of waste materials, especially waste glycerol. The focus of using microwave pyrolysis is to provide an alternative pyrolysis process by making use of the high temperatures that the carbonaceous material can achieve when subjected to a microwave field. This alternative way of heating is reported to have advantages over other conventional pyrolysis processes on account of better heat transfer to the waste materials, good control over the heating process, as well as offering a sustainable processing route. However, in view of mainly positive findings reported in the literature on the microwave pyrolysis studies, it would be worthwhile to carry on researching further aspects of microwave pyrolysis of waste materials, especially waste glycerol from the biodiesel process in order to explore its full potential. The optimization of this process and the subsequent scale-up to a commercial scale is the next level of progression, and hence better understanding of the microwave technology knowledge is crucial.
8. Conclusions
Pyrolysis of agricultural residues and waste glycerol from the biodiesel industry can help to meet renewable energy targets by displacing fossil fuels and, thereby, deal with concerns about global warming. Besides the use of bio-oil and syn-gases, the other pyrolysis product, which is bio-char, can also be used for soil amendment and as a carbon-sequestering agent. With the growing production of biodiesel in the coming years, managing crude glycerol produced will become an increasingly difficult task. Although the development of glycerol-free biodiesel production is making progress significantly, implementation of these processes at a large scale still faces a number of challenges such as high costs, low efficiency, and lack of relevant technologies. It is crucial to utilize this waste stream generated from biodiesel production efficiently. Most of the current methods of utilization of crude glycerol are only able to uptake small volumes of the waste glycerol. Furthermore, the real costs of its utilization are uncertain. Pyrolysis of glycerol is a viable process for clean energy production. Liquid bio-oils, produced from the pyrolysis process, are a promising route to utilize large quantities of the waste glycerol. However, several key technical barriers must be addressed – (a) optimization of process conditions and catalyst performance to maximize bio-oil yield and quality while reducing the impact of feedstock variability and impurities; (b) improving the thermal stability of bio-oil and impurities be removed to facilitate economical upgrading to biofuels, and (c) maximizing carbon efficiency during bio-oil deoxygenation. One of the promising technologies for enhancement of bio-oil quality and quantity is by using the microwave-assisted pyrolysis process. Only few literatures are available on the microwave-assisted pyrolysis of waste glycerol. A more detailed study on the production of bio-oil from waste glycerol by microwave-assisted pyrolysis is needed in order to have a better understanding of the process parameters. Further research and development on microwave-assisted pyrolysis should focus on: (i) the types of microwave absorbents since it is necessary to achieve desired temperatures, (ii) improving catalyst selectivity, (iii) optimizing reaction conditions such as flow rate of inert gas to improve yield, (iv) study of the reaction kinetics of the overall process, and (v) study on the mechanism for microwave-assisted crude glycerol pyrolysis. The raw glycerol may also present some difficulties in feeding since it is liquid. For this reason, further studies must be done to reduce the water content in order to obtain a smaller concentrated volume of waste glycerol. The concentrated waste could be blended with a small portion of sawdust or similar waste to make the glycerol into a semi-solid paste. Finally, it can be concluded that the utilization of waste glycerol into higher value products through pyrolysis can potentially improve the issue of excess glycerol within the biodiesel industry. More work is needed to extend the existing understanding of the microwave technology for pyrolysis in order to improve the process and ultimately to transform it into a commercially viable route to recover energy from waste materials.
Acknowledgements
The authors would like to thank the University Tenaga Nasional (UNITEN) for the research facilities. SN and RH conceived and designed the study, and formatted the article. All the authors gathered the data. SN and RH wrote the main manuscript text and performed the discussions. SN checked, edited, and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Prof. Dr. Saifuddin Nomanbhay, In the last 18 years at UNITEN, he has been actively involved in the research the field of microwave assisted reactions, biocatalyst immobilization and biodiesel/bioethanol production. He is currently the principal researcher at the Institute for Sustainable Energy (ISE) of UNITEN. His research now is more focus on renewable resources and enabling technologies. He has substantial volume of refereed research publications (more than 53 journal papers and 13 conference papers), and a book.
Refal Hussein, a graduate from Universiti Tenaga Nasional (UNITEN) with PhD. in Mechanical Engineering. She has two years working experience as a graduate research assistance in Institute of Sustainable Energy (UNITEN) where she is currently in as a postdoctoral researcher fellow.
Mei Yin Ong graduated on March 2016 with Bachelor of Mechanical Engineering (Hons.) at UNITEN where she continued her master study in Mechanical Engineering on November 2016. Currently, she is working as graduate research officer in Institute of Sustainable Energy (UNITEN).
Additional information
Funding
References
- Environmental Protection Agency (EPA). Inventory of U.S. Greenhouse Gas Emissions and Sinks, 1990–2015. https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2015 (accessed 2017).
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. doi: 10.1038/nature11475
- Budzianowski, W.M.; Postawa, K. Renewable Energy from Biogas with Reduced Carbon Dioxide Footprint: Implications of Applying Different Plant Configurations and Operating Pressures. Renew. Sust. Energ. Rev. 2017, 68, 852–868. doi: 10.1016/j.rser.2016.05.076
- Soltanieh, A.A.; Rezaeian, M. Nuclear and Renewable Energy in Iran: Nuclear Challenges and Opportunities. Int. J.Hydrog. Energy 2017, xx, 1–5.
- Demirbas, A. Waste Energy for Life Cycle Assessment, Chapter 2; Future Energy Sources, Green Energy and Technology;Springer International Publishing: Switzerland, 2016, doi:10.1007/978-3-319-40551-3_2.
- Bagheri, S., Julkapli, N.M., Dabdawb, W.A.Y., Mansouri, N. Biodiesel-Derived Raw Glycerol to Value-added Products: Catalytic Conversion Approach. In Handbook of Composites from Renewable Materials, Physico-Chemical and Mechanical Characterization, 3rd ed.;Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.;John Wiley: Hoboken, NJ, 2017, pp 309–366.
- Hajjari, M.; Ardjmand, M.; Tabatabaei, M. Experimental Investigation of the Effect of Cerium Oxide Nanoparticles as a Combustion-improving Additive on Biodiesel Oxidative Stability: Mechanism. Roy. Soc. Chem. Adv. 2014, 4 (28), 14352–14356.
- Tabatabaei, M.; Karimi, K.; Kumar, R.; Horváth, I.S. Renewable Energy and Alternative Fuel Technologies. BioMed Res. Int. 2015, 2015, 1–2. doi: 10.1155/2015/245935
- Johari, A.; Nyakuma, B.B.; Mohd Nor, S.H.; Mat, R.; Hashim, H.; Ahmad, A.; Yamani Zakaria, Z.; Tuan Abdullah, T.A. The Challenges and Prospects of Palm Oil Based Biodiesel in Malaysia. Energy 2015, 81, 255–261. doi: 10.1016/j.energy.2014.12.037
- Wahab, A.G. Malaysia-Biofuels;USDA Foreign Agricultural Service, United States Department of Agriculture;Kuala Lumpur, 2012.
- Ilham, Z.; Saka, S. Esterification of Glycerol from Biodiesel Production to Glycerol Carbonate in Non-Catalytic Supercritical Dimethyl Carbonate. Springer Plus 2016, 5, 466. doi: 10.1186/s40064-016-2643-1
- Garlapati, V.K.; Shankar, U.; Budhiraja, A. Bioconversion Technologies of Crude Glycerol to Value Added Industrial Products. Biotechnol. Rep. 2016, 9, 9–14. doi: 10.1016/j.btre.2015.11.002
- Anuar, M.R.; Abdullah, A.Z. Challenges in Biodiesel Industry with Regards to Feedstock, Environmental, Social and Sustainability Issues: A Critical Review. Renew. Sust. Energ. Rev. 2016, 58, 208–223. doi: 10.1016/j.rser.2015.12.296
- Food and Agriculture Organization of the United Nations (OECD), OECD-FAO Agricultural Outlook; OECD: Paris, 2015.
- Nanda, M.R.; Yuan, Z.; Qin, W.; Poirier, M.A.; Chunbao, X. Purification of Crude Glycerol using Acidification: Effects of Acid Types and Product Characterization. Austin J. Chem. Eng. 2014, 1 (1), 1–7.
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The Potential of Glycerol as a Value-Added Commodity. Chem. Eng. J. 2016, 295, 119–130. doi: 10.1016/j.cej.2016.03.012
- Konstantinović, S.S.; Danilović, B.R.; Ćirić, J.T.; Ilić, S.B.; Savić, D.S.; Veljković, V.B. Valorization of Crude Glycerol from Biodiesel Production. Chem. Ind. Chem. Eng. Q. 2016, 22 (4), 461–489. doi: 10.2298/CICEQ160303019K
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis -A Technological Review. Energies 2012, 5, 4952–5001. doi: 10.3390/en5124952
- Kusuma, H.S.; Mahfud, M. Preliminary Study: Kinetics of Oil Extraction from Basil (Ocimum Basilicum) by Microwave-Assisted Hydrodistillation and Solvent-Free Microwave Extraction. S. Afr. J. Chem. Eng. 2016, 21, 49–53.
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Palm Oil Empty Fruit Bunch Based Magnetic Biochar Composite Comparison for Synthesis by Microwave-Assisted and Conventional Heating. J. Anal. Appl. Pyrolysis 2016, 120, 521–528. doi: 10.1016/j.jaap.2016.06.026
- Ghasali, E.; Yazdani-rad, R.; Asadian, K.; Ebadzadeh, T. Production of Al-SiC-TiC Hybrid Composites Using Pure and 1056 Aluminum Powders Prepared through Microwave and Conventional Heating Methods. J. Alloys Comp. 2017, 690, 512–518. doi: 10.1016/j.jallcom.2016.08.145
- Pradima, J.; Kulkarni, M.R. Review on Enzymatic Synthesis of Value Added Products of Glycerol, a By-Product Derived from Biodiesel Production. Res.-Efficient Technol. 2017. doi.org/10.1016/j.reffit.2017.02.009.
- Torres, J.J.; Rodriguez, N.E.; Arana, J.T.; Ochoa, N.A.; Marchese, J.; Pagliero, C. Ultrafiltration Polymeric Membranes for the Purification of Biodiesel from Ethanol. J. Cleaner Prod. 2017, 141, 641–647. doi: 10.1016/j.jclepro.2016.09.130
- Motasemi, F.; Afzal, M.T. A Review on the Microwave-Assisted Pyrolysis Technique. Renewable Sustainable Energy Rev. 2013, 28, 317–330. doi: 10.1016/j.rser.2013.08.008
- Shah, P.; Chiu, F.; Lan, J.C. Aerobic Utilization of Crude Glycerol by Recombinant Escherichia Coli for Simultaneous Production of Poly 3-Hydroxy-Butyrate and Bioethanol. Journal of Bioscience and Bioengineering 2014, 117 (3), 343–350. doi: 10.1016/j.jbiosc.2013.08.018
- Plácido, J.; Capareda, S. Conversion of Residues and By-Products from the Biodiesel Industry into Value-Added Products. Bioresources and Bioprocessing 2016, 3, 733.
- Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum. Fermentation 2016, 2, 13–13. doi: 10.3390/fermentation2020013
- Caetano de Souza, A.C.; Silveira, J.L. Hydrogen Production Utilizing Glycerol from Renewable Feedstocks-the Case of Brazil. Renew. Sust. Energ. Rev. 2011, 15 (4), 1835–1850. doi: 10.1016/j.rser.2010.12.001
- Menezes, A.O.; Rodrigues, M.T.; Zimmaro, A.; Borges, L.E.P.; Fraga, M.A. Production of Renewable Hydrogen from Aqueous-Phase Reforming of Glycerol over Pt Catalysts Supported on Different Oxides. Renew. Energ. 2011, 36 (2), 595–599. doi: 10.1016/j.renene.2010.08.004
- Tuza, P.; Manfro, R.; Ribeiro, N.; Souza, M. Production of Renewable Hydrogen by Aqueous-Phase Reforming of Glycerol over Ni–Cu Catalysts Derived from Hydrotalcite Precursors. Renew. Energ. 2013, 50, 408–414. doi: 10.1016/j.renene.2012.07.006
- Wang, C.; Dou, B.; Chen, H.; Song, Y.; Xu, Y.; Du, X.Zhang, L.; Luo, T.; Tan, C. Renewable Hydrogen Production from Steam Reforming of Glycerol by Ni–Cu–Al, Ni–Cu–Mg, Ni–Mg Catalysts. Int. J. Energ. 2013, 38 (9), 3562–3571.
- Kamonsuangkasem, K.; Therdthianwong, S.; Therdthianwong, A. Hydrogen Production from Yellow Glycerol via Catalytic Oxidative Steam Reforming. Fuel Process Technol. 2013, 106, 695–703. doi: 10.1016/j.fuproc.2012.10.003
- Gutiérrez Ortiz, F. J.; Ollero, P.; Serrera, A.; Galera, S. Optimization of Power and Hydrogen Production from Glycerol by Supercritical Water Reforming. Chem. Eng. J. 2013, 218, 309–318. doi: 10.1016/j.cej.2012.12.035
- Reungsang, A.; Sittijunda, S.; O-thong, S. Bio-Hydrogen Production from Glycerol by Immobilized Enterobacter Aerogenes ATCC 13048 on Heat-Treated UASB Granules as Affected by Organic Loading Rate. Int. J. Hydrog. Energy. 2013, 38 (17), 6970–6979. doi: 10.1016/j.ijhydene.2013.03.082
- Lin, Y. Catalytic Valorization of Glycerol to Hydrogen and Syngas. Int. J. Hydrog. Energy. 2013, 38 (6), 2678–700. doi: 10.1016/j.ijhydene.2012.12.079
- Beatrice, C.; Di Blasio, G.; Lazzaro, M.; Cannilla, C.; Bonura, G.; Frusteri, F.; Asdrubali, F.; Baldinelli, G.; Presciutti, A.; Fantozzi, F.; Bidini, G.; Bartocci, P. Technologies for Energetic Exploitation of Biodiesel Chain Derived Glycerol: Oxy-Fuels Production by Catalytic Conversion. Appl. Energy. 2013, 102, 63–71. doi: 10.1016/j.apenergy.2012.08.006
- Kiatkittipong, W.; Suwanmanee, S.; Laosiripojana, N.; Praserthdam, P.; Assabumrungrat, S. Cleaner Gasoline Production by Using Glycerol as Fuel Extender. Fuel Process. Technol. 2010, 91 (5), 456–460. doi: 10.1016/j.fuproc.2009.12.004
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent Progress on Innovative and Potential Technologies for Glycerol Transformation into Fuel Additives: A Critical Review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. doi: 10.1016/j.rser.2009.11.010
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Esterification of Glycerol with Acetic Acid over Dodecamolybdophosphoric Acid Encaged in USY Zeolite. Catal. Commun. 2009, 10, 481–484. doi: 10.1016/j.catcom.2008.10.015
- Gutiérrez Ortiz, F.J.; Serrera, A.; Galera, S.; Ollero, P. Methanol Synthesis from Syngas Obtained by Supercritical Water Reforming of Glycerol. Fuel. 2013, 105, 739–751. doi: 10.1016/j.fuel.2012.09.073
- Tsang, S.C.E.; Oduru, W.O.; Redman, D.J. European Patent Application. Methanol Production Process, World Intellectual Property Organization: Geneva, Switzerland, 2009. WO130452A1.
- Duan, S.; Chen, L.; Su, F.; Lin, J.; He, C.; Weckbecker, C. European patent Application. Oxygenated Hydrocarbon Reforming, World Intellectual Property Organization: Geneva, Switzerland, 2010. WO104467A1.
- Posada, J.A.; Cardona, C.A. Design and Analysis of Fuel Ethanol Production from Raw Glycerol. Energy. 2010, 35 (12), 5286–5293. doi: 10.1016/j.energy.2010.07.036
- Oh, B.R.; Seo, J.W.; Heo, S.Y.; Hong, W.K.; Luo, L.H.; Joe, M.H.; Park, D.-H.; Kim, C.H. Efficient Production of Ethanol from Crude Glycerol by a Klebsiella pneumoniae Mutant Strain. Bioresour. Technol. 2011, 102 (4), 3918–3922. doi: 10.1016/j.biortech.2010.12.007
- Carvalho, E.R.; Schmelz-Roberts, N.S.; White, H.M.; Doane, P.H.; Donkin, S.S. Replacing Corn with Glycerol in Diets for Transition Dairy Cows. J. Dairy Sci. 2011, 94 (2), 908–916. doi: 10.3168/jds.2010-3581
- Walter Borges de Oliveira, S.V; Leoneti, A.B.; Magrini Caldo, G.M.; Borges de Oliveira, M.M. Generation of Bioenergy and Biofertilizer on a Sustainable Rural Property. Biomass Bioenerg. 2011, 35 (7), 2608–2618. doi: 10.1016/j.biombioe.2011.02.048
- Schieck, S.J.; Shurson, G.C.; Kerr, B.J.; Johnston, L.J. Evaluation of Glycerol, a Biodiesel Co-Product, in Grow-Finish Pig Diets to Support Growth and Pork Quality. J. Anim. Sci. 2010, 88, 3927–3935. doi: 10.2527/jas.2010-2858
- Li, M.H.; Minchew, C.D.; Oberle, D.F.; Robinson, E.H. Evaluation of Glycerol from Biodiesel Production as a Feed Ingredient for Channel Catfish, Ictalurus Punctatus. J. World Aquaculture Society 2010, 41, 1.
- Wang, C.; Liu, Q.; Huo, W.J.; Yang, W.Z.; Dong, K.H.; Huang, Y.X. Effects of Glycerol on Rumen Fermentation, Urinary Excretion of Purine Derivatives and Feed Digestibility in Steers. Animal Feed Sci. Technol. 2009, 121, 15–20.
- Irieb. Vegetable glycerine, 2009. http://www.vaporden.com/notes/Vegetable Glycerin (accessed Dec 26, 2012).
- Ning, L.; Ding, Y.; Chen, W.; Gong, L.; Lin, R.; Yuan, L.; Xin, Q. Glycerol Dehydration to Acrolein over Activated Carbon-Supported Silicotungstic Acids. Chin. J. Catal. 2008, 29, 212–214. doi: 10.1016/S1872-2067(08)60026-1
- Rosa, D.S.; Bardi, M.A.G.; Machado, L.D.B.; Dias, D.B.; Silva, L.G.A.; Kodama, Y. Starch Plasticized with Glycerol from Biodiesel and Polypropylene Blends: Mechanical and Thermal Properties. J. Therm. Anal. Calorim. 2010, 102, 181–186. doi: 10.1007/s10973-010-0828-3
- Lee, S.H.; Park, D.R.; Kim, H.; Lee, J.; Jung, J.C.; Woo, S.Y.; Song, W.S.; Kwon, M.S.; Song, I.K. Direct Preparation of Dichloropropanol (DCP) from Glycerol using Heteropolyacid (HPA) Catalysts: A Catalyst Screen Study. Catal. Commun. 2008, 9, 1920–1923. doi: 10.1016/j.catcom.2008.03.020
- Pollington, S.D.; Enache, D.I.; Landon, P.; Meenakshisundaram, S.; Dimitratos, N.; Wagland, A.; Hutchings, G.J.; Stitt, E.H. Enhanced Selective Glycerol Oxidation in Multiphase Structured Reactors. Catal. Today. 2009, 145, 169–175. doi: 10.1016/j.cattod.2008.04.020
- Chi, Z.; Pyle, D.; Wen, Z.; Frear, C.; Chen, S. A Laboratory Study of Producing Docosahexaenoic Acid from Biodiesel-Waste Glycerol by Microalgal Fermentation. Process Biochem. 2007, 42, 1537–1545. doi: 10.1016/j.procbio.2007.08.008
- Skoulou, V.; Zabaniotou, A. Co-Gasification of Crude Glycerol with Lignocellulosic Biomass for Enhanced Syngas Production. J. Anal. Appl. Pyrolysis. 2013, 99, 110–116. doi: 10.1016/j.jaap.2012.10.015
- Robra, S.; Serpa da cruz, R.; de Oliveira, A.M.; Neto, J.A.A.; Santos, J.V. Generation of Biogas using Crude Glycerine from Biodiesel Production as a Supplement to Cattle Slurry. Biomass Bioenerg. 2010, 34 (9), 1330–1335. doi: 10.1016/j.biombioe.2010.04.021
- Siles, J.A.; Martín, M.A.; Chica, A.F.; Martín, A. Anaerobic Co-Digestion of Glycerol and Wastewater Derived from Biodiesel Manufacturing. Bioresour. Technol. 2010, 101 (16), 6315–6321. doi: 10.1016/j.biortech.2010.03.042
- Fountoulakis, M.S.; Manios, T. Enhanced Methane and Hydrogen Production from Municipal Solid Waste and Agro-industrial By-products Co-Digested with Crude Glycerol. Bioresour. Technol. 2009, 100 (12), 3043–3047. doi: 10.1016/j.biortech.2009.01.016
- Mizielińska, M.; Kowalska, U.; Łabuda, M.; Furgała, J.; Bartkowiak, A. Fed-Batch Bioconversion of Glycerol to 1,3-PD by Using Immobilized Citrobacter freundii Cells. J. Biotechnol. Biomater. 2015, 5, 3.
- Nomanbhay, S.M.; Hussain, R. Immobilization of Escherichia Coli Mutant Strain for Efficient Production of Bioethanol from Crude Glycerol. Journal of Applied Science 2015, 15 (3), 415–430. doi: 10.3923/jas.2015.415.430
- Đurišić-Mladenović, N.; Škrbić, B.D.; Zabaniotou, A. Chemometric Interpretation of Different Biomass Gasification Processes Based on the Syngas Quality: Assessment of Crude Glycerol Co-Gasification with Lignocellulosic Biomass. Renew. Sust. Energ Rev. 2016, 59, 649–661. doi: 10.1016/j.rser.2016.01.002
- Dianningrum, L.W.; Choi, H.; Kim, Y.; Jung, K.-D.; Susanti, R.F.; Kim, J.; Sang, B.I. Hydrothermal Gasification of Pure and Crude Glycerol in Supercritical Water: A Comparative Study. Int. J. Hydrogen Energy. 2014, 39, 1262–1273. doi: 10.1016/j.ijhydene.2013.10.139
- Yang, F.; Hanna, M.A.; Marx, D.B.; Sun, R. Optimization of Hydrogen Production from Supercritical water Gasification of Crude Glycerol-Byproduct of Biodiesel Production. Int. J. Energy Res. 2013, 37, 1600–1609. doi: 10.1002/er.2969
- Wei, L.; Pordesimo, L.O.; Haryanto, A.; Wooten, J. Co-Gasification of Hardwood Chips and Crude Glycerol in a Pilot Scale Downdraft Gasifier. Bioresources Technol. 2011, 102, 6266–6272. doi: 10.1016/j.biortech.2011.02.109
- Valliyappan, T.; Ferdous, D.; Bakhshi, N.N.; Dalai, A.K. Production of Hydrogen and Syngas via Steam Gasification of Glycerol in a Fixed-Bed Reactor. Topics Catal. 2008, 49 (1–2), 59–67. doi: 10.1007/s11244-008-9062-7
- Cengiz, NÜ; Yıldız, G.; Sert, M.; Selvi Gökkaya, D.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal Gasification of a Biodiesel by-Product Crude Glycerol in the Presence of Phosphate Based Catalysts. Int. J. Hydrogen Energy. 2015, 40 (43), 14806–14815. doi: 10.1016/j.ijhydene.2015.08.097
- Yoon, S.J.; Choi, Y.C.; Son, Y.I.; Lee, S.H.; Lee, J.G. Gasification of Biodiesel By-Product with Air or Oxygen to make Syngas. Bioresour. Technol. 2010, 101 (4), 1227–1232. doi: 10.1016/j.biortech.2009.09.039
- Yoon, S.-J.; Yun, Y.-M.; Seo, M.-W.; Kim, Y.-K.; Ra, H.-W.; Lee, J.-G. Hydrogen and Syngas Production from Glycerol through Microwave Plasma Gasification. Int. J. Hydrogen Energy. 2013, 38, 14559–14567. doi: 10.1016/j.ijhydene.2013.09.001
- Marcilla, A.; Catalá, L.; García-Quesada, J.C.; Valdés, F.J.; Hernández, M.R. A Review of Thermochemical Conversion of Microalgae. Renew. Sust. Energ. Rev. 2013, 27, 11–19. doi: 10.1016/j.rser.2013.06.032
- Dou, B.; Dupont, V.; Williams, P.T.; Chen, H.; Ding, Y. Thermogravimetric Kinetics of Crude Glycerol. Bioresour. Technol. 2009, 100, 2613–2620. doi: 10.1016/j.biortech.2008.11.037
- Ben Hassen-Trabelsi, A.; Kraiem, T.; Naoui, S.; Belayouni, H. Pyrolysis of Waste Animal Fats in a Fixed-Bed Reactor: Production and Characterization of Bio-oil and Bio-char. Waste Manage. 2014, 34, 210–218. doi: 10.1016/j.wasman.2013.09.019
- Jimenez-Cordero, D.; Heras, F.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J. Porous Structure and Morphology of Granular Chars from Flash and Conventional Pyrolysis of Grape Seeds. Biomass Bioenergy 2013, 54, 123–132. doi: 10.1016/j.biombioe.2013.03.020
- Delgado, R.; Rosas, J.G.; Gómez, N.; Martínez, O.; Sanchez, M.E.; Cara, J. Energy Valorisation of Crude Glycerol and Corn Straw by Means of Slow Co-Pyrolysis: Production and Characterisation of Gas, Char and Bio-Oil. Fuel. 2013, 112, 31–37. doi: 10.1016/j.fuel.2013.05.005
- Manara, P.; Zabaniotou, A. Co-Pyrolysis of Biodiesel-Derived Glycerol with Greek Lignite: A Laboratory Study. J. Anal. Appl. Pyrolysis. 2013, 100, 166–172. doi: 10.1016/j.jaap.2012.12.013
- Skoulou, V.K.; Manara, P.; Zabaniotou, A.A. H2 Enriched Fuels from Co-Pyrolysis of Crude Glycerol with Biomass. J. Anal. Appl. Pyrolysis 2012, 97, 198–204. doi: 10.1016/j.jaap.2012.05.011
- Xiu, S.; Shahbazi, A.; Shirley, V.; Mims, M.R.; Wallace, C.W. Effectiveness and Mechanisms of Crude Glycerol on the Biofuel Production from Swine Manure through Hydrothermal Pyrolysis. J. Anal. Appl. Pyrolysis 2010, 87, 194–198. doi: 10.1016/j.jaap.2009.12.002
- Ganesapillai, M.; Manara, P.; Zabaniotou, A. Effect of Microwave Pretreatment on Pyrolysis of Crude Glycerol–Olive Kernel Alternative Fuels. Energy Convers. Manage. 2016, 110, 287–295. doi: 10.1016/j.enconman.2015.12.045
- Xiu, S.; Shahbazi, A. Bio-Oil Production and Upgrading Research: A Review. Renewable Sustainable Energy Rev. 2012, 16, 4406–4414. doi: 10.1016/j.rser.2012.04.028
- Dickerson, T.; Soria, J. Catalytic Fast Pyrolysis: A Review. Energies. 2013, 6, 514–538. doi: 10.3390/en6010514
- Duan, P.; Savage, P.E. Upgrading of Crude Algal Bio-Oil in Supercritical Water. Bioresour. Technol. 2011, 102 (2), 1899–1906. doi: 10.1016/j.biortech.2010.08.013
- Gunawan, R.; Li, X.; Lievens, C.; Gholizadeh, M.; Chaiwat, W.; Hu, X.; Mourant, D.; Bromly, J.; Li, C.-Z. Upgrading of Bio-oil into Advanced Biofuels and Chemicals. Part I. Transformation of GC-Detectable Light Species during the Hydrotreatment of Bio-Oil Using Pd/C Catalyst. Fuel. 2013, 111, 709–717. doi: 10.1016/j.fuel.2013.04.002
- Zhang, X.; Zhang, Q.; Wang, T.; Li, B.; Xu, Y.; Ma, L. Efficient Upgrading Process for Production of Low Quality Fuel from Bio-oil. Fuel. 2016, 179, 312–321. doi: 10.1016/j.fuel.2016.03.103
- Umeki, E.R.; de Oliveira, C.F.; Torres, R.B.; Santos, R.G.D. Physico-Chemistry Properties of Fuel Blends Composed of Diesel and Tire Pyrolysis oil. Fuel. 2016, 185, 236–242. doi: 10.1016/j.fuel.2016.07.092
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric Parameters Relevant to Microwave Dielectric Heating. Chem. Soc. Rev. 1998, 27, 213–224. doi: 10.1039/a827213z
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermudez, J.M. Microwave Heating Processes Involving Carbon Materials. Fuel Process. Technol. 2010, 91, 1–8. doi: 10.1016/j.fuproc.2009.08.021
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.D.; Kingman, S.W.; Miles, N.J. Microwave Heating Applications in Environmental Engineering: A Review. Resour. Conserv. Recycl. 2002, 34, 75–90. doi: 10.1016/S0921-3449(01)00088-X
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating strategies. Materials. 2016, 9, 231. doi: 10.3390/ma9040231
- Surat, M.A.; Jauhari, S.; Desak, K.R. A Brief Review: Microwave Assisted Organic Reaction. J. Appl. Sci. Res. 2012, 4, 645–661.
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M.; Sano, S.; Hayashi, Y.; Takizawa, H. In-Situ Kinetic Study on Non-Thermal Reduction Reaction of CuO During Microwave Heating. Matererals Lett. 2013, 91, 252–254. doi: 10.1016/j.matlet.2012.09.114
- Xu, W.; Zhou, J.; Su, Z.; Ou, Y.; You, Z. Microwave Catalytic Effect: A New Exact Reason for Microwave-Driven Heterogeneous Gas-Phase Catalytic Reactions. Catal. Sci. Technol. 2016, 6, 698–702. doi: 10.1039/C5CY01802A
- Zhou, J.; Xu, W.; You, Z.; Wang, Z.; Luo, Y.; Gao, L.; Yin, C.; Peng, R.; Lan, L. A New Type of Power Energy for Accelerating Chemical Reactions: The Nature of a Microwave-Driving Force for Accelerating Chemical Reactions. Scientific Reports. 2016, 6 (6), 279.
- Saifuddin, N.; Ong, M.Y. A Review of Microwave-assisted Reactions for Biodiesel Production. Bioengineering. 2017, 4 (2), 57. doi: 10.3390/bioengineering4020057
- Ingole, P.M.; Ranveer, A.C.; Deshmukh, S.M.; Deshmukh, S.K. Microwave Assisted Pyrolysis of Biomass: A review. Int. J. Adv. Technol. Eng. Sci. 2016, 4 (6), 78–84.
- Salema, A.A.; Ani, F.N. Pyrolysis of Oil Palm Empty Fruit Bunch Biomass Pellets Using Multimode Microwave Irradiation. Bioresour. Technol. 2012, 125, 102–107. doi: 10.1016/j.biortech.2012.08.002
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave Assisted Pyrolysis of Biomass: Catalysts to Improve Product Selectivity. J. Anal. Appl. Pyrolysis 2009, 86 (1), 161–167. doi: 10.1016/j.jaap.2009.05.006
- Kuan, W.-H.; Huang, Y.-F.; Chang, C.-C.; Lo, S.-L. Catalytic Pyrolysis of Sugarcane Bagasse by Using Microwave Heating. Bioresour. Technol. 2013, 146, 324–329. doi: 10.1016/j.biortech.2013.07.079
- Zhang, B.; Zhong, Z.; Chen, P.; Ruan, R. Microwave-Assisted Catalytic Fast Pyrolysis of Biomass for Bio-Oil Production Using Chemical Vapour Deposition Modified HZSM-5 Catalyst. Bioresour. Technol. 2015, 197, 79–84. doi: 10.1016/j.biortech.2015.08.063
- Menéndez, J.A.; Domínguez, A.; Fernandez, Y.; Pis, J.J. Evidence of Self-Gasification During the Microwave-Induced Pyrolysis of Coffee Hulls. Energy Fuels. 2007, 21, 373–378. doi: 10.1021/ef060331i
- Nomanbhay, S.; Salman, B.; Hussain, R.; Ong, M.Y. Microwave Pyrolysis of Lignocellulosic Biomass – a Contribution to Power Africa. Energy, Sustainability and Society. 2017, 7 (23), 1–24.
- Miura, M.; Kaga, H.; Sakurai, A.; Kakuchi, T.; Takahashi, K. Rapid Pyrolysis of Wood Block by Microwave Heating. J. Anal. Appl. Pyrolysis. 2004, 71, 187–199. doi: 10.1016/S0165-2370(03)00087-1
- Chen, M.Q.; Wang, J.; Zhang, M.X.; Chen, M.G.; Zhu, X.F.; Min, F.F.; Tan, Z.C. Catalytic Effects of Eight Inorganic Additives on Pyrolysis of Pine Wood Sawdust by Microwave Heating. J. Anal. Appl. Pyrolysis. 2008, 82, 145–150. doi: 10.1016/j.jaap.2008.03.001
- Wang, X.H.; Chen, H.P.; Luo, K.; Shao, J.G.; Yang, H.P. The Influence of Microwave Drying on Biomass Pyrolysis. Energy Fuels 2008, 22, 67–74. doi: 10.1021/ef700300m
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Total Recovery of Resources and Energy from Rice Straw using Microwave-induced Pyrolysis. Bioresour. Technol. 2008, 99, 8252–8258. doi: 10.1016/j.biortech.2008.03.026
- Budarin, V.L.; Clark, J.H.; Lanigan, B.A.; Shuttleworth, P.; Breeden, S.W.; Wilson, A.J.; Macquarrie, D.J.; Milkowski, K.; Jones, J.; Bridgeman, T.; Ross, A. The Preparation of High-Grade Bio-oils through the Controlled, Low Temperature Microwave Activation of Wheat Straw. Bioresour. Technol. 2009, 100 (23), 6064–6068. doi: 10.1016/j.biortech.2009.06.068
- Salema, A.A.; Ani, F.N. Microwave Induced Pyrolysis of Oil Palm Biomass. Bioresour. Technol. 2011, 102, 3388–3395. doi: 10.1016/j.biortech.2010.09.115
- Suriapparao, D.V.; Pradeep, N.; Vinu, R. Bio-oil Production from Prosopis Juliflora via Microwave Pyrolysis. Energy Fuels. 2015, 29 (4), 2571–2581. doi: 10.1021/acs.energyfuels.5b00357
- Shang, H.; Lu, R.-R.; Shang, L.; Zhang, W.-H. . Effect of Additives on the Microwave-Assisted Pyrolysis of Sawdust. Fuel Process. Technol. 2015, 131, 167–174. doi: 10.1016/j.fuproc.2014.11.025
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renewable Sustainable Energy Rev. 2016, 57, 1126–1140. doi: 10.1016/j.rser.2015.12.185
- Borges, F.C.; Du, Z.; Xie, Q.; Trierweiler, J.O.; Cheng, Y.; Wan, Y.; Liu, Y.; Zhu, R.; Lin, X.; Chen, P.; Ruan, R. Fast Microwave Assisted Pyrolysis of Biomass using Microwave Absorbent. Bioresour. Technol. 2014, 156, 267–274. 110. doi: 10.1016/j.biortech.2014.01.038
- Omar, R.; Idris, A.; Yunus, R.; Khalid, K.; Aida Isma, M.I. Characterization of Empty Fruit Bunch for Microwave-assisted Pyrolysis. Fuel 2011, 90 (4), 1536–1544. doi: 10.1016/j.fuel.2011.01.023
- Motasemi, F.; Ani, F.N. A Review on Microwave-assisted Production of Biodiesel. Renew. Sust. Energ. Rev. 2012, 16 (7), 4719–4733. doi: 10.1016/j.rser.2012.03.069
- Budarin, V.L.; Shuttleworth, P.S.; De bruyn, M.; Farmer, T.J.; Gronnow, M.J.; Pfaltzgraff, L.; Macquarrie, D.J.; Clark, J.H. The Potential of Microwave Technology for the Recovery, Synthesis and Manufacturing of Chemicals from Bio-Wastes. Catalysis Today. 2015, 239, 80–89. doi: 10.1016/j.cattod.2013.11.058
- Mamaeva, A.; Tahmasebi, A.; Tian, L.; Yu, J. Microwave-Assisted Catalytic Pyrolysis of Lignocellulosic Biomass for Production of Phenolic-Rich Bio-oil. Bioresour. Technol. 2016, 211, 382–389. doi: 10.1016/j.biortech.2016.03.120
- Mohamed, B.A.; Kim, C.S.; Ellis, N.; Bi, X. Microwave-Assisted Catalytic Pyrolysis of Switchgrass for Improving Bio-Oil and Biochar Properties. Bioresour. Technol. 2016, 201, 121–132. doi: 10.1016/j.biortech.2015.10.096
- Liu, H.; Ma, X.; Li, L.; Hu, Z.; Guo, P.; Jiang, Y. The Catalytic Pyrolysis of Food Waste by Microwave Heating. Bioresour. Technol. 2014, 166, 45–50. doi: 10.1016/j.biortech.2014.05.020
- Li, L.; Ma, X.; Xu, Q.; Hu, Z. Influence of Microwave Power, Metal Oxides and Metal Salts on the Pyrolysis of Algae. Bioresour. Technol. 2013, 142, 469–474. doi: 10.1016/j.biortech.2013.05.080
- Yu, Y.; Yu, J.; Sun, B.; Yan, Z. Influence of Catalyst Types on the Microwave-induced Pyrolysis of Sewage Sludge. J. Anal. Appl. Pyrolysis. 2014, 106, 86–91. doi: 10.1016/j.jaap.2014.01.003
- Hascakir, B.; Akin, S. Recovery of Turkish Oil Shales by Electromagnetic Heating and Determination of the Dielectric Properties of Oil Shales by an Analytical Method. Energy Fuels. 2010, 24 (1), 503–509. doi: 10.1021/ef900868w
- Du, Z.; Hu, B.; Ma, X.; Cheng, Y.; Liu, Y.; Lin, X.; Wan, Y.; Lei, H.; Chen, P.; Ruan, R. Catalytic Pyrolysis of Microalgae and Their Three Major Components: Carbohydrates, Proteins, and Lipids. Bioresour. Technol. 2013, 130, 777–782. doi: 10.1016/j.biortech.2012.12.115
- Farag, S.; Fu, D.; Jessop, P.G.; Chaouki, J. Detailed Compositional Analysis and Structural Investigation of a Bio-Oil from Microwave Pyrolysis of Kraft Lignin. J. Anal. Appl. Pyrolysis. 2014, 109, 249–257. doi: 10.1016/j.jaap.2014.06.005
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and Slow Pyrolysis Biochar-comparison of Physical and Functional Properties. J. Anal. Appl. Pyrolysis. 2013, 100, 41–48. doi: 10.1016/j.jaap.2012.11.015
- Suriapparao, D.V.; Vinu, R. Bio-Oil Production via Catalytic Microwave Pyrolysis of Model Municipal Solid Waste Component Mixtures. Royal Society of Chemistry Advances. 2015, 5 (71), 57619–57631.
- Patil, P.; Reddy, H.; Muppaneni, T.; Ponnusamy, S.; Sun, Y.; Dailey, P.; Cooke, P.; Patil, U.; Deng, S. Optimization of Microwave-Enhanced Methanolysis of Algal Biomass to Biodiesel Under Temperature Controlled Conditions. Bioresour. Technol. 2013, 137, 278–285. doi: 10.1016/j.biortech.2013.03.118
- Wang, N.; Tahmasebi, A.; Yu, J.; Xu, J.; Huang, F.; Mamaeva, A. A Comparative Study of Microwave-Induced Pyrolysis of Lignocellulosic and Algal Biomass. Bioresour. Technol. 2015, 190, 89–96. doi: 10.1016/j.biortech.2015.04.038
- Beneroso, D.; Bermúdez, J.; Arenillas, A.; Menéndez, J. Microwave Pyrolysis of Microalgae for High Syngas Production. Bioresour. Technol. 2013, 144, 240–246. doi: 10.1016/j.biortech.2013.06.102
- Bermúdez, J.M.; Francavilla, M.; Calvo, E.G.; Arenillas, A.; Franchi, M.; Menéndez, J.A.; Luque, R. Microwave-induced Low Temperature Pyrolysis of Macroalgae for Unprecedented Hydrogen-Enriched Syngas Production. R. Soc. Chem. Adv. 2014, 4 (72), 38144–38151.
- Ferrera-Lorenzo, N.; Fuente, E.; Bermúdez, J.; Suárez-Ruiz, I.; Ruiz, B. Conventional and Microwave Pyrolysis of a Macroalgae Waste from the Agar-Agar Industry, Prospects for Bio-Fuel Production. Bioresour. Technol. 2014, 151, 199–206. doi: 10.1016/j.biortech.2013.10.047
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Microwave Assisted Pyrolysis of Corn Derived Plastic Bags. J. Anal. Appl. Pyrolysis 2014, 108, 86–97. doi: 10.1016/j.jaap.2014.05.013
- Bartoli, M.; Rosi, L.; Frediani, M.; Undri, A.; Frediani, P. Depolymerization of Polystyrene at Reduced Pressure through a Microwave Assisted Pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 281–287. doi: 10.1016/j.jaap.2015.01.026
- Yang, A.L.C.; Ani, F.N. Controlled Microwave-Induced Pyrolysis of Waste Rubber Tires. Int. J. Technol. 2016, 7 (2), 314–322. doi: 10.14716/ijtech.v7i2.2973
- Leong, S.K.; Ani, F.N.; Chong, C.T. Production of Syngas from Controlled Microwave Assisted Pyrolysis of Crude Glycerol. Key Eng. Mater. 2016, 723, 584–588. doi: 10.4028/www.scientific.net/KEM.723.584
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of Glycerol for the Production of Hydrogen or Syngas. Bioresour. Technol. 2008, 99, 4476–83. doi: 10.1016/j.biortech.2007.08.069
- Fernández, Y.; Arenillas, A.; Díez, M.A.; Pis, J.J.; Menéndez, J.A. Pyrolysis of Glycerol Over Activated Carbons For Syngas Production. J. Anal. Appl. Pyrolysis 2009, 84 (2), 145–150. doi: 10.1016/j.jaap.2009.01.004
- Fernández, Y.; Arenillas, A.; Bermúdez, J.M.; Menéndez, J.A. Comparative Study of Conventional and Microwave-Assisted Pyrolysis, Steam and Dry Reforming of Glycerol for Syngas Production, Using a Carbonaceous Catalyst. J. Anal. Appl. Pyrolysis. 2010, 88, 155–159. doi: 10.1016/j.jaap.2010.03.009
- Qadariyah, L.; Mahfud, M.; Prihatini, P.; Hadi, S.; Kurniati, Y. The Pyrolysis of Glycerol Using Microwave for the Production of Hydrogen. Mod. Appl. Sci. 2015, 9 (7), 74–79. doi: 10.5539/mas.v9n7p74
- Leong, S.K.; Lam, S.S.; Ani, F.N.; Ng, J.H.; Chong, C.T. Production of Pyrolyzed Oil from Crude Glycerol Using a Microwave Heating Technique. Int. J. Technol. 2016, 7 (2), 323–331. doi: 10.14716/ijtech.v7i2.2979
- Bridgwater, A.V.; Meier, D.; Radlein, D. An Overview of Fast Pyrolysis of Biomass. Org. Geochem. 1999, 30 (12), 1479–1493. doi: 10.1016/S0146-6380(99)00120-5
- Domínguez, A.; Menéndez, J.A.; Fernández, Y.; Pis, J.J.; Nabais, J.M.V.; Carrott, P.J.M.; Carrott, M.M.L.R. Conventional and Microwave Induced Pyrolysis of Coffee Hulls for the Production of a Hydrogen Rich Fuel Gas. J. Anal. Appl. Pyrolysis. 2007, 79, 128–135. doi: 10.1016/j.jaap.2006.08.003
- Khaghanikavkani, E.; Farid, M.M. Thermal Pyrolysis of Polyethylene: Kinetic Study. Energy Sci. Technol. 2011, 2 (1), 1–10.
- Ng, J.H.; Leong, S.W.; Lam, S.S.; Ani, F.N.; Chong, C.T. Microwave-assisted and Carbonaceous Catalytic Pyrolysis of Crude Glycerol from Biodiesel Waste for Energy production. Energy Convers. Manage. 2017, 143, 399–409. doi: 10.1016/j.enconman.2017.04.024
- Huang, Y.F.; Chiueh, P.T.; Lo, S.L. A Review on Microwave Pyrolysis of Lignocellulosic Biomass. Sust. Environ. Rev. 2016, 26, 103–109.
- Namazi, A.B.; Allen, D.G.; Jia, C.Q. Probing Microwave Heating of Lignocellulosic Biomasses. J. Anal. Appl. Pyrolysis 2015, 112, 121–128. doi: 10.1016/j.jaap.2015.02.009
- Salema, A.A.; Afzal, M.T. Numerical Simulation of Heating Behaviour in Biomass bed and Pellets under Multimode Microwave System. International Journal of Thermal Science. 2015, 91, 12–24. doi: 10.1016/j.ijthermalsci.2015.01.003
- Yu, F.; Deng, S.; Chen, P.; Liu, Y.; Wan, Y.; Olson, A.; Kittelson, D.; Ruan, R. Physical and Chemical Properties of Bio-oils from Microwave Pyrolysis of Corn Stover. Applied Biochemistry and Biotechnology 2007, 137–140 (1–12), 957–970. doi: 10.1007/s12010-007-9111-x
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels. 2004, 18, 590–598. doi: 10.1021/ef034067u
- Li, H.; Qu, Y.; Xu, J. Microwave-Assisted Conversion of Lignin. In Production of Biofuels and Chemicals with Microwave; Fang, Z., Smith, J.L.R., Qi, X. Eds.; Springer: Dordrecht, 2015; pp 61–82.
- Chen, P., Xie, Q., Du, Z., Borges, F.C., Peng, P., Cheng, Y., Wan, Y., Lin, X., Liu, Y., Ruan, R. Microwave-assisted Thermochemical Conversion of Biomass for Biofuel Production. In Production of Biofuels and Chemicals with Microwave; Fang, Z., Smith, J.L.R., Qi, X. Eds.; Springer: Dordrecht, 2015; pp 83–98.
- Zhang, X.; Lei, H.; Wang, L.; Zhu, L.; Wei, Y.; Liu, Y.; Yadavalli, G.; Yan, D. Renewable Gasoline-Range Aromatics and Hydrogen-enriched Fuel Gas from Biomass via Catalytic Microwave-induced Pyrolysis. Green Chem. 2015, 17, 4029–4036. doi: 10.1039/C5GC00516G
- Luque, R.; Menéndez, J.A.; Arenillas, A.; Cot, J. Microwave-Assisted Pyrolysis of Biomass Feedstocks: The Way Forward. Energy Environ. Sci. 2012, 5 (2), 5481–5488. doi: 10.1039/C1EE02450G
- Macquarrie, D.J.; Clark, J.H.; Fitzpatrick, E. The Microwave Pyrolysis of Biomass. Biofuels, Bioprod. Biorefin. 2012, 6 (5), 549–560. doi: 10.1002/bbb.1344
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Fuel Oil Quality and Combustion of Fast Pyrolysis Bio-oils. VTT Technol. 2013, 87, 79.
- Hou, S.-S.; Huang, W.-C.; Rizal, F.M.; Lin, T.-H. Co-Firing of Fast Pyrolysis Bio-oil and Heavy Fuel Oil in a 300-kWth Furnace. Appl. Sci. 2016, 6, 326. doi: 10.3390/app6110326
- Zheng, J.L.; Kong, Y.P. Spray Combustion Properties of Fast Pyrolysis Bio-oil Produced from Rice Husk. Energy Convers. Manage. 2010, 51, 182–188. doi: 10.1016/j.enconman.2009.09.010
- Krutof, A.; Hawboldt, K. Blends of Pyrolysis Oil, Petroleum, and other Bio-Based Fuels: A Review. Renew. Sust. Energ. Rev. 2016, 59, 406–419. doi: 10.1016/j.rser.2015.12.304
- ASTM D 7544. Standard Specification for Pyrolysis Liquid Biofuel;American Society for Testing and Materials: Easton, MD, 2012.
- Bühler, W.; Dinjus, E.; Ederer, H.J.; Kruse, A.; Mas, C. Ionic Reactions and Pyrolysis of Glycerol as Competing Reaction Pathways in Near- and Supercritical Water. The Journal of Supercritical Fluids. 2002, 22, 37–53. doi: 10.1016/S0896-8446(01)00105-X
- Antal, M.J.; Mok, W.S.L.; Roy, J.C.; Raissi, A.T.; Anderson, D.G.M. Pyrolytic Sources of Hydrocarbons, From Biomass. J. Anal. Appl. Pyrolysis 1985, 8, 291–303. doi: 10.1016/0165-2370(85)80032-2
- Yaws, C.L. Chemical Properties Handbook: Physical, Thermodynamic, Environmental, Transport, Safety and Health related properties for Organic and Inorganic Chemicals, McGraw-Hill: New York, USA, 1999.
- Järvik, O.; Oja, V. Molecular Weight Distributions and Average Molecular Weights of Pyrolysis Oils from Oil Shales: Literature Data and Measurements by Size Exclusion Chromatography (SEC) and Atmospheric Solids Analysis Probe Mass Spectroscopy (ASAP MS) for Oils from Four Different Deposits. Energy Fuels 2017, 31 (1), 328–339. doi: 10.1021/acs.energyfuels.6b02452
- Ciriminna, R.; Della Pinna, C.; Rossi, M.; Pagliaro, M. Understanding the Glycerol Market. Eur. J. Lipid Sci. Technol. 2014, 116 (10), 1432–1439. doi: 10.1002/ejlt.201400229
- Shemfe, M.B.; Gu, S.; Ranganathan, P. Techno-economic Performance Analysis of Biofuel Production and Miniature Electric Power Generation from Biomass Fast Pyrolysis and Bio-oil Upgrading. Fuel. 2015, 143, 361–372. doi: 10.1016/j.fuel.2014.11.078
- Thomas, J.M. Heterogeneous Catalysis and the Challenges of Powering the Planet, Securing Chemicals for Civilised Life, and Clean Efficient Utilization of Renewable Feedstocks. ChemSusChem. 2014, 7 (7), 1801–1832. doi: 10.1002/cssc.201301202
- ICIS. Propylene Prices and Pricing Information 2014 [cited November, 2017]; https://www.icis.com/chemicals/propylene/.
- Oleo Line, the Dependent Oleo Reporter, Oleo Line Glycerine Market Report 2017, No 117, 2, http://www.hbint.com/datas/media/590204fd077a6e381ef1a252/sample-quarterly-glycerine.pdf.
- U.S. Department of Energy. How to Calculate the True Cost of Steam, Industrial Technologies Program Energy Efficiency and Renewable Energy, Washington, DC, 2003.
- U.S. Energy Information Administration (EIA). Electric Power Monthly with Data for July 2017. In dependent Statistics & Analysis;U.S. Department of Energy: Washington, DC, 20585, 2017.
- Liu, L.; Ye, X.P.; Bozell, J.J. A Comparative Review of Petroleum-Based and Bio-Based Acrolein Production. ChemSusChem. 2012, 5 (7), 1162–1180. doi: 10.1002/cssc.201100447
- Wang, L., Lei, H., Ruan, R.: Techno-Economic Analysis of Microwave-Assisted Pyrolysis for Production of Biofuels. In: Production of Biofuels and Chemicals with Microwave;Fang, Z., Smith, J.L.R., Qi, X. Eds.;Springer: Dordrecht, 2015; pp 251–263.
- Zhao, X.; Zhang, J.; Song, Z.; Liu, H.; Li, L.; Ma, C. Microwave Pyrolysis of Straw Bale and Energy Balance Analysis. J. Anal. Appl. Pyrolysis 2011, 92, 43–49. doi: 10.1016/j.jaap.2011.04.004
- Stals, M.; Carleer, R.; Reggers, G.; Schreurs, S.; Yperman, J. Flash Pyrolysis of Heavy Metal Contaminated Hardwoods from Phytoremediation: Characterisation of Biomass, Pyrolysis Oil and Char/Ash Fraction. J. Anal. Appl. Pyrolysis 2010, 89, 22–29. doi: 10.1016/j.jaap.2010.05.001
