ABSTRACT
Grass waste was used for transform an inexpensive waste into health. Silver nanoparticles (AgNPs) have been synthesized using waste material (dried grass). The average size of silver nanoparticles observed in transmission electron images was estimated to be about 15 nm. The anticancer, antifungal and antibacterial effect of AgNPs were studied in vitro. The minimum inhibitory concentration of AgNPs against Pseudomonas aeruginosa and Acinetobacter baumannii was calculated about 3 µg/ml. The highest level of inhibitory effect of AgNPs against Fusarium solani was close to 90% at a concentration of 20 μg/ml of AgNPs. An inhibitory effect on the cancer cell growth is reach, by increasing the concentration of AgNPs to 5 µg/ml; the cancer cells’ survival decreases about 30%. Western results showed that the expression of Cyclin D1 protein of MCF-7 cell line decreased after treatment with the effective concentration of AgNPs.
GRAPHICAL ABSTRACT

Introduction
Cancer is a defect in the mechanisms of regulating of normal growth, proliferation and cell death ( Citation1,Citation2). Cancer cells lose their control over the cell cycle and continue to proliferate in a continuous way, regardless of cellular messages and growth factors ( Citation3). They can invade the adjacent tissues and ultimately spread to other areas of the body (metastasis) ( Citation4). Cancer has been a common term to describe all tumors, and its main action is the growing out of cell control, cell death resistance and the ability to invade other tissues ( Citation5,Citation6). It is the second leading cause of death in the world ( Citation7). In a broad sense, therapies often in the cancer combat do not work well and have harmful side effects on the patient ( Citation8). Researches are essential in discover of the new therapies for treating this disease. In this way, resistance to conventional antimicrobial agents in the market has been weekly reported at fungal species, leishmaniasis and pathogenic bacteria every week ( Citation9). Patients with cancer can undergo serious risk of infection as function these resistant infectious agents, in especial at the burn centers of many hospitals around the world ( Citation10). Therefore, research on the discovery of potential anticancer, antimicrobial (aseptic) and antifungal agent brings a clear vision for controlling and treating these diseases.
Nanotechnology is the manipulation or self-assembly of the atoms, molecules or molecular chains, in such a way that the formed structures lead to the production of materials and structures with new or completely different properties ( Citation11–17). Metallic nanoparticles (particles with at least one dimension between 1 and 100 nm) show different electrical, magnetic, catalytic and optical properties in comparison with the bulk state of the same metal ( Citation18–26). Among metallic nanoparticles, silver nanoparticles have strong, light, catalytic and antimicrobial properties ( Citation27,Citation28). One of the goals of research in the field of green technology (environmentally friendly) in the field of nanoparticles synthesis is to minimize potential risks in the production process and use for humans, as well as the environment ( Citation29,Citation30). One of these measures is finding a route to synthesize nanoparticles that decrease the potential risks and dangerous by products of synthesis and optimize the use of these nanomaterials. This field of nano-studies is in the early stages, and research on the development of this branch of science is very effective and valuable, and will bring us a bright future ( Citation31).
The biosynthesis of nanoparticles is consistent with the principles set of green chemistry ( Citation32). Green method of preparation or green production (biosynthesis) of silver nanoparticles was investigated using waste material (dried grass).
During the synthesis of nanoparticles biomolecule, microbial biomass, fungi, plants or their derivatives (biosynthesis) act as both reducing and stabilizing agents of synthesized nanoparticles ( Citation33–35). These reactions can be considered as a green chemistry process that can minimize the environmental hazards in the chemical synthesis processes ( Citation36,Citation37). In comparison of the different biosynthesis methods of nanoparticles, it should be noted that the synthesis of nanoparticles by microbial agents has the following disadvantages:
The need for specialized facilities and long-term maintenance to protect the growth of microorganisms.
Problems in proper control of the size, shape and crystallization of nanoparticles.
The manipulation of reaction parameters such as pH and temperature may interfere with the microbial growth and biological induction processes.
A special precaution is required when the user is going to work manually on the bacteria.
Biosafety issues should be considered in microbiological work as well as large-scale nanoparticle microorganisms and in commercial applications (Citation33).
Nanomaterials may be harmful to human or may lead to the spread of dangerous bacterial diseases.
The use of dried grass to synthesize nanoparticles is relatively simple and cost-effective compared to microorganisms of even some other plants. Use of agricultural wastes can be an alternative to remove waste. This feature can be used for converting “waste to wealth,” in especial if it is made with low waste. The biosynthesis of nanoparticles can be both in the living plant and biomass ( Citation38–40). In order to facilitate the biosynthesis of nanoparticles by plant extract, it is recommended that plant extracts should be used instead of live plants, in which the compounds are needed to reduce the metal ion, which are extracted and added to the reaction mixture to produce extracellular nanoparticles. Nanoparticles are produced with a specific size range and shape by setting reaction parameters such as pH, concentration and temperature ( Citation41–45). The ability of various plant systems to synthesize silver and gold nanoparticles has been reported in various forms by various research groups. These studies have proved the effect of these parameters on the structure of nanoparticles in some studies.
In this study, the fast and simple synthesis of silver nanoparticles was performed using a waste material (dried grass). The antibacterial and antifungal were determined. The cytotoxicity effects of the Ago Green synthesized nanoparticles is discussed.
Materials and method
Reagent
Silver nitrate (Merck), 2–4,5 dimethyl-thiazole 2,5-diphenyl-tetrazolim bromide (MTT), an initial monoclonal antibody beta-actin (β-actine) (Sigma, USA), 2-mercaptoethanol, sodium chloride, Tween 20, Glycine, acrylamide, blue color, 2-propanol, ethanol, methanol, orthophosphoric acid and sodium docksilate (Merck, Germany); Dulbecco’s modified Eagle’s medium (DMEM), calf fetal serum, penicillin-streptomycin and trypsin EDTA (Biosera, UK); Polyvinylidene Difluoride film and paper laminate (Roche, Germany); Protein and ECL Advance and non-fat dry milk (GE-Healthcare, UK); Primary polyclonal antibody (Mouse) Caspase-3 Primary antibody Cyclin D1 (Mouse), the primary polyclonal antibody (Rabbit) cytochrom c, secondary antibodies, polyclonal anti-rabbit (Goat Anti Rabbit) and anti-mouse (Goat Anti Muose) (Santa Cruz Biotechnology, United States).
Synthesis detail
The waste based on the dried grass was washed with clean water. Their surface was disinfected for 4 min by sodium hypochlorite solution. Then, the grass was washed twice with distilled water for 2 min. The specimens were then placed in alcohol 70% for 2 min and immediately washed with sterilized deionized water (DI) for three times. 20 g of clean and dry waste grass was added to an Erlenmeyer flask (250-ml) containing 100 ml of deionized water and boiled for 15 min at room temperature. The resulting extract was filtered with Whatman® Cellulose Filter Paper No. 42 and kept at 4°C.
Different volumes of silver nitrate 0, 450, 1125, 1800 and 2250 µl form silver nitrate solution stock (0.1 mol) was added to 15 ml of the extract; then, the final volume of reaction mixture (extract + silver nitrate) reached 45 ml with deionized water which lead to prepared final concentrations of silver nitrate at 0, 1, 2.5, 4 and 5 mM µg/ml, respectively. The reaction mixture was kept in the dark at 28°C.
Physicochemical characterization of nanoparticles
The biosynthesized Ag nanoparticles were characterized using UV–Vis spectrophotometer (Analytik Jena; Germany), X-ray diffraction (XRD), (PANalytical X'Pert PRO; The Netherlands) and Transmission electron microscopy (TEM), (Carl ZIESS, Germany) to determine the physicochemical properties of biosynthesized nanoparticles.
Antibacterial effect study
Both Pseudomonas aeruginosa and Acinetobacter baumannii culture medium, (ATCC 19606) strains, containing standard amounts (5. McFarland) were added to ELISA plate wells separately. After incubation, the absorbance of the above systems was measured by spectrophotometer at 530 nm, and the antimicrobial effect was evaluated after the comparison with the negative controls. Also, to determine the antimicrobial effects of the substance, the broth macro-dilution method was used to determine minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC). In this experiment, for each bacterial strain, a series of 10 tubes containing one milliliter of the culture medium of the broth Muller was prepared serially at different concentrations (1.56–800 μg/ml) of silver nanoparticles. One milliliter of one-day-old cultivated bacteria was added to all dilutions in the tubes. It was then incubated at 37°C for 24 h. After incubation, the turbidity of the tubes was examined with naked eyes. The turbidity was caused by bacterial growth. To ensure the bacterial cell death and determine the lowest bactericidal concentration, the tubes that were free from turbidity were cultured on Hinton agar medium. The lowest concentration of silver nanoparticles that prevents microbial growth has the MIC and the MBC is the lowest concentration of nanoparticles that results in more than 99.9% killing of the bacteria being tested.
Antifungal effects of synthesized nanoparticles
The antifungal effects of the synthesized silver nanoparticles were evaluated using the agar dilution method on Fusarium solani and Rhizoctonia solani cultures as previously described by Khatami et al. ( Citation32,Citation46). Briefly, different concentrations (0, 2, 5, 10 and 20 µg ml) of AgNPs were mixed with Potato dextrose agar (PDA) medium in Petri plates. The Petri plates were inoculated with a 6 mm agar plug of F. solani and R. solani at the center of each Petri plates and incubated at 28oC. The fungal growth in all treatments was recorded, when the fungus mycelia on control plates relatively covered the entire surface of the PDA medium.
Cytotoxic effects of silver nanoparticles
Cell culture
Cancer cells (MCF-7 cell line) were cultured in DMEM medium with 25 mM of glucose, 10% of fetal calf serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml). The cells were kept in a humidified incubator with 5% CO2 at 37°C. 200 μl complete DMEM and 5000 counted cells were added to each well of ELISA plate. It was kept in the incubator for 24 h so that the cells stock to the bottom. Twenty-four hours after incubation, the wells were completely drained, and various cell groups were treated with waste material extract alone, AgNPs and a new DMEM environment.
The well containing the cells was returned to the incubator and incubated for 24 h. Wells were completely evacuated and replaced with a new culture medium. The MTT stock solution (15 mg/ml) was provided, and then 20 μl was injected to the wells with the capacity of 200 μl, and the MTT value in each well was 0.25 mg/ml. After adding MTT, the plate containing the cells was kept in the incubator for 2 h. All of the liquid contained in the wells was drained and 100 μl Dimethyl sulfoxide (DMSO) was added to each of them. By penetrating the cell membrane and dissolving Formosan in itself, its purple color spread in the wells. The plate was shaken for a few times; it has been reported that the purple color has been thoroughly distributed in the wells. The optical absorbance of purple color in the wells was read by an automatic spectrophotometer at a wavelength of 540 nm.
Tested samples in MTT test:
Control group: No treatment was performed on the cells in this group. Wells containing the control cells were filled with only 200 μl of complete DMEM medium (at concentration of 25 μm glucose).
Cell groups treated with extracts: Wells containing the cells of this group were treated with different concentrations (1–5 μg/ml) of grass extract and complete DMEM medium.
Cells treated with extract containing synthesized silver nanoparticles: Wells containing this group of cells were treated with different concentrations (1–5 μg/ml), including an extract containing silver nanoparticles and a complete DMEM medium.
Finally, all the tested samples were kept in the incubator for 24 h.
Western blot
The extraction of protein from mCf7 cells
TNE (Tris–HCl, NaCl and EDTA) lysis buffer was used to extract the total protein of the MCF7 cells. This buffer, by breaking down the tissues and cell components, caused the proteins to be separated from other parts of the cell. In addition, this buffer has protease inhibitors to prevent the destruction of cell proteins by protein digestive enzymes.
To extract the protein from the MCF7 cells, the cells were cultured in six wells in equal proportions. The plate was kept in the incubator for 24 h. Then, the wells were completely discharged. Cells were washed once with Phosphate Buffered Saline (PBS). The cell plate was placed on ice to prevent the cellular proteins from altering and degrading.
Inside each well, 350 µl of TNE buffer was poured. The buffer was kept in a plate for 10 min. The broth was purified from the bottom of the wells for all cellular residues and was poured into separate tubes. The tubes were centrifuged 14,000 at 4°C for 15 min. After centrifuging, the contents of the tubes were separated into two phases of the material and solution. Cell proteins were in the fluid phase (upper). The liquid portion (by sampler) was carefully separated and placed in other tubes; the specimens were transferred to the freezer −80 for storage (until used in the test).
Investigation of the amount of proteins involved in apoptosis (Cytochrome C, caspase 3) and cell proliferation (Cyclin d1) using western blot method
In order to perform the electrophoresis of proteins on the SDS-PAGE gel, 40 μg of each sample was used. At first, proteins were boiled for 5 min with a Laemmli sample buffer at 100°C to break down the spatial structures of the proteins. Under these conditions, the SDS is joined to polypeptide strands (for each molecule of the amino acid, an SDS molecule binds to the polypeptide). This causes the entire polypeptide to be negatively charged, and hydrophobic proteins are easily soluble. In addition, the disulfide, hydrophobic and hydrogen bonds break down; as a result, the protein structure is completely open from one another, and the polypeptide strands only maintain their linear structure. The samples were loaded and electrophoresed in 12% SDS-PAGE gel. Electrophoresis was carried out in a running-buffer with a voltage of 120. Protein markers were used to determine the protein content based on molecular weight on SDS-PAGE. After electrophoresis, the proteins separated in the gel were transferred to PVDF (Polyvinylidene Difluoride) paper.
Blocking
Non-fat dry milk (2%), which completely covers all the binding sites of the protein on the PVDF paper, except for the area where the antigen is located, had no effect on proteins fixed on paper.
The PVDF paper was placed in a small glass plate and immersed in the blocking solution for a period; Caspase 3, Cyclin D1 and β-actine antibodies need 1 h to block (room temperature); and BAX and BCL2 antibodies need 4°C overnight. The refrigerator incubator was used to keep the temperature of the blocking solution overnight and uniformly spread on paper.
Addition of the initial antibody
After washing, PVDF paper was immersed in a solution containing the primary antibody. First, the antibody was diluted in the blocking buffer (Caspase 3: 1000/1, BAX: 1000/1, BCL2: 1000/1). For Caspase 3 and Cyclin D1 antigens, the PVDF paper was kept in a solution containing primary antibody for 1 night (4°C) and the same process was done for BAX and BCL2 antigens at 25°C (with shaking by shaker) for 3 h. After this step, the paper was washed three times with TBS-T solution. The secondary anti-rabbit antibody was used for Caspase 3 and BAX, and anti-mouse antibody was used for Cyclin D1, β-actine and BCL2. At first, the secondary antibody was diluted 1:15,000 in the blocking buffer. After this step, the PVDF paper was washed three times with T-BST solution. Finally, the image of the bands recorded on the film was evaluated using lab work software in terms of density.
Statistical analysis
All the tests were repeated three times. SPSS software (version 15) was used for statistical analysis. T-test was used to compare the groups. A p-value of <.05 was considered to be significant.
Results
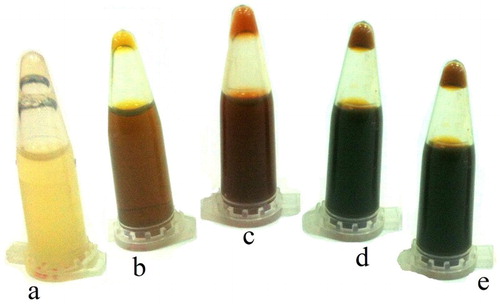
The change in the color of the dried grass extract from light yellow to reddish black was the first visible sign for the synthesis of silver nanoparticles ().
Figure 1. Color change of grass extract treated with silver nitrate. From left to right, there are grass extract 48 h after treatment with 0, 1, 2.5, 4 and 5 mM µg/ml of silver nitrate.
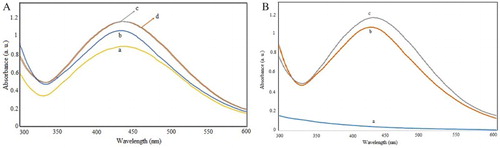
The ultraviolet spectroscopy of nanoparticles synthesized by waste material extract showed maximum peak absorption in the range of 430–450 nm. The spectra were measured at different concentrations and over different times, as shown in .
Figure 2. UV absorption spectra of (A) reaction mixture treated by different concentrations a: 1, b: 2.5, c: 4, d: 5 mM of AgNO3 and (B) at different times a: 0, b: 1 day and c: 5 months.
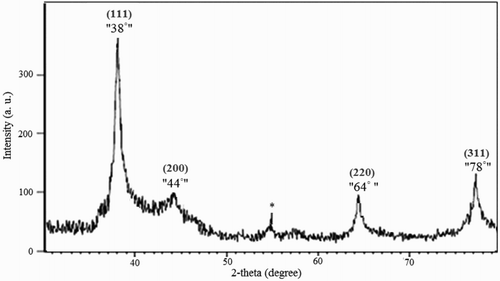
The XRD pattern of AgNPs is shown in . Based on the XRD pattern, it has been determined that the highest synthesis phase is related to the silver element, which indicates the formation of Ago in the sample. The diffraction lines positioned 38°, 44°, 64° and 78° are respectively related to the (111), (200), (220) and (311) (hkl) planes of metallic silver and the extra peak of 54.6 (noted by *) is related to other compounds that are probably in the extract and have a crystalline structure. Based on studies and according to set (hkl) planes of the crystal, there is further evidence that silver is crystallized in the face-centered cubic FCC. Results of this study were consistent with previously reported researches.
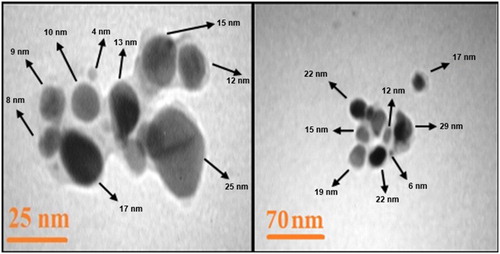
TEM was used to determine the characteristics of particles including the shape, size, and distribution of particles. TEM images show that the nanoparticles exhibits spherical-oblate profile with a size ranging from 4 to 34 nm. The average size of nanoparticles was estimated to be about 15 nm ().
Results of both minimum inhibitory concentration MIC and minimum bactericidal concentration MBC studies are listed in .
Table 1. The MIC and MBC of silver nanoparticles.
The inhibitory results of waste material extract containing silver nanoparticles on both F. solani and R. solani fungi are as follows:
The highest growth-inhibitory effect of AgNPs against R. solani was about 55% at a concentration of 20 μg/ml. The lowest growth inhibitory area was reported about 5% at a concentration of 2 μg/ml. Concentrations of 5 μg/ml and 10 μg/ml have been reported with growth inhibitory of 29% and 43%, respectively (, part 1).
Figure 5. Mycelium growth of: (1) R. solani cultures at concentrations of (A): 0, (B): 2, (C): 5, (D): 10 and (E) 20 μg/ml of silver nanoparticles. (2) F. solani cultures at concentrations of (A):0, (B): 2, (C): 5, (D): 10 and (E) 20 μg/ml of silver nanoparticles.
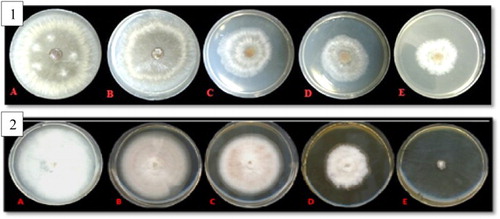
The highest growth inhibitory effect of AgNPs against F. solani was about 90% at a concentration of 20 μg/ml of AgNPs. The concentrations of 2, 5 and 10 μg/ml of the treated AgNPs were reported with an inhibitory growth percentage of 20%, 38% and 60%, respectively. According to results, the inhibition zone of fungal growth depended strongly on the increase in AgNPs’ concentration in the growth medium (, part 2).
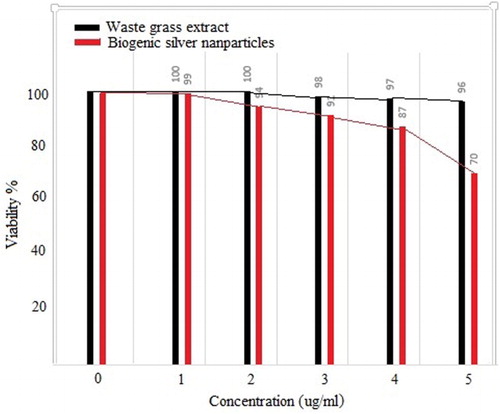
Evaluation of the effects of different doses of waste material extracts on MCF-7 cancer cells in the MTT assay. First, the effects of different concentrations of waste grass extract on MCF-7 cell survival were investigated using the MTT test. The effects of different concentrations of the extract and AgNPs showed that increasing the concentration had a negative effect on the survival of MCF-7 cells. By increasing of the concentration to 5 µg/ml, the number of cancer cells’ survival decreased to about 30% ().
Figure 6. The effect of different concentrations of AgNPs and grass extract on MCF-7 cell survival using MTT test.
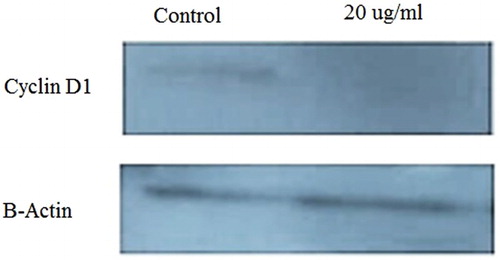
The expression of Cyclin D1 protein was investigated to determine the effect of the extract on growth and MCF-7 cell division. As shown in the , the expression of this protein decreased after treatment with the effective dose of AgNPs. The reduction was 64.42% at a concentration of 20 μg/ml.
Discussion
Waste grass extract acts as both reducing (electron donor) and capping agent for ecofriendly synthesis silver nanoparticles, without using any harmful chemical compounds (). Silver nanoparticles with an average size 15 nm were synthesized by exposing the waste grass extract and silver nitrate solution at ambient laboratory condition.
Figure 8. Schematic design of potential green synthesis mechanism of silver nanoparticles with waste grass extract.

For centuries, silver and its derivatives have been used to treat acute burns and deep wounds. There are documents which prove that Egyptians used silver coins in ancient wars to prevent the infections of their soldiers’ wounds. Historical texts mention that hundreds of years ago, silver was used to disinfect drinking water. Silver nanoparticles have been successfully applied against a wide range of bacteria, viruses, protozoa and fungi. Today, the nanostructures of silver have become stable, for example, zeolite systems impregnated with silver and activated carbon nanotubes systems containing silver. Silver zeolite is one of the antimicrobial forms of silver. After the discovery of penicillin in the 1940s, the use of silver for the treatment of microbial infections was reduced. However, in the 1965s, when Moyer used 5% silver nitrate solution to improve the burns, silver again attracted attentions ( Citation47). Moyer found that this solution did not interfere with the growth and reproduction of the new skin and had antimicrobial activity. Then, silver nitrate was combined with sulfonamide to produce silver sulfadiazine (an antimicrobial agent and a healing agent for burns) ( Citation48). Silver Sulfadiazine (AgSD) is a combination of silver and sulfadiazine. This compound is used as a 1% soluble cream in water. AgSD is a broad-spectrum antibiotic that is especially used in the treatment of burn injuries. AgSD acts like a silver-containing reservoir in the wound and slowly releases silver ions. At around twenty years later, in 1988, the antibacterial properties of colloidal silver were expressed against other bacteria, viruses and fungi. In 2006, food bags and containers containing antibacterial silver nanoparticles were introduced and, subsequently, the properties of silver nanoparticles were widely used in the production of consumables such as soaps and sprays. It is also known that silver nanoparticles and Ag+ are effective in improving diabetic ulcers. Silver nanoparticles also have antiviral properties ( Citation49,Citation50). According to Elechiguerra et al. ( Citation51), silver nanoparticles with a diameter of 5–20 nm and a mean diameter of 10 nm are inhibitors of the proliferation of the 1-HIV virus.
The antibacterial effect of silver nanoparticles depends on the size and shape of these particles ( Citation52). In this process, various forms of nanoparticles can exhibit a variety of antibacterial activities. Triangular silver nanoparticles with a MIC of 1 g/ml can stop the bacterial growth. While spherical nanoparticles with a MIC of 12.5 g/ml, and nanosized pellets with a MIC of 100–80 g/ml can stop bacterial growth. The stability of silver nanoparticles is influenced by the size and shape of the nanoparticles, the covering factor, the pH, dissolved ionic strength of the specific ions and ligands, and the coarse molecules.
The antifungal power of silver nanoparticles with amphotericin B and fluconazole, two of the strongest fungicides, have been compared against several different fungi ( Citation53). The results show that the antifungal power of silver nanoparticles is similar to one of the strongest fungicides (amphotericin B) and is much stronger than fluconazole. They showed that the normal form of cell wall will be damaged, or they completely destroy a part of the cell wall and create a cavity in the fungus membrane.
Conclusion
Silver nanoparticles with an average size close to 15 nm were synthesized by exposing the waste grass extract and silver nitrate solution at ambient laboratory conditions. Waste grass extract acts as both reducing and capping agent for ecofriendly synthesis silver nanoparticles without using any harmful chemical compounds. The physicochemical properties of the resulting silver nanoparticles were characterized using UV–Vis spectroscopy, XRD and TEM. Antimicrobial activity of Ago was verified against P. aeruginosa and A. baumannii. Also, antifungal was proofed. Significant level of cytotoxicity of the Ago was derived in the expression of Cyclin D1 protein.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mehrdad Khatami was born and raised in the Kerman province of Kerman, Iran. His area of interest is medical nano-biotecnology, nanocomposites, green synthesis of nanostructures. Currently he is academic staff and head of central research laboratory of Bam University of Medical Sciences, Bam, Iran.
Iraj Sharifi is a Professor of Medical Parasitology, obtained his PhD from University of Illinois College of Medicine-Urbana: Urbana, IL, United States (USA) and ever since, he has been teaching and doing research studies at the School of Medicine and Leishmanisis Research Center, Kerman University of Medical Sciences, Iran. Curentlly he is director of Leishmaniasis Research Center, School of Medicine,Kerman University of Medical Sciences, Kerman, Iran.
Marcos Augusto Lima Nobre is an assistant Professor of Sao Paulo State University, Brazil (2006) Physics Department. Head of Lab. PostDoc fellow (1999-2000). Dr in Science (1999). Master in Chemistry (1995). Licentiate in Physics (1993). From 2006, Undergraduate courses taught: Gen. Physics I, II, II and IV; Experimental Physics I and II; Elements of Mineralogy; Materials Science. From 2009, Graduate courses taught: Nanoscience and Nanotechnology Applied to Fuels and Biofuels; Thermal Methods of Analysis; Advanced Chemical Physics; Colloids and Surface Chemistry.
Niloofar Zafarnia curently is an assistant Professor of Bam University of Medical Sciences, Bam. Iran. She obtained her PhD from Kerman University of Medical Sciences, Kerman, Iran.
Mohammadreza R. Aflatoonian is an assistant Professor of Bam University of Medical Sciences, Bam. Iran also, he is director of Research Center of Tropical and Infectious Diseases, Kerman University of Medical Sciences, Kerman, Iran. He has published more than 50 scientific papers.
ORCID
Mehrdad Khatami http://orcid.org/0000-0002-7519-6998
Iraj Sharifi http://orcid.org/0000-0002-6894-6834
Marcos A. L. Nobre http://orcid.org/0000-0003-4843-3975
Mohammadreza R. Aflatounian http://orcid.org/0000-0003-4834-5086
Additional information
Funding
References
- Poor, M.H.S.; Khatami, M.; Azizi, H.; Abazari, Y. Cytotoxic Activity of Biosynthesized Ag Nanoparticles by Plantago Major Towards a Human Breast Cancer Cell Line. Rendiconti Lincei 2017, 28 (4), 693–699. doi: 10.1007/s12210-017-0641-z
- Nazari-Vanani, R.; Azarpira, N.; Heli, H.; Karimian, K.; Sattarahmady, N. A Novel Self-Nanoemulsifying Formulation for Sunitinib: Evaluation of Anticancer Efficacy. Colloids Surf. B. Biointerfaces 2017, 160, 65–72. doi: 10.1016/j.colsurfb.2017.09.008
- Collins, K.; Jacks, T.; Pavletich, N.P. The Cell Cycle and Cancer. Proc. Natl. Acad. Sci. USA 1997, 94 (7), 2776–2778. doi: 10.1073/pnas.94.7.2776
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying Collective Cancer Cell Invasion. Nat. Cell Biol. 2012, 14 (8), 777–783. doi: 10.1038/ncb2548
- Zbar, B.; Tanaka, T. Immunotherapy of Cancer: Regression of Tumors After Intralesional Injection of Living Mycobacterium bovis. Science 1971, 172, 271–273. doi: 10.1126/science.172.3980.271
- Alfano, R.R.; Das, B.B.; Cleary, J.; Prudente, R.; Celmer, E. Light Sheds Light on Cancer – Distinguishing Malignant Tumors from Benign Tissues and Tumors. Bull. NY Acad. Med. 1991, 67 (2), 143.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA: Canc. J. Clin. 2016, 66 (1), 7–30.
- Shapiro, C.L.; Recht, A. Side Effects of Adjuvant Treatment of Breast Cancer. N. Engl. J. Med. 2001, 344 (26), 1997–2008. doi: 10.1056/NEJM200106283442607
- Salari, S.; Ayatollahi Mousavi, S.A.; Hadizadeh, S.; Izadi, A. Epidemiology of Dermatomycoses in Kerman Province, Southeast of Iran: A 10-years Retrospective Study (2004–2014). Microb. Pathog. 2017, 110 (Suppl. C), 561–567. doi: 10.1016/j.micpath.2017.07.043
- Safaei, H.G.; Moghim, S.; Isfahani, B.N.; Fazeli, H.; Poursina, F.; Yadegari, S.; Nasirmoghadas, P.; Hosseininassab Nodoushan, S.A. Distribution of the Strains of Multidrug-resistant, Extensively Drug-resistant, and Pandrug-resistant Pseudomonas aeruginosa Isolates from Burn Patients. Adv. Biomed. Res. 2017, 6, 74. doi: 10.4103/abr.abr_239_16
- Hamedi, S.; Shojaosadati, S.A.; Shokrollahzadeh, S.; Hashemi-Najafabadi, S. Mechanism Study of Silver Nanoparticle Production Using Neurospora intermedia. IET Nanobiotechnol. Inst. Eng. Technol. 2017, 11, 157–163. doi: 10.1049/iet-nbt.2016.0038
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Nanostructured CdO-NiO Composite for Multifunctional Applications. J. Phys. Chem. Solids 2018, 112 (Suppl. C), 106–118. doi: 10.1016/j.jpcs.2017.09.016
- Karthik, K.; Dhanuskodi, S.; Prabu Kumar, S.; Gobinath, C.; Sivaramakrishnan, S. Microwave Assisted Green Synthesis of MgO Nanorods and Their Antibacterial and Anti-breast Cancer Activities. Mater. Lett. 2017, 206 (Suppl. C), 217–220. doi: 10.1016/j.matlet.2017.07.004
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Multifunctional Properties of Microwave Assisted CdO–NiO–ZnO Mixed Metal Oxide Nanocomposite: Enhanced Photocatalytic and Antibacterial Activities. J. Mater. Sci: Mater. Electron. 2018, 29, 1–13.
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Dielectric and Antibacterial Studies of Microwave Assisted Calcium Hydroxide Nanoparticles. J. Mater. Sci: Mater. Electron. 2017, 28 (21), 16509–16518.
- Khazaei Feizabad, M.H.; Sharafi, S.; Khayati, G.R.; Ranjbar, M. Effect of Process Control Agent on the Structural and Magnetic Properties of Nano/amorphous Fe0.7Nb0.1Zr0.1Ti0.1 Powders Prepared by High Energy Ball Milling. J. Magn. Magn. Mater. 2018, 449, 297–303. doi: 10.1016/j.jmmm.2017.10.018
- Pishahang, J.; Amiri, H.B.; Heli, H. Synthesis of Carbon Nanoparticles-Poly(Ortho-Aminophenol) Nanocomposite and Its Application for Electroanalysis of Iodate. Sens. Actuat. B: Chem. 2018, 256, 878–887. doi: 10.1016/j.snb.2017.10.030
- Baghbani, F.; Moztarzadeh, F.; Mohandesi, J.A.; Yazdian, F.; Mokhtari-Dizaji, M.; Hamedi, S. Formulation Design, Preparation and Characterization of Multifunctional Alginate Stabilized Nanodroplets. Int. J. Biol. Macromol. 2016, 89, 550–558. doi: 10.1016/j.ijbiomac.2016.05.033
- Tripathi, R.M.; Kumar, N.; Singh Bhadwal, A.; Gupta, R.K.; Shrivastav, B.R.; Shrivastav, A. Facile and Rapid Biomimetic Approach for Synthesis of HAp Nanofibers and Evaluation of Their Photocatalytic Activity. Mater. Lett. 2015, 140, 64–67. doi: 10.1016/j.matlet.2014.10.149
- Tripathi, R.M.; Gupta, R.K.; Singh, P.; Bhadwal, A.S.; Shrivastav, A.; Kumar, N.; Shrivastav, B.R. Ultra-Sensitive Detection of Mercury(II) Ions in Water Sample Using Gold Nanoparticles Synthesized by Trichoderma harzianum and Their Mechanistic Approach. Sens. Actuators B: Chem. 2014, 204, 637–646. doi: 10.1016/j.snb.2014.08.015
- Bhadwal, A.S.; Tripathi, R.M.; Gupta, R.K.; Kumar, N.; Singh, R.P.; Shrivastav, A. Biogenic Synthesis and Photocatalytic Activity of CdS Nanoparticles. RSC Adv. 2014, 4 (19), 9484–9490. doi: 10.1039/c3ra46221h
- Moghaddam, H.M.; Beitollahi, H.; Tajik, S.; Jahani, S.; Khabazzadeh, H.; Alizadeh, R. Voltammetric Determination of Droxidopa in the Presence of Carbidopa Using a Nanostructured Base Electrochemical Sensor. Russ. J. Electrochem. 2017, 53 (5), 452–460. doi: 10.1134/S1023193517050123
- Beitollai, H.; Garkani Nejad, F.; Tajik, S.; Jahani, S.; Biparva, P. Voltammetric Determination of Amitriptyline based on Graphite Screen Printed Electrode Modified with a Copper Oxide Nanoparticles. Int. J. Nano Dimension 2017, 8 (3), 197–205.
- Jahani, S.; Khorasani-Motlagh, M.; Noroozifar, M. DNA Interaction of Europium(III) Complex Containing 2,2′-bipyridine and Its Antimicrobial Activity. J. Biomol. Struct. Dyn. 2016, 34 (3), 612–624. doi: 10.1080/07391102.2015.1048481
- Khorasani-Motlagh, M.; Noroozifar, M.; Jahani, S. Preparation and Characterization of Nano-sized Magnetic Particles LaCoO3 by Ultrasonic-assisted Coprecipitation Method. Synth. React. Inorg. Metal-Organic Nano-Metal Chem. 2015, 45 (10), 1591–1595. doi: 10.1080/15533174.2015.1031010
- Jahani, S.; Beitollahi, H. Carbon Paste Electrode Modified with TiO2/Fe3O4/MWCNT Nanocomposite and Ionic Liquids as a Voltammetric Sensor for Sensitive Ascorbic Acid and Tryptophan Detection. Anal. Bioanal. Electrochem. 2016, 8 (2), 158–168.
- Singh, P.; Singh, H.; Ahn, S.; Castro-Aceituno, V.; Jiménez, Z.; Simu, S.Y.; Kim, Y.J.; Yang, D.C. Pharmacological Importance, Characterization and Applications of Gold and Silver Nanoparticles Synthesized by Panax Ginseng Fresh Leaves. Artif Cells Nanomed. Biotechnol. 2016, 45, 1415–1424. doi: 10.1080/21691401.2016.1243547
- Soshnikova, V.; Kim, Y.J.; Singh, P.; Huo, Y.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Kang, J.; Chokkalingam, M.; Mathiyalagan, R.; Yang D.C. Cardamom Fruits as a Green Resource for Facile Synthesis of Gold and Silver Nanoparticles and Their Biological Applications. Artif Cells Nanomed. Biotechnol. 2018, 46, 108–117. doi: 10.1080/21691401.2017.1296849
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium Oxide Nanoparticles: Green Synthesis and Biological Applications. Int. J. Nanomed. 2017, 12, 1401–1413. doi: 10.2147/IJN.S124855
- Allafchian, A.; Jalali, S.A.H.; Aghaei, F.; Farhang, H.R. Green synthesis of silver nanoparticles using Glaucium corniculatum (L.) Curtis extract and evaluation of its antibacterial activity. IET Nanobiotechnol. 2018, 12, 1–6. doi: 10.1049/iet-nbt.2017.0068
- Khatami, M.; Heli, H.; Jahani, P.M.; Azizi, H.; Nobre, M.A.L. Copper/Copper Oxide Nanoparticles Synthesis Using Stachys lavandulifolia and Its Antibacterial Activity. IET Nanobiotechnol. 2017, 11, 709–713. doi: 10.1049/iet-nbt.2016.0189
- Soltanzadeh, M.; Soltani Nejad, M.; Bonjar, G.H.S. Application of Soil‐borne Actinomycetes for Biological Control against Fusarium Wilt of Chickpea (Cicer arietinum) caused by Fusarium solani fsp pisi. J Phytopath. 2016, 164 (3), 967–978. doi: 10.1111/jph.12517
- Mortazavi, S.M.; Khatami, M.; Sharifi, I.; Heli, H.; Kaykavousi, K.; Sobhani Poor, M.H.; Kharazi, S.; Lima Nobre, M.A. Bacterial Biosynthesis of Gold Nanoparticles Using Salmonella enterica subsp. Enterica Serovar Typhi Isolated from Blood and Stool Specimens of Patients. J. Cluster Sci. 2017, 28, 2997–3007. doi: 10.1007/s10876-017-1267-0
- Tripathi, R.M.; Gupta, R.K.; Bhadwal, A.S.; Singh, P.; Shrivastav, A.; Shrivastav, B.R. Fungal Biomolecules Assisted Biosynthesis of Au-Ag Alloy Nanoparticles and Evaluation of Their Catalytic Property. IET Nanobiotechnol. 2015, 9 (4), 178–183. doi: 10.1049/iet-nbt.2014.0043
- Jamdagni, P.; Khatri, P.; Rana, J.S. Nanoparticles based DNA Conjugates for Detection of Pathogenic Microorganisms. Int. Nano Lett. 2016, 6 (3), 139–146. doi: 10.1007/s40089-015-0177-0
- Tripathi, R.M.; Kumar, N.; Shrivastav, A.; Singh, P.; Shrivastav, B.R. Catalytic Activity of Biogenic Silver Nanoparticles Synthesized by Ficus panda Leaf Extract. J. Mol. Catal. B: Enzym. 2013, 96, 75–80. doi: 10.1016/j.molcatb.2013.06.018
- Saxena, A.; Tripathi, R.M.; Zafar, F.; Singh, P. Green Synthesis of Silver Nanoparticles Using Aqueous Solution of Ficus benghalensis Leaf Extract and Characterization of Their Antibacterial Activity. Mater. Lett. 2012, 67 (1), 91–94. doi: 10.1016/j.matlet.2011.09.038
- Darroudi, M.; Sarani, M.; Kazemi Oskuee, R.; Khorsand Zak, A.; Amiri, M.S. Nanoceria: Gum Mediated Synthesis and In Vitro Viability Assay. Ceram. Int. 2014, 40 (2), 2863–2868. doi: 10.1016/j.ceramint.2013.10.026
- Sharifi, F.; Sharififar, F.; Sharifi, I.; Alijani, H.; Khatami, M. Cytotoxicity, Leishmanicidal, and Antioxidant Activity of Biosynthesized Zinc Sulfide Nanoparticles Using Phoenix dactylifera. IET Nanobiotechnol. 2018, 12, 1–6. doi: 10.1049/iet-nbt.2017.0068
- Khatami, M.; Alijani, H.; Sharifi, I.; Sharifi, F.; Pourseyedi, S.; Kharazi, S.; Lima Nobre, M.A.; Khatami, M. Leishmanicidal Activity of Biogenic Fe3O4 Nanoparticles. Sci. Pharm. 2017, 85 (4), 36. doi: 10.3390/scipharm85040036
- Miri, A.; Darroudi, M.; Entezari, R.; Sarani, M. Biosynthesis of gold nanoparticles using Prosopis farcta extract and its in vitro toxicity on colon cancer cells. Res. on Chem. Intermed. 2018, 44, 1–9. doi: 10.1007/s11164-017-3028-y
- Rahi, A.; Sattarahmady, N.; Heli, H. Zepto-molar Electrochemical Detection of Brucella Genome Based on Gold Nanoribbons Covered by Gold Nanoblooms. Sci. Rep. 2016, 5, 1030. doi: 10.1038/srep18060
- Hossein, H.; Masoud, N. Applications of Nanoflowers in Biomedicine. Recent Pat. Nanotechnol. 2017, 11, 1–12.
- Sattarahmady, N.; Tondro, G.H.; Gholchin, M.; Heli, H. Gold Nanoparticles Biosensor of Brucella spp. Genomic DNA: Visual and Spectrophotometric Detections. Biochem. Eng. J. 2015, 97, 1–7. doi: 10.1016/j.bej.2015.01.010
- Sattarahmady, N.; Rahi, A.; Heli, H. A Signal-On Built In-Marker Electrochemical Aptasensor for Human Prostate-Specific Antigen based on a Hairbrush-like Gold Nanostructure. Sci. Rep. 2017, 7 (1), 594. doi: 10.1038/s41598-017-11680-5
- Khatami, M.; Mortazavi, S.M.; Kishani-Farahani, Z.; Amini, A.; Amini, E.; Heli, H. Biosynthesis of Silver Nanoparticles Using Pine Pollen and Evaluation of the Antifungal Efficiency. Iran. J. Biotechnol. 2017, 15 (2), 95–101. doi: 10.15171/ijb.1436
- Moyer, C.A.; Brentano, L.; Gravens, D.L.; Margraf, H.W.; Monafo, W.W.Jr. Treatment of Large Human Burns with 0.5% Silver Nitrate Solution. Arch. Surg. 1965, 90 (6), 812–867. doi: 10.1001/archsurg.1965.01320120014002
- Chung, J.Y.; Herbert, M.E. Myth: Silver Sulfadiazine Is the Best Treatment for Minor Burns. Western J. Med. 2001, 175 (3), 205–206. doi: 10.1136/ewjm.175.3.205
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20 (5), 595–601. doi: 10.1016/j.drudis.2014.11.014
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed. Res. Int. 2014, 2014, 1–12. doi: 10.1155/2014/186864
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3 (1), 6. doi: 10.1186/1477-3155-3-6
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6 (4), 74. doi: 10.3390/nano6040074
- Kim, K.-J.; Sung, W.S.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal Effect of Silver Nanoparticles on Dermatophytes. J. Microbiol. Biotechnol. 2008, 18 (8), 1482–1484.