ABSTRACT
The inhibiting impact of natural aqueous extracts of some plants such as curcumin, parsley and cassia bark extracts for the corrosion of carbon steel (C-steel) in 0.5 M H2SO4 solution was inspected utilizing some techniques such as galvanostatic and potentiodynamic anodic polarization and weight loss measurements. Outcomes indicated that the percentage inhibition efficiency increases with increasing the concentration of the extract due to its horizontal adsorption on the C-steel surface. The process of adsorption is followed by the Temkin isotherm. These natural extracts acted as pitting corrosion inhibitors by shifting the pitting potential to more noble values. The sequence of inhibition efficiency of the natural extracts decreases in the following order: cassia bark extract > parsley extract > curcumin extract. This arrangement is related to the molecular size of the major components of the three natural extracts used.
GRAPHICAL ABSTRACT
1. Introduction
Sulfuric acid is used in many industrial applications, for example pickling and chemical cleaning of steel. Unluckily, the acidic solution caused the corrosion damage. The addition of the inhibitors is one of the effective methods used to protect the steel from the corrosive acid attack. Most of the inhibitors used are inorganic or organic compounds containing heteroatoms (Citation1–16). These compounds are more efficient for the protection of steel from corrosion damage by its adsorption on the steel surface. Unfortunately, most of the synthetic compounds are toxic and cause damage to public health and the environment. Therefore, most researchers now tend to use some natural extracts for some plants to inhibit the corrosion attack of some metals and alloys in aqueous solutions (Citation17–26).
In the previous work, aqueous extract of the leaves of henna (Lawsonia) was studied as an inhibitor for the corrosion of carbon steel (C-steel) in acidic solutions (Citation27). The inhibiting action of the extract is discussed in view of adsorption of the complex formed between metal cations and Lawsonia molecules on the steel surface. Also, Guar gum (Citation28) and some natural oils such as rosemary (Citation29), parsley, lettuce and radish oils (Citation30) are used as corrosion inhibitors for C-steel in aqueous solutions.
The purpose of this manuscript is to find an environmental-friendly, nontoxic, inexpensive and harmless to human health, efficient inhibitor created from the aqueous extract of some plants such as curcumin, parsley and cassia bark extracts to inhibit the corrosion of carbon steel in 0.5 M H2SO4 solution. The study is conducted by some techniques such as galvanostatic polarization, potentiodynamic anodic polarization at scanning rates of 1 and 50 mV/s and weight loss measurements.
2. Experimental methods
2.1. Electrochemical measurements
The experiments were performed with C-steel of type L-52 used in Egyptian petroleum pipelines and has the following chemical composition (wt-%): 0.14 C, 0.6 Mn, 0.05 S, 0.04 P and the rest is Fe.
A cylindrical rod entrenched in Araldite with exposed surface area of 1 cm2 was used for the electrochemical measurements such as galvanostatic and potentiodynamic polarization measurements. The exposed area was refined with different emery papers starting from coarser to finer, followed by degreasing with acetone and finally washed with distilled water twice, just before insertion in the electrolytic cell. The experiments were performed at the 23 ± 1°C using an air thermostat. The cell used in the electrochemical measurements contains three electrodes, C-steel as the working electrode, saturated calomel reference electrode (SCE) and a platinum foil auxiliary electrode.
The galvanostatic and potentiodynamic polarization at scan rates of 50 and 1 mV/s experiments were carried out using a PS remote potentiostat with PS6 software for calculation of some corrosion parameters.
2.2. Weight loss measurements
The weight loss measurements were accomplished in large test tubes suspended in a thermostated water bath. Each tube was open to air. In each experiment, 50 ml of the test solution was used. The test species were cut into 1.0 × 2.0 × 0.3 cm elements. Treatment of carbon steel coupons such as those mentioned in the electrochemical measurements. The cleaned C-steel coupons were weighed before and after immersion in 50 ml of the test solution for a period up to 8 h. The average weight loss for each identical experiments was taken and expressed in mg cm−2.
2.3. Natural extracts
The natural extracts were obtained from a natural product company, Cairo, Egypt.
The main components in the three natural extracts are given in .
Table 1. The IUPAC name, molecular weight and molecular formula of the major component found in the three natural extracts used as green corrosion inhibitors.
3. Results and discussion
3.1. Galvanostatic polarization
clarifies the impact of different concentrations of the cassia bark extract on the galvanostatic anodic and cathodic polarization curves of C-steel electrode in 0.5 M H2SO4 solutions. Analogous curves were also obtained for the parsley extract and curcumin extract, but it is not shown here. Some corrosion parameters were anodic (ba) and cathodic (bc) Tafel constants, corrosion potential (Ecorr), corrosion current density (icorr.), surface coverage (θ) and the percentage inhibition efficiency (%IE). The corrosion parameters were calculated from the intercept of the anodic and cathodic Tafel lines and are present in .
Figure 1. Effect of various concentrations of cassia bark extract on the galvanostatic polarization curves for C-steel in 0.5 M H2SO4. (1) 0.00 ppm, (2) 100 ppm, (3) 200 ppm, (4) 300 ppm, (5) 400 ppm and (6) 500 ppm.
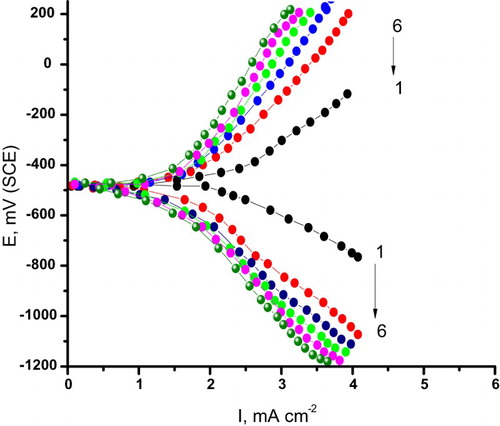
Table 2. Corrosion parameters obtained from galvanostatic polarization measurements of C-steel in 0.5 M H2SO4 solution containing different concentrations of natural extracts of curcumin, parsley and cassia bark.
The percentage inhibition efficiency (%IE) and surface coverage (θ) were calculated using equations (1) and (2):(1)
(2) where Iuninh. and Inh are the corrosion current densities in uninhibited and inhibited solution, respectively.
It is evident that from and , as the concentrations of the extract of curcumin, parsley and cassia bark increase, the polarization curves shift toward more negative potential and lower current density values. The values of ba and bc are diverse a bit, indicating the inhibition impact of these extracts by its adsorption on the C-steel due to blocking adsorption mechanism (Citation31). Also, these natural extracts are categorized as a mixed type inhibitor. The values of Ecorr are slightly shifted toward negative direction, the values of icorr are lowered and the values of θ and consequently %IE increase. These results confirm the inhibitory effect of these extracts. The efficiency of inhibition of natural extracts is reduced in the following order:
3.2. Potentiodynamic anodic polarization
The potentiodynamic anodic polarization curves of C-steel electrode in 0.5 M H2SO4 containing different concentrations of cassia bark extract at a scanning rate of 50 mV/s is presented in . Analogous curves were also obtained for the parsley extract and curcumin extract, but are not shown here.
Figure 2. Potentiodynamic anodic polarization curves of C-steel in 0.5 M H2SO4 containing different concentrations of cassia bark extract at a scan rate of 50 mV/s. (1) 0.00 ppm, (2) 100 ppm, (3) 200 ppm, (4) 300 ppm, (5) 400 ppm and (6) 500 ppm.
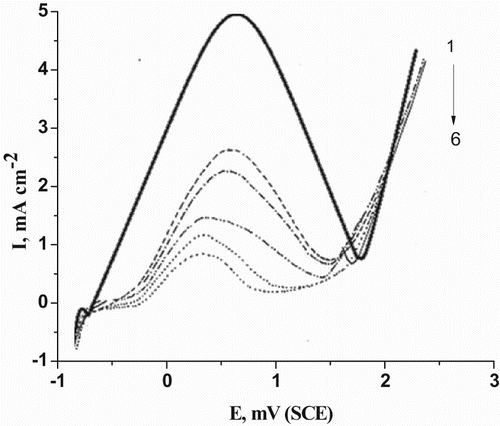
It is obvious from this figure that there is only one anodic peak (A) followed by passive region before O2 evolution.
Peak (A) was explicated due to the active dissolution of Fe into Fe+2 ion according to a previous mechanism (Citation32). At certain potential, the current drops to low values, indicating the onset of passivity. The electrode is considered now to be covered by a passivating oxide film of mainly ferric oxide (γFe2O3). This oxide can be created in the film covering the electrode surface by the oxidation of Fe2+ ions according to Abdallah and Megahed (Citation33):(3)
As the concentration of the extract increases, the passive film increases as shown in . As the potential become positive, the current rises again due to the evolution of oxygen according to(4)
The values of peak current density (iP) and the peak potential (Ep) are calculated and listed in .
Table 3. Corrosion parameters obtained from potentiodynamic anodic polarization measurements of C-steel in 0.5 M H2SO4 containing different concentrations of natural extracts of curcumin, parsley and cassia bark at a scan rate 50 mV/s.
The percentage inhibition efficiency (% IE) was calculated from the following equation (Citation34):(5) where Ip(inh) and Ip(uninh) are the peak current densities in the presence and absence of inhibitors.
Inspection of the curves of and indicates that, as the concentration of natural extracts increases, the values of Ep are shifted to more positive values and Ip to lower values and the values of %IE increases. This indicates an increased resistance to the active dissolution C-steel.
The values of % IE of the natural extracts decrease in the following order:
3.3. Natural extract as pitting corrosion inhibitors
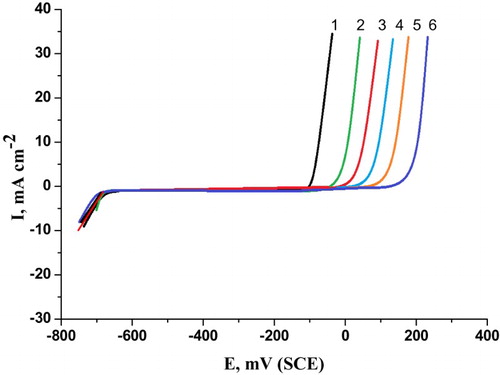
represents the effect of the addition of different concentrations of the natural cassia bark extract on the potentiodynamic anodic polarization curves of C-steel electrode in 0.5 M H2SO4 containing 0.5 M NaCl as pitting corrosion agent at a scanning rate of 1 mV/s. Analogous curves were also obtained for the parsley extract and curcumin extract, but are not shown here. It was found that the pitting potential of the C-steel electrode shifts to more positive (noble) values with increasing the concentration of these natural extracts. This indicates an increased resistance to pitting attack (Citation35, Citation36).
Figure 3. Potentiodynamic anodic polarization curves of carbon steel in 0.5 M H2SO4 + 0.5 M NaCl solutions containing different concentrations of cassia bark extract at a scan rate1 mV/s. (1) 0.00 ppm, (2) 100 ppm, (3) 200 ppm, (4) 300 ppm, (5) 400 ppm and (6) 500 ppm.
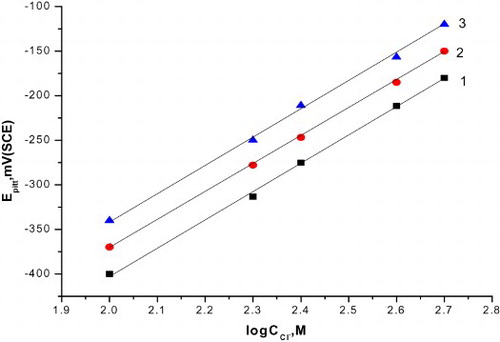
represents the relationship between pitting potential and the logarithm of the molar concentration of the added compounds. Straight lines were obtained and the following conclusions can be drawn:
Figure 4. The relation between the pitting potential of C-steel and logarithm the concentration of natural extracts. (1) Curcumin extract, (2) parsley extract and (3) cassia bark extract.
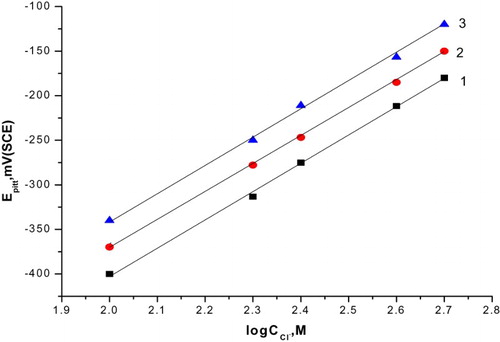
The increase of inhibitor concentration causes the shift of the pitting potential into more positive values in accordance with the following equation (Citation28, Citation37):(6) where a2 and b2 are constants which depend on both the composition of additives and the nature of the electrode.
Inhibition afforded at the same concentrations of the natural extracts decreases in the following order:
3.4. Weight loss measurements
The effect of increasing concentrations of the natural cassia bark extract on the weight loss of C-steel electrode in 0.5 M H2SO4 solution is presented in . The same curves were obtained for the parsley extract and curcumin extract, but are not shown. As shown from these figures, it is obvious that by increasing the concentration of the natural extract, the weight loss of C-steel is decreased. This means that the presence of these extracts retards the corrosion of C-steel in 0.5 M H2SO4 solution.
Figure 5. Weight loss as a function of time of C-steel in 0.5 M H2SO4 solution without and with cassia bark extract: (1) 0.00 ppm, (2) 100 ppm, (3) 200 ppm, (4) 300 ppm, (5) 400 ppm and (6) 500 ppm.
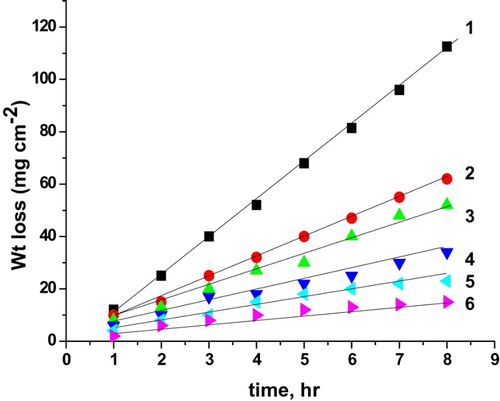
The linear relationship obtained in indicates the absence of insoluble surface film during corrosion. In the absence of any surface films, the inhibitors are first adsorbed onto the metal surface and thereafter affect corrosion.
The corrosion rate values (k) (mg cm−2 min−1) were calculated from equation (7):(7)
The inhibition efficiency (%IE) and the surface coverage (θ) of the natural extract were calculated from the following equations:(8)
(9) where, Kinh and Kuninh are the corrosion rate in devoid of and containing the natural extracts, respectively.
The values of k, θ and %IE for various concentrations of three natural extracts are given in . The obtained data from reveal that the inhibition efficiency of these natural extracts is arranged in the following order:
Table 4. Variation of degree of surface coverage (θ), corrosion rate (k) and percentage inhibition efficiency (%IE) of C-steel in the presence of different concentrations of curcumin, parsley and cassia bark extracts.
cassia bark extract > parsley extract > curcumin extract.
3.5. Adsorption isotherms and explanation of inhibition
The inhibition of general and localized pitting corrosion of C-steel in 0.5 M H2SO4 solution by some natural extracts, e.g. curcumin extract, parsley extract and cassia bark extract, were inspected. The primary step of the inhibitory action of these aqueous extract toward the corrosion of C-steel in 0.5 M H2SO4 solution is usually by the adsorption of these extracts on the steel surface The adsorption of the natural extracts on the steel surface is considered as an alternative adsorption process between the natural extract compounds in the aqueous solution (Extaq) and the water molecules adsorbed on the C-steel surface (H2O)(10) where x is the ratio of the number of water molecules substituted by one molecule of extract adsorbate. The adsorption process depends on the chemical structure of the extract and the presence of the active group in it, the number of adsorption active centers in the molecule and their charge density, molecular size, mode of adsorption, the nature of the metal surface used, temperate, type of the corrosive acidic solution, and the potential of the metal–solution interface
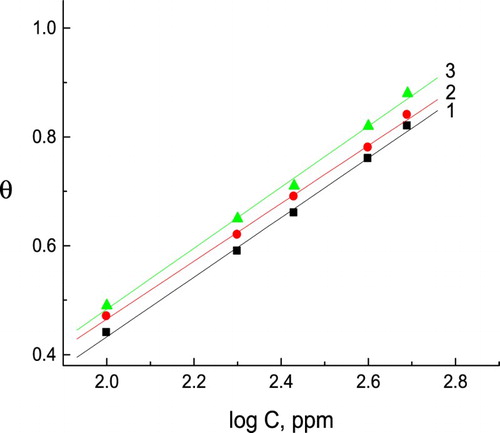
Trials were made to insert the θ values to some adsorption isotherm such as Temkin, Frumkin, Frundich and Langmuir isotherms. In the present work, the adsorption process obeys the Temkin’s adsorption isotherm (Citation38), according to the below equation:(11) where K is the equilibrium constant of the adsorption process, a is the molecule interaction parameter and C is the inhibitor concentration in the bulk solution.
clarifies the relation between θ and log C for C-steel in the presence of natural extracts. Straight lines were obtained indicating that the adsorption of these natural extracts obeys Temkin’s adsorption isotherm. The natural extracts block the reaction sites on the surface of C-steel samples by adsorption and reduce the available area for further corrosion reaction.
Figure 6. Curve fitting of the corrosion data for C-steel in 0.5 M H2SO4 solution in presence of different concentrations of natural extracts to the Temkin’s adsorption isotherm. (1) Curcumin extract, (2) parsley extract and (3) cassia bark extract.
The values of %IE obtained by galvanostatic and potentiodynamic anodic polarization and weight loss techniques indicate that the extent of inhibition efficiency of the natural extracts toward the corrosion of C-steel in 0.5 M H2SO4 solution obeys the following order:
The inhibiting vigor of natural extracts could be interpreted by strong blocking adsorption on the C-steel surface due to the high molecular weight of these natural extracts. We expect horizontal adsorption of its components of the natural extracts on the C-steel surface. The adsorbed layer acts as a barrier between the C-steel surface and corrosive H2SO4 solution leading to a decrease in the corrosion rate. This difference in the inhibition efficiencies could be explained on the basis of the molecular size. The three natural extracts had a high molecular size and this led to facilitate the adsorption process and hence increase the surface coverage.
The values of %IE which were evaluated for the three natural extracts used toward the corrosion of carbon steel in 0.5 M H2SO4 solution using different techniques show an agreement and conformity of the experimental results. However, there is a small difference in the values obtained from the different techniques. This observed discrepancy could be attributed to the differences of the experimental condition.
4. Conclusions
Curcumin, parsley and cassia bark extracts inhibit corrosion of C-steel in 0.5 M H2SO4 solution.
The inhibition efficiency of the three extracts used increases with increasing the concentration of the extract.
The polarization curves proved that the natural extracts act as a mixed inhibitor.
The inhibition was explained in view of its horizontal adsorption on the surface of the steel.
The process of adsorption follows Temkin’s isotherm.
The order of the inhibition efficiency depends on the molecular size of the major component of three natural extract used.
The natural extracts inhibit the pitting corrosion of C-steel by shifting the pitting potential to more noble values.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
M. Abdallah is a Professor of physical chemistry since 2004. He published more than 130 papers in international journals. Most publications are in the field of corrosion inhibition of some metals and alloys in aqueous solutions. Focusing on plant extract, natural oils, surfactant compounds, polymer compounds, organic and inorganic compounds. He supervisor of 40 Master Degree and 18 Ph.D. Degree.
Hatem M. Altass is an assistant professor of physical chemistry, Dean of Faculty of Applied Science, Umm Al Qura university, Makkah, Saudi Arabia. He published some papers in nanotechnology science and corrosion inhibition of metals and alloys in international journals.
B. A. AL Jahdaly is an assistant professor of physical chemistry, Faculty of Applied Science, Umm Al Qura university, Makkah, Saudi Arabia. She has published some papers in corrosion and electrochemistry fields.
M. M. Salem is an assistant professor of physical chemistry Faculty of Science in Zulfi, Majmaah University, Saudi Arabia. She has published some papers in corrosion inhibition of metals and alloys in different media.
ORCID
M. Abdallah http://orcid.org/0000-0002-6132-8849
References
- Hmamou, D.B.; Salghi, R.; Zarrouk, A.; Zarrok, H.; Hammouti, B.; Al-Deyab, S.S.; El Assyry, A.; Benchat, N.; Bouachrine, M. Electrochemical and Gravimetric Evaluation of 7-Methyl-2-Phenylimidazo[1,2-α]Pyridine of Carbon Steel Corrosion in Phosphoric Acid Solution. Int. J. Electrochem. Sci. 2013, 8, 11526–11545.
- Zhiyong, H.; Meng, Y.; Ma, X.; Zhu, H.; Cao, D. Experimental and Theoretical Studies of Benzothiazole Derivatives as Corrosion Inhibitors for Carbon Steel in 1M HCl. Corros. Sci. 2016, 112, 563–575. doi: 10.1016/j.corsci.2016.08.012
- Abdallah, M.; Al-Tass, H.M.; AL Jahdaly, B.A.; Fouda, A.S. Inhibition Properties and Adsorption Behavior of 5-Arylazothiazole Derivatives on 1018 Carbon Steel in 0.5M H2SO4 Solution. J. Mol. Liq. 2016, 216, 590–597. doi: 10.1016/j.molliq.2016.01.077
- Deyab, M.A.; Zaky, M.T.; Nessim, M.I. Inhibition of Acid Corrosion of Carbon Steel Using Four Imidazolium Tetrafluoroborates Ionic Liquids. J Mol. Liq. 2017, 229, 396–404. doi: 10.1016/j.molliq.2016.12.092
- Salhi, A.; Tighadouini, S.; El-Massaoudi, M.; Elbelghiti, M.; Zarrouk, A. Keto-enol Heterocycles as New Compounds of Corrosion Inhibitors for Carbon Steel in 1M HCl: Weight Loss, Electrochemical and Quantum Chemical Investigation. J. Mol. Liq. 2017, 248, 340–349. doi: 10.1016/j.molliq.2017.10.040
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Mohamed, M.A. Corrosion Inhibition of Carbon Steel Pipelines by Some Novel Schiff Base Compounds During Acidizing Treatment of Oil Wells Studied by Electrochemical and Quantum Chemical Methods. J. Mol. Struct. 2017, 1130, 522–542. doi: 10.1016/j.molstruc.2016.10.078
- Kannan, P.; Rao, T.S.; Rajendran, N. Improvement in the Corrosion Resistance of Carbon Steel in Acidic Condition Using Naphthalen-2-Ylnaphthalene-2-Carboxammide Inhibitor. J. Coll. Interface Sci. 2018, 512, 618–628. doi: 10.1016/j.jcis.2017.09.061
- Abdallah, M; Eltass, H.M.; Hegazy, M.A.; Ahmed, H. Adsorption and Inhibition Effect of Novel Cationic Surfactant for Pipelines Carbon Steel in Acidic Solution. Prot. Met. Phys. Chem. Surf. 2016, 52 (4), 721–730. doi: 10.1134/S207020511604002X
- Bouanis, M.; Tourabi, M.; Nyassi, A.; Zarrouk, A.; Bentiss, E. Corrosion Inhibition Performance of 2,5-bis(4-Dimethylaminophenyl)-1,3,4-Oxadiazole for Carbon Steel in HCl Solution: Gravimetric, Electrochemical and XPS Studies. Appl. Surf. Sci. 2016, 389, 952–966. doi: 10.1016/j.apsusc.2016.07.115
- Zhang, Z.; Tian, N.; Zhang, L.; Wu, L. Inhibition of the Corrosion of Carbon Steel in HCl Solution by Methionine and its Derivatives. Corros. Sci. 2015, 98, 438–449. doi: 10.1016/j.corsci.2015.05.048
- Abdel Hameed, R.S.; Shamroukh, A.H.; Abdallah, M. Synthesis, Evaluation of Pyrazolo[3,4-d]Pyrimidinone Derivatives as Corrosion Inhibitors for Carbon Steel in 1 M HCl Acidic Medium. Jokull J. 2016, 66 (2), 1–24.
- Sobhi, M.; El-Sayed, R.; Abdallah, M. Synthesis, Surface Properties and Inhibiting Action of Novel Nonionic Surfactants on C-Steel Corrosion in 1M Hydrochloric Acid Solution. Chem. Eng. Commun. 2016, 203 (6), 758–768.
- Abdallah, M.; Salem, M.M.; AL Jahdaly, B.A.; Awad, M.I.; Helal, E.; Fouda, A.S. Corrosion Inhibition of Stainless Steel Type 316 L in 1.0 M HCl Solution Using 1,3-Thiazolidin-5-One Derivatives. Int. J. Electrochem. Sci. 2017, 12 (5), 4543–4562. doi: 10.20964/2017.05.35
- Fouda, A.S.; El-Sayyed, S.A.; Abdallah, M. N-3-Hydroxyl-2-Naphthoyl Hydrazone Derivatives as Inhibitors for Corrosion of Carbon Steel in H2SO4 Acid Solution. Anti-Corros. Methods Mater. 2011, 58 (2), 63–69. doi: 10.1108/00035591111110705
- Abdallah, M.; Al Jahdaly, B.A.; Al-Malyo, O.A. Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution Using Non-Ionic Surfactants Derived From Phenol Compounds. Int. J. Electrochem. Sci. 2015, 10, 2740–2754.
- Al-Nowaiser, F.M.; Abdallah, M.; El-Mossalamy, E.H. N,N-di (Polyoxyethylene)-4-Dodecylaniline as an Inhibitors of Iron in Hydrochloric Acid Solutions. Chem. Tech. Fuels Oils 2012, 47 (6), 453–463. doi: 10.1007/s10553-012-0324-5
- Parthipan, P.; Narenkumar, J.; Sujatha, P.S.; Preethi, S.; Rajasekar, A. Neem Extract as a Green Inhibitor for Microbiologically Influenced Corrosion of Carbon Steel API 5LX in a Hypersaline Environments. J. Mol. Liq. 2017, 240, 121–127. doi: 10.1016/j.molliq.2017.05.059
- Muthukrishnan, P.; Jeyaprabha, B.; Prakash, P. Adsorption and Corrosion Inhibiting Behavior of Lannea coromandelica Leaf Extract on Mild Steel Corrosion. Arabian J. Chem. 2017, 10 (2), 2343–2354. doi: 10.1016/j.arabjc.2013.08.011
- Mourya, P.; Banerjee, S.; Singh, M.M. Corrosion Inhibition of Mild Steel in Acidic Solution by Tagetes erecta (Marigold Flower) Extract as a Green Inhibitor. Corros. Sci. 2014, 85, 352–363. doi: 10.1016/j.corsci.2014.04.036
- Mhiri, N.; Renaux, D.V; Rocca, E.; Irinaloannu, I.; Boudhrioua, M.N. Corrosion Inhibition of Carbon Steel in Acidic Medium by Orange Peel Extract and its Main Antioxidant Compounds. Corros. Sci. 2016, 102, 55–62. doi: 10.1016/j.corsci.2015.09.017
- Díaz-Cardenas, M.Y.; Valladares-Cisneros, M.G.; Lagunas-Rivera, S.; Lopez-Sesenes, V.M.R.; Gonzalez-Rodríguez, J.G. Peumus boldus Extract as Corrosion Inhibitor for Carbon Steel in 0.5 M Sulfuric Acid. Green Chem. Lett. Rev. 2017, 10 (4), 257–268. doi: 10.1080/17518253.2017.1369167
- Johnsirani, V.; Sathiyabama, J.; Rajendran, S.; Rajan, R.N. Corrosion Inhibition by an Aqueous Extract of Curcumin Dye for Carbon Extract in Sea Water. Eur. Chem. Bull. 2013, 2, 401–406.
- Al Juhaiman, L.A. Curcumin Extract as Corrosion Inhibitor for Al. Green Sustainable Chem. 2016, 6, 57–70. doi: 10.4236/gsc.2016.62005
- Farooqi, I.H.; Hussain, L.A.; Saini, P.A.; Quraishi, M.A. Study of Low Cost Eco-Friendly Compounds as Corrosion Inhibitors for Cooling Systems. Anti-Corros. Methods Mater. 1999, 46 (5), 328–335. doi: 10.1108/00035599910295508
- Vimala, J.R.; Rose, A.L.; Raja, S. Cassia auriculata Extract as Corrosion Inhibitor for Mild Steel in Acid Medium. Int. J. Chem. Tech. Res. 2011, 3 (4), 1791–1801.
- Al-Senani, G.M. The Use of Green Leafy Vegetables Extracts as Corrosion Inhibitors for Carbon Steel in Acidic Medium. World Appl. Sci. J. 2015, 33 (10), 1659–1666.
- El-Etre, A.Y.; Abdallah, M.; El-Tantawy, Z. Corrosion Inhibition of Some Metals Using Lawasonia Extract. Corros. Sci. 2005, 47 (2), 385–395. doi: 10.1016/j.corsci.2004.06.006
- Abdallah, M. Guar Gum as Corrosion Inhibitor for Dissolution of Carbon Steel in NaCl Solution. Port. Electrochim Acta 2004, 22, 161–175. doi: 10.4152/pea.200402161
- Al-Nowaiser, F.M.; Abdallah, M.; El-Mossalamy, E.H. Rosemary Oil as an Inhibitor for Corrosion of Carbon Steel in 0.5 M H2SO4 Solution. Chem. Tech. Fuels Oils 2011, 47 (1), 66–74. doi: 10.1007/s10553-011-0258-3
- Abdallah, M.; Radwan, M.A.; Shohayeb, S.M.; Abdelhamed, S. Use of Some Natural Oils as Crude Pipeline Corrosion Inhibitors in Sodium Hydroxide Solutions. Chem. Tech. Fuels Oils 2010, 46 (5), 354–362. doi: 10.1007/s10553-010-0234-3
- Abdallah, M. Antibacterial Drugs as Corrosion Inhibitors for Corrosion of Aluminum in Hydrochloric Acid Solution. Corros. Sci. 2004, 46 (8), 1981–1996. doi: 10.1016/j.corsci.2003.09.031
- Abdallah, M.; Zaafarany, I.; Khairou, K.S.; Sobhi, M. Inhibition of Carbon Steel Corrosion by Iron(III) and Imidazole in Sulfuric Acid. Int. J. Electrochem. Soc. 2012, 7 (2), 1564–1579.
- Abdallah, M.; Megahed, H.E. Cyclic Voltammograms of Iron and C-Steels in Oxalic Acid Solution and Study the Effect of Phenyl Phthalilmide as Corrosion Inhibitors. Monatsh fur Chemie. 1995, 126, 519–527. doi: 10.1007/BF00807424
- Abdallah, M.; El-Dafrawy, M.A.M.; Sobhi, M.; Elwahy, A.H.M.; Shaaban, M.R. Inhibiting Effects and Theoretical Studies of Synthesized Novel Bisaminothiazole Derivatives as Corrosion Inhibitors for Carbon Steel in Sulphuric Acid Solutions. Int. J. Electrochem. Sci. 2014, 9 (5), 2186–2207.
- Abdallah, M.; Al Karane, S.A.; Abdel Fattah, A.A. Inhibition of the Corrosion of Nickel and Its Alloys by Natural Clove Oil. Chem. Eng. Comm. 2009, 196, 1406–1416. doi: 10.1080/00986440902939053
- Abd El-Haleem, S.M. Initiation and Inhibition of Pitting Corrosion of Incoloy 800 and 316 Stainless Steel. Bull. Electrochem. 1996, 12 (7–8), 449–458.
- Abdallah, M.; Mead, A.I. Pitting Corrosion of an Iron Electrode in HCO-3/Cl- Solutions and Its Inhibition by Some Nonionic Surfactants. Ann. Chim. 1993, 83, 424–430.
- Deyab, M.A.; Fouda, A.S; Osman, M.M.; Abdel-Fattah, S. Mitigation of Acid Corrosion on Carbon Steel by Novel Pyrazolone Derivatives. RSC Adv. 2017, 7, 45232–45240. doi: 10.1039/C7RA08761F
