ABSTRACT
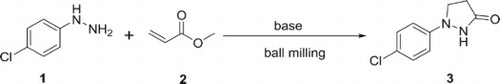
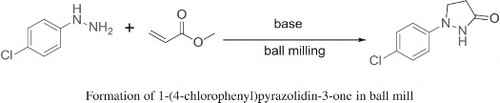
An easy, solvent-free method for the synthesis of 1-(4-chlorophenyl)pyrazolidin-3-one has been achieved by grinding 4-chlorophenyl hydrazine and methyl acrylate in the presence of base in a ball mill. A systematic investigation of the reaction has been made in order to study the various factors affecting the reaction.
GRAPHICAL ABSTRACT
1. Introduction
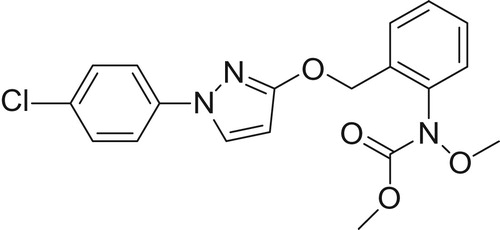
Ball milling is a mechanochemical technique which is primarily used in the grinding of minerals and the preparation and modification of inorganic solids ( Citation1,Citation2). Nowadays, its use in synthetic organic chemistry is a strong emerging field of research, some recent examples of ball milling in organic synthesis include catalyzed C–C bond forming reactions ( Citation3,Citation4) (metal-catalyzed C–C, C–N and C–F bond formation ( Citation5–12), organocatalyzed C–C bond formation ( Citation13–21), cycloaddition reaction ( Citation22)), synthesis of heterocycles ( Citation23), protecting group chemistry ( Citation24), redox processes ( Citation25–27), reactions with fullerenes ( Citation28) and bromination reactions ( Citation29). Often the described reactions take advantage of the fact that different and usually increased selectivities and reactivities have been found when working in a ball mill compared with conventional solution-phase reactions. Therefore, during the further exploration of the applications of ball mill in synthesis (Citation11,Citation21), we wondered whether it would be possible to develop an organic reaction in the ball mill for the formation of 1-(4-chlorophenyl)pyrazolidin-3-one, which is an important intermediate for the synthesis of strobilurin fungicide pyraclostrobin ().
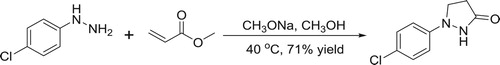
Compared with conventional solution-phase reaction ( Citation30) (), which requires strict condition to keep the reaction system absolutely anhydrous and to use a relatively strong base, our attempts in the ball mill are under mild conditions using relatively weak base instead of sodium methylate, and the solvent-free method decreasing the amount of harmful organic reagents proved to be more environmental friendly ().
2. Results and discussion
To study the influence of the base in the ball mill, some commonly used bases were used (). A milling cycle consisting of a 5 min milling period at a rotational speed of 600 rpm, followed by a 4 min pause to avoid overheating, was used. What surprised us was that the common inorganic base potassium hydroxide and sodium hydroxide functioned well in this solvent-free process to give moderate yields (entries 1–2). However, the reaction exhibited the worse performance when the stronger base like sodium methylate and sodium ethylate was used (entries 3–4). Further, the organic base DABCO and triethylamine could not initiate the reaction well in these conditions (entries 5–6). On the other hand, the same reaction almost could not proceed in conventional solution-phase using potassium hydroxide as the base (entry 7). Therefore, potassium hydroxide was chosen as the base for the following optimization.
Table 1. Screening of bases for the formation of 1-(4-chlorophenyl)pyrazolidin-3-onea.
Once the base chosen, we turned to explore the effect of the base amount on the reaction (), and an increase of the reaction yield was observed if the base amount was changed from 1:1 to 2.5:1 (entries 1–4). The excessive base reducing the sufficient contact of the reactants may cause the yield erosion (entry 5).
Table 2. Influence of potassium hydroxide/4-chlorophenyl hydrazine (1) ratioa.
Subsequently, the ratio of methyl acrylate (2) and 4-chlorophenyl-hydrazine (1) () was optimized, the results clearly show an increase of yields from 44% to 63% by raising the ratio from 1:1 to 1.8:1, then it became constant if the ratio was further increased.
Table 3. Influence of methyl acrylate (2)/4-chlorophenyl hydrazine (1) ratioa.
Having optimized the common reaction parameters, we proceeded now to investigating the influence of the ball milling conditions (), including the ball types, the frequency of milling and the milling time. Initially, Φ10 mm agate balls were chosen to evaluate the frequency and the milling time effects. The yield increased by raising frequency within the restriction speed of the apparatus (entries 1, 3 vs. 2, 4), and it tended to be constant if we continued to prolong the milling time (entries 3, 4). Next, some different types of balls were examined (, entries 5–9), the results reveal that different types of balls, including the ball materials, diameters and ball numbers, may significantly influence the final yield. Generally, the Φ10 mm balls were proved better than others (entries 5, 7 vs. 6, 8); agate ball as well as stainless steel ball gave better yields than zirconium oxide ball (entries 4, 7 vs. 5).
Table 4. Optimization of the milling conditions for the formation of 1-(4-chlorophenyl)pyrazolidin-3-onea.
As the grinding bowl volume is fixed in the ball mill vessel, too much or little raw material may affect the milling process, therefore, an investigation to search for the optimized milling capacity was then performed. As shown in , the highest yield reached 68% when 4-chlorophenyl hydrazine (1) amount was 0.2 g (entry 2). Actually, the yield dropped if the reactant amount is increased probably for the inefficient mixing.
Table 5. Influence of the reactant amounta.
In addition, we also investigated the reaction by adding grinding aids such as alumina and silica powder, but no evidence showed a positive influence in this reaction.
Eager to improve the yield of this reaction, we continued to explore other efficient bases. In recent years, the crown ether complex cation ionic liquids (CECILs) have been applied in various reactions, such as Michael addition, Henry reaction, Knoevenagel condensation and Heck reaction, with excellent results ( Citation31). Moreover, the complexes of ionic liquids with poly(ethylene glycol)s (PEG-ILs) were also reported ( Citation32). According to these pioneering works, a series of novel poly(ethylene glycol) complex cation ionic liquids(PEG-CILs) were synthesized and applied in this reaction (). To our delight, better results were obtained by [15-Crown-5Na+][OH−], [18-Crown-6K+][OH−] and [PEG400 K+][OH−] (entries 1, 2, 4), and the yield could be maintained even reducing the base from 2.5 to 1.0 mol equiv. However, the reaction gave relatively low yield when [PEG200 K+][OH−] or [PEG600 K+][OH−] was used (entries 3, 5), presumably because the chain length of PEG400 (most close to 18-crown-6) was more suitable to wrap around potassium cations. Tetrabutylammonium and benzyl trimethylammonium hydroxide were chosen to investigate whether PEG-CILs could further enhance the efficiency of the reaction. It was found that all the complexes (entries 6–8, 10–13), particularly PEG400 (entries 7, 11), gave much better yields than the comparative one (entry 9), and the desired product could be obtained in 78% yield when PEG400-benzyl trimethylammonium hydroxide complex was used, but the yield was decreased when reducing the base loading from 1.0 to 0.5 equiv. (entry 11).
Table 6. Influence of diferent types of PEG-CILsa.
3. Experimental materials
3.1. General methods
Flash chromatography (FC) was carried out using silica gel (200–300 mesh). Monitoring of reactions was performed by TLC on silica gel precoated on glass plates, and spots were visualized with UV light at 254 nm.1H and 13C NMR were recorded in CDCl3 on Bruker AVANCE III (500 MHz for 1H NMR and 126 MHz for 13C NMR). TMS served as internal standard (δ = 0 ppm) for 1H NMR and CDCl3 was used as internal standard (δ = 77.0 ppm) for 13C NMR; 1H NMR data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), coupling constants (Hz) and integration. IR were recorded on an EQUINOX 55. High-resolution electrospray ionization mass spectra (HR-ESI-MS) were recorded on an Agilent 6545 Q-TOF LCMS spectrometer equipped with an ESI source and controlled by using the MassHunter software. All experiments were carried out under air. Reactions in the ball mill were conducted using a Fritsch Planetary Micro Mill model “Pulverisette 7”. The milling instrument accommodates two grinding bowls (45 mL) which are made of stainless steel.
3.2. General procedure for the synthesis of 1-(4-Chlorophenyl)pyrazolidin-3-one
A clean, dry ball milling vessel was charged with 6 Φ10 mm stainless steel balls, 4-chlorophenyl hydrazine (0.2 g), methyl acrylate (0.22 g, 1.8 mol equiv.) and KOH powder (0.2 g, 2.5 mol equiv.) were ball milled over periods of 12 cycles (1 cycle = 5 min. rotation + 4 min. pause). Afterwards, the vessel and the balls were rigorously washed with 30 ml water; adjust to a pH of 7 using 5% HCl. The aqueous phase was extracted three times with ethyl acetate, and the combined organic phase were dried over Na2SO4, filtered off, and was concentrated to dryness under reduced pressure. The residue was purified via silica gel column chromatography. White solid; mp: 108.5–110.5 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.96 (s, 1H, NH), 7.29 (m, part AA’ of the AA’BB’ system, J = 9.0 Hz, 3.0 Hz, 2H, H-2 and H-6, ClPh), 6.99 (m, part BB’ of the AA’BB’ system, J = 9.0 Hz, 3.0 Hz, 2H, H-3 and H-5, ClPh), 3.93 (t, J = 8.0 Hz, 2H, NCH2), 2.60 (t, J = 8.0 Hz, 2H, COCH2). 13C NMR (126 MHz, Chloroform-d) δ 175.38, 149.93, 129.22, 127.77, 117.59, 55.34, 29.85. IR (film) 1685, 1594, 1497, 1465, 1276 cm−1. HRMS (ESI) exact mass calculated for C9H8ClN2O ([M-H]−) requires m/z 195.0331. Found m/z 195.0327.
3.3. Preparation of the PEG-CILs complexes
Potassium hydroxide or quaternary ammonium hydroxide (10 mmol), PEG (10 mmol) were added to methanol (30 mL), and the mixture stirred at room temperature overnight. The organic phase was concentrated to dryness under reduced pressure to give the PEG-CIL complexes.
4. Conclusions
In summary, a novel and convenient method for the synthesis of 1-(4-chlorophenyl)pyrazolidin-3-one in a ball mill has been developed. A series of poly(ethylene glycol) complex cation ionic liquids (PEG-CILs) were applied to afford the product with good yield. Simplicity of the reaction, solvent-free condition, fast reaction time and its potential application, led us to conclude that the ball mill procedure is a significantly improved version of the described reaction.
Supplementary_Material
Download MS Word (898.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Xiong Zhang is working at the School of Science and Technology, Zhejiang International Studies University. His research has focused on green chemistry.
Biao Wang is studying as a master candidate in the College of Chemical Engineering, Zhejiang University of Technology. His research has focused on the asymmetric catalysis and green chemistry.
Binbin Zhang is studying as a master candidate in the College of Chemical Engineering, Zhejiang University of Technology. His research has focused on green chemistry.
Yifeng Wang is working at College of Chemical Engineering, Zhejiang University of Technology. His research has focused on the asymmetric catalysis and green chemistry.
Additional information
Funding
References
- Kaupp, G.; Naimi-Jamal, M.R.; Ren, H.; Zoz, H. Advanced Technologies Based on Self-Propagating and Mechanochemical Reactions for Environmental Protection; Research Signpost: Kerala, 2003, 83.
- Kipp, S.; Sepelák, V.; Becker, K.D. Chem Unsere Zeit 2005, 39, 384. doi: 10.1002/ciuz.200500355
- Rodríguez, B.; Bruckmann, A.; Rantanen, T.; Bolm, C. Adv Synth Catal 2007, 349, 2213. doi: 10.1002/adsc.200700252
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Chem Soc Rev 2011, 40, 2317. doi: 10.1039/c0cs00195c
- Schneider, F.; Ondruschka, B. ChemSusChem 2008, 1, 622. doi: 10.1002/cssc.200800086
- Thorwirth, R.; Stolle, A.; Ondruschka, B. Green Chem 2010, 12, 985. doi: 10.1039/c000674b
- Stolle, A.; Ondruschka, B. Pure Appl Chem 2011, 83, 1343. doi: 10.1351/PAC-CON-10-09-26
- Thorwirth, R.; Stolle, A.; Ondruschka, B.; Wild, A.; Schubert, U.S. Chem Commun 2011, 47, 4370. doi: 10.1039/c0cc05657j
- Cravotto, G.; Garella, D.; Tagliapietra, S.; Stolle, A.; Schüßler, S.; Leonhardt, S.E.S.; Ondruschka, B. New J Chem 2012, 36, 1304. doi: 10.1039/c2nj40064b
- Lou, S.-J.; Mao, Y.-J.; Xu, D.-Q.; He, J.-Q.; Chen, Q.; Xu, Z.-Y. ACS Catal 2016, 6, 3890. doi: 10.1021/acscatal.6b00861
- Wang, Y.-F.; Wang, H.-J.; Jiang, Y.-D.; Zhang, C.; Shao, J.-J.; Xu, D.-Q. Green Chem 2017, 19, 1674. doi: 10.1039/C6GC03306G
- Shao, Q.-L.; Jiang, Z.-J.; Su, W.-K. Tetrahedron Lett 2018, 59, 2277. doi: 10.1016/j.tetlet.2018.04.078
- Rodríguez, B.; Rantanen, T.; Bolm, C. Angew Chem Int Ed 2006, 45, 6924. doi: 10.1002/anie.200602820
- Rodríguez, B.; Bruckmann, A.; Bolm, C. Chem Eur J 2007, 13, 4710. doi: 10.1002/chem.200700188
- Gérard, E.M.C.; Sahin, H.; Encinas, A.; Bräese, S. Synlett 2008, 17, 2702.
- Baron, A.; Martinez, J.; Lamaty, F. Tetrahedron Lett 2010, 51, 6246. doi: 10.1016/j.tetlet.2010.09.069
- Schmidt, R.; Thorwirth, R.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Chem Eur J 2011, 17, 8129. doi: 10.1002/chem.201100604
- Hernández, J.G.; Juaristi, E. J Org Chem 2011, 76, 1464. doi: 10.1021/jo1022469
- Hernández, J.G.; Juaristi, E. Tetrahedron. 2011, 67, 6953. doi: 10.1016/j.tet.2011.06.042
- Su, W.-K.; Yu, J.-B.; Li, Z.-H.; Jiang, Z.-J. J Org Chem 2011, 76, 9144. doi: 10.1021/jo2015533
- Wang, Y.-F.; Chen, R.-X.; Wang, K.; Zhang, B.-B.; Li, Z.-B.; Xu, D.-Q. Green Chem 2012, 14, 893. doi: 10.1039/c2gc16521j
- Hsu, C.-C.; Chen, N.-C.; Lai, C.-C.; Liu, Y.-H.; Peng, S.-M.; Chiu, S.-H. Angew Chem. Int. Ed. 2008, 47, 7475. doi: 10.1002/anie.200803056
- Wang, G.-W.; Dong, Y.-W.; Wu, P.; Yuan, T.-T.; Shen, V.-B. J Org Chem 2008, 73, 7088. doi: 10.1021/jo800870z
- Patil, P.R.; Kartha, K.P.R. J Carbohydr Chem 2008, 27, 279. doi: 10.1080/07328300802218713
- Mack, J.; Fulmer, D.; Sofel, S.; Santos, N. Green Chem 2007, 9, 1041. doi: 10.1039/b706167f
- Thorwirth, R.; Bernhardt, F.; Stolle, A.; Ondruschka, B.; Asghari, J. Chem Eur J 2010, 16, 13236. doi: 10.1002/chem.201001702
- Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopfe, W. Green Chem 2010, 12, 1288. doi: 10.1039/c002819c
- Cheng, X.; Wang, G.-W.; Murata, Y.; Chin, K.-K. Chem Lett 2005, 16, 1327.
- Wang, G.-W.; Gao, J. Green Chem 2012, 14, 1125. doi: 10.1039/c2gc16606b
- Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Org Biomol Chem 2011, 9, 1443. doi: 10.1039/c0ob00686f
- Song, Y.-Y.; Jing, H.-W.; Li, B.; Bai, D.-S. Chem Eur J 2011, 17, 8731. doi: 10.1002/chem.201100112
- Luo, S.-P.; Zhang, S.; Wang, Y.-F.; Xia, A.-B.; Zhang, G.-C.; Du, X.-H.; Xu, D.-Q. J Org Chem 2010, 75, 1888. doi: 10.1021/jo902521w