ABSTRACT
A high-throughput and selective method for the determination of ʟ-dopa (levodopa) in complex formulations was developed. The method is based on the oxidation of ʟ-dopa to yield dopachrome using tyrosinase-labeled magnetic nanoparticles (TYR-MNPs) as the oxidation catalyst. TYR-MNP activity was retained at 75% after 20 reuse cycles, which is superior to previously reported systems that employ other substrates or cross-linkers for the immobilization of tyrosinase. In addition, the precision (< 3%), accuracy (recovery = 95–102%), and selectivity of the newly developed quantitative-analysis method for ʟ-dopa in complex polypill formulations meets the pharmaceutical industry’s quality-control requirements; consequently this method can be applied to the routine analysis of complex formulations. The quality-control assay uses 96-well microplates, which reduces the required volume of reagents, and the tyrosinase can easily be recycled and reused using an in-house-prepared magnetic microplate, thereby rendering the proposed method economical and less wasteful than existing methods.
GRAPHICAL ABSTRACT
1. Introduction
3,4-Dihydroxy-ʟ-phenylalanine (ʟ-dopa, levodopa) is the precursor of dopamine, and its supplementation can increase dopamine levels in the body. This compound is the most efficacious and best-tolerated treatment for Parkinson’s disease, which is caused by a significant depletion of dopamine resulting from the death of dopamine-producing neurons in the brain. However, as dopamine cannot penetrate the brain-blood barrier, ʟ-dopa therapy is employed to treat this disease ( Citation1). More specifically, ʟ-dopa is rapidly converted into dopamine in peripheral tissue through the action of dopamine decarboxylase enzymes. Since the use of ʟ-dopa can result in excessive dopaminergic firing as a possible side-effect, it is commonly paired with carbidopa, which is an inhibitor of decarboxylase enzymes ( Citation2).
A number of methods for the analysis of ʟ-dopa in pharmaceutical formulations have been reported to date, including spectrophotometry ( Citation3,Citation4), multivariate calibration of kinetic-spectrophotometric methods ( Citation5,Citation6), H-point standard addition methods ( Citation7), chemiluminescence ( Citation8), fluorometry ( Citation9), voltammetry ( Citation10), high-performance liquid chromatography ( Citation11–13), capillary electrophoresis ( Citation14), and liquid chromatography tandem-mass spectrometry ( Citation15). Of the various methods reported, spectrophotometry is perhaps the most convenient analytical technique for routine analysis because of its inherent simplicity, low cost, and wide availability in quality-control laboratories. However, existing spectrophotometric methods for the determination of ʟ-dopa in complex formulations have a number of disadvantages, including poor selectivity, complex sample preparation procedures, and long analysis times ( Citation3, Citation7).
With this background in mind, we describe herein a high-throughput spectrophotometric method for the determination of ʟ-dopa in complex polypill formulations that overcomes these drawbacks. This assay is performed using reusable tyrosinase-immobilized magnetic nanoparticles (TYR-MNPs) and an in-house-prepared 96-well magnetic microplate, which provides a convenient system for the detection of multiple samples in a single run with low sample and reagent volumes. In our introduced system, detection is based on the tyrosinase-catalyzed oxidation of ʟ-dopa; we quantified ʟ-dopa by determining the absorption of dopachrome, the oxidation product of ʟ-dopa, at 475 nm. The reusability and stability of the TYR-MNPs were investigated, and two complex formulations, namely Madopar® and Stalevo®, were analyzed using this high-throughput spectrophotometric method.
2. Material and methods
2.1 . Chemicals
Glutaraldehyde (GA, grade I, 25 wt% in water), iron(III) chloride hexahydrate (97%), and iron(II) chloride tetrahydrate (98%) were purchased from Alfa Aesar (Reston, VA, USA). Tyrosinase from mushroom lyophilized powder (EC 1.14.18.1), ʟ-dopa, sodium phosphate monobasic monohydrate (NaH2PO4·H2O, ACS reagent, ≥98%), and sodium phosphate dibasic (Na2HPO4, ACS reagent, ≥99.0%) salts were used for the preparation of the phosphate buffer (PB) solution; tetraethoxysilane (reagent grade, 98%) and triethoxysilane (3-aminopropyl) (≥98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ammonium hydroxide (28.0–30.0%, Baker Analyzed™ ACS Reagent) was obtained from Fisher Scientific (New Jersey, USA). All chemicals were used as received without further purification. Samples of Madopar® and Stalevo® were purchased as commercial goods in a community pharmacy in Taiwan and tested within their expiry dates.
2.2. Quantitative determination of ʟ-dopa and TYR-MNP reusability
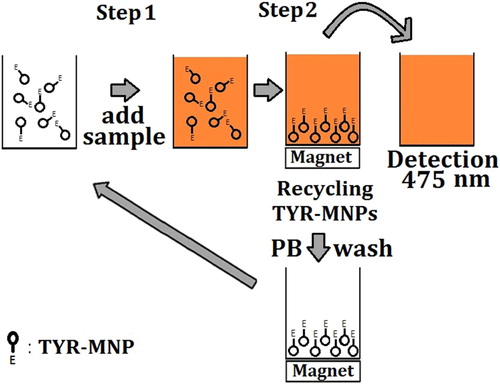
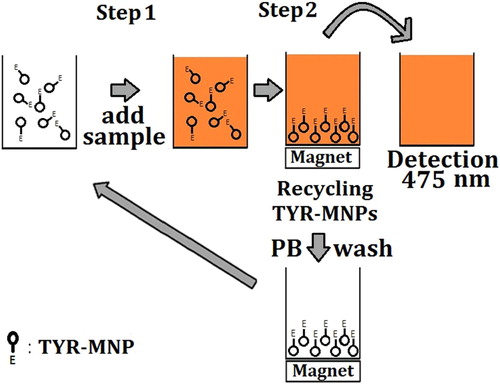
ʟ-Dopa was quantitatively determined using an indirect assay based on the relationship between the TYR-MNP-promoted oxidation of ʟ-dopa and the quantity of dopachrome produced and detected. The preparation of the TYR-MNPs is described in the Supplementary Information. TYR-MNPs were prepared twice (labeled as “batch 1” and “batch 2”) in order to verify that the preparation was repeatable. A schematic representation of the procedures employed for the detection of ʟ-dopa and the recycling of the TYR-MNPs are shown in . In Step 1, an aliquot (200 μL) of the desired ʟ-dopa standard solution or test sample was added to microplate wells containing 80 μL of 0.1 mg/mL TYR-MNPs prepared in 0.1 M PB (pH 6.0). ʟ-Dopa standard solutions with a range of concentrations were prepared in 0.1 M PB (pH 6.0) and mixed using a pipette prior to incubation at 30°C for 13 min. In Step 2, the microplate was placed on top of an in-house-prepared magnetic microplate that attracted the TYR-MNPs to the bottom of the plate, and a portion of the solution (150 μL) was transferred to another microplate. The absorbance of dopachrome at 475 nm was then measured using a FlexStation 3 MultiMode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA), and the TYR-MNPs were recycled by washing three times with PB (0.1 M, pH 6.0). Relative tyrosinase activity (Equation 1) following storage or recycling demonstrated the TYR-MNPs were stable.(1) where A0 is the absorbance measured using fresh TYR-MNPs, A is the absorbance measured using the recycled TYR-MNPs or TYR-MNPs stored in a refrigerator for a period of time, and b is the absorbance of a blank well.
2.3. Determination of ʟ-dopa in Madopar® and Stalevo® tablets
Five tablets of each formulation (Madopar® and Stalevo®) were accurately weighed and the average weights of the tablets calculated (Madopar® = 572.2 mg, Stalevo® = 587.4 mg); the resulting samples were separately ground into powders using a mortar and pestle. The desired quantity of each powder was subsequently accurately weighed and dissolved in 0.1 M PB (pH 6.0) under ultrasonication for 20 min. Following filtration through a hydrophilic polyvinylidene fluoride syringe filter (membrane diameter and pore size of 25 mm and 0.22 μm, respectively), the filtered sample solutions were diluted with the required volumes of 0.1 M PB (pH 6.0) to give the desired ʟ-dopa concentrations. All prepared solutions were within the linear range of the resulting calibration curve.
2.4. Statistical analyses
All experiments were conducted in triplicate (n = 3) and average values were calculated along with the corresponding standard deviations. Paired t-tests, using Microsoft Excel 2013 software, were used to compare the stabilities of the tyrosinase solution and the TYR-MNPs.
3. Results and discussion
3.1. TYR-MNP reusability and stability
The optimal time for the reaction between ʟ-dopa and the TYR-MNPs was determined prior to TYR-MNP reusability and stability testing. As indicated in Figure S5 (see Supporting Information), the absorbance of dopachrome plateaued after 7 min, indicating that the oxidation of ʟ-dopa was complete. Hence, a reaction time of 7 min was selected for all subsequent experiments.
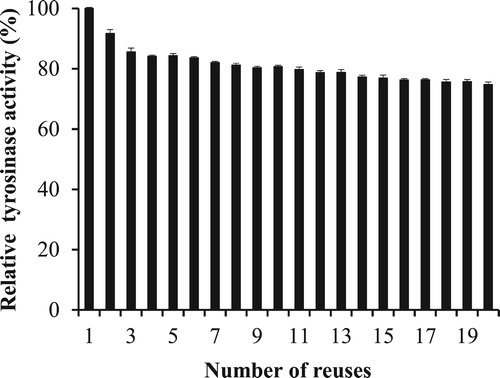
TYR-MNP reusability was then examined by recycling the nanoparticles 20 times over a single day. Although TYR-MNP activity was observed to gradually decrease to 75% after 20 reuse cycles (), these nanoparticles exhibited superior reusability compared to previously reported systems that employed other substrates or cross-linkers for the immobilization tyrosinase, as summarized in .
Table 1. Tyrosinase activities of systems using various immobilization methods.
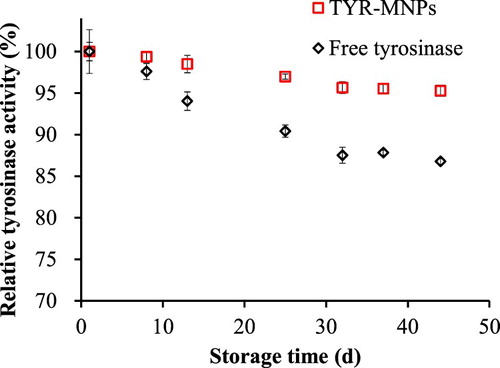
The activities of TYR-MNP suspensions stored at 4°C for 0–44 d were then measured. As shown in , the free tyrosinase solution retained 87% of its original activity after 44 d, while the TYR-MNPs retained 95% of their initially recorded activity. As expected from these observations, the results of the paired t-test (p < 0.01) revealed that the immobilized tyrosinase was more stable during storage than the free tyrosinase solution. In addition, when compared to the stability results of tyrosinase immobilized using a range of methods (see ), these data confirm that the immobilization method used herein is superior to previously reported methods ( Citation16–19).
Table 2. Stabilities of tyrosinase immobilized by a range of methods.
3.2. Assay repeatability for the determination of ʟ-dopa
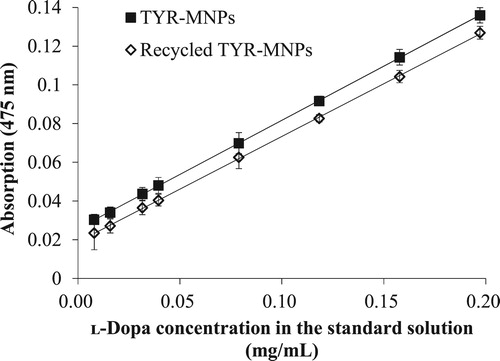
displays regression plots of ʟ-dopa obtained using both freshly prepared and recycled TYR-MNPs; the regression plots are linear in the 0.0158–0.197 mg/mL concentration range, with the regression equation for the freshly prepared TYR-MNPs determined to be: A = 0.5588 C + 0.02582 (R² = 0.9999), where A is the absorbance and C is the ʟ-dopa concentration (mg/mL). In addition, the limit of detection was determined to be 5.6 μg/mL based on 3.3 sb/m, where sb is the standard deviation of the blank and m is the slope of the regression line. Furthermore, the limit of quantitation is 0.017 mg/mL based on 10 sb/m. The regression equation for the recycled TYR-MNPs was found to be: A = 0.5440 C + 0.0190 (R² = 0.9998), leading to a less than 2.7% difference in the slopes of the equations for the fresh and recycled TYR-MNPs.
Regression-plot repeatability was determined using TYR-MNPs from batch 1, the results of which are listed in . The average slope for the inter-day assays was 0.533 ± 0.009, with a 1.7% relative standard deviation in the slope, and the precision was < 3% at an ʟ-dopa concentration of ≤0.1 mg/mL. The reproducibility of the slope of the assay using TYR-MNPs from both batches 1 and 2 was 3%.
Table 3. Regression equations of inter-day assays using TYR-MNPs from batch 1.
3.3. Application of the TYR-MNP assay
Our newly developed method for the determination of ʟ-dopa was then applied to complex formulations containing this target compound. The accuracy and selectivity of the method were determined by spiking prepared solutions of pharmaceutical formulations with known concentrations of ʟ-dopa. For this purpose, we used Madopar® and Stalevo® at concentrations of 0.2138 and 0.147 mg/mL, respectively. As shown in , the recoveries in the Madopar® and Stalevo® assays were in the 95.6–96.3% and 98.0–101.2% ranges, respectively. Based on this assay, the measured ʟ-dopa contents for each purchased Madopar® and Stalevo® tablet were 206 (± 3.4) and 101 (± 7.7) mg, respectively, which are comparable to the manufacturers’ specified quantities of 200 and 100 mg, respectively. These results clearly confirm that this high-throughput spectrophotometric method is accurate and unaffected by other tablet components.
Table 4. Recoveries using the new method following the additions of various quantities of an ʟ-dopa standard solution to known concentrations of Madopar® and Stalevo® solutions.
4. Conclusions
We developed a high throughput and selective method for the determination of ʟ-dopa in complex formulations. In this system, ʟ-dopa was oxidized to dopachrome using TYR-MNPs, which were stable and recyclable. Recovery and reuse were achieved through the implementation of an in-house-prepared magnetic microplate, which renders the method both economical and less wasteful than existing methods. This method was then employed to determine the ʟ-dopa contents of two complex pharmaceutical formulations (i.e. Madopar® and Stalevo®); the new method proved to be highly accurate and was unaffected by other tablet components. Furthermore, the accuracy and selectivity of this method meets the pharmaceutical industry’s quality-control requirements. The 8th pharmacopoeia published by the Food and Drug Administration of Taiwan states that accuracy can be expressed in terms of percent recovery, with a value of 95–105% being the criterion for acceptance; using this standard, our proposed method can be applied to the routine analysis of complex polypill formulations. The quality control assay uses a 96-well microplate, which reduces the reagent volume and facilitates tyrosinase recycling and reuse, in turn resulting in a method that is economical and less wasteful than other methods.
Supplementary_Material
Download MS Word (718.7 KB)Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST) [grant number 105-2113-M-040-006].
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Hweiyan Tsai is a professor of Medical Applied Chemistry at Chung Shan Medical University, Taiwan. She received her PhD from University of Pittsburgh and worked as a postdoc research fellow at biomedical engineering department of Cleveland Clinic Foundation in USA and a project manager of Pharmaceutical Technology Development Programs at Industrial Technology Research Institute in Taiwan. Her major fields of research interest include electrochemical biosensors, magnetic functional nanoparticles for biochemical analyses and screening of functional foods.
Chia-Yi Chen received the B.S. degree from Medical Applied Chemistry at Chung Shan Medical University, Taiwan, in 2012. She is currently a student of master program at National Cheng Kang University, Taiwan.
Yi-Hsuan Lu received the B.S. degree from Medical Applied Chemistry at Chung Shan Medical University, Taiwan. She was a student of master program at National Chi Nan University, Taiwan and worked on the synthesis of nanoparticles.
Ya-Yun Lai received the Ph.D. degree from China medical University, Taiwan, in 2002. From 2003 to 2011, she was an assistant professor of Medical Applied Chemistry at Chung Shan Medical University, Taiwan. She is currently an associate professor in National Tainan Junior college of Nursing, Taiwan. Her research interests include synthesis and analysis of pharmaceutical compounds, evaluation of total flavonoids of herbs.
Chwan-Bor Fuh is a professor of Applied Chemistry at National Chi Nan University, Taiwan. He received his PhD from University of Utah and worked as a postdoc research fellow at biomedical engineering department of Cleveland Clinic Foundation in USA. He was a guesting professor at Osaka University in Japan. His major fields of research interest include thin channel, magnetic separation, and functional nanoparticles for analytical and biochemical analyses.
ORCID
Hweiyan Tsai http://orcid.org/0000-0002-4070-0058
Additional information
Funding
References
- Foster, S.B.; Wrona, M.Z.; Han, J.; Dryhurst, G. Chem. Res. Toxicol. 2003, 16, 1372–1384.
- Feyder, M.; Bonito-Oliva, A.; Fisone, G. Front. Behav. Neurosci. 2011, 5, 1–11. doi:10.3389/fnbeh.2011.00071.
- Elshiekh, B.; Basheir, A.; Elbashir, A.A. Eur. J. Pharm. Med. Res. 2015, 2, 304–316.
- Pecanac, D.; Karljikovic-Rajic, K.; Radulovic, D. Anal. Lett. 1997, 30, 1833–1841.
- Pistonesi, M.; Centurión, M.E.; Band, B.S.F.; Damiani, P.C.; Olivieri, A.C. J. Pharm. Biomed. Anal. 2004, 36, 541–547.
- Blanco, M.; Coello, J.; Iturriaga, H.; Maspoch, S.; Villegas, N. Anal. Lett. 2000, 33, 2701–2718.
- Madrakian, T.; Mohammadnejad, M. Chem. Pharm. Bull. 2007, 55, 865–870.
- Alam, S.M.; Karim, M.M.; Lee, S.H.; Wabaidur, S.M.; Chung, H.Y.; Choi, J.H.; Kang, M. Luminescence. 2008, 23, 327–332.
- Jie, N.; Yang, D.; Zhang, Q.; Yang, J.; Song, Z. Talanta. 1998, 46, 1163–1168.
- Bergamini, M.F.; Santos, A.L.; Stradiotto, N.R.; Zanoni, M.V.B. J. Pharm. Biomed. Anal. 2005, 39, 54–59.
- Bugamelli, F.; Marcheselli, C.; Barba, E.; Raggi, M.A. J. Pharm. Biomed. Anal. 2011, 54, 562–567.
- Ribeiro, R.P.; Gasparetto, J.C.; de Oliveira Vilhena, R.; de Francisco, T.M.G.; Martins, C.A.F.; Cardoso, M.A.; Pontarolo, R.; de Carvalho, K.A.T. Bioanalysis. 2015, 7, 207–220.
- Muzzi, C.; Bertocci, E.; Terzuoli, L.; Porcelli, B.; Ciari, I.; Pagani, R.; Guerranti, R. Biomed. Pharmacother. 2008, 62, 253–258.
- Wang, J.; Zhou, Y.; Liang, J.; He, P.G.; Fang, Y.Z. Chromatographia. 2005, 61, 265–270.
- Lv, L.; Jiang, W.; Zhou, S.; Huang, X.; Shi, X.; Lv, C.; Wu, L.; Xu, C. Chromatographia. 2010, 72, 239–243.
- Vandeput, M.; Patris, S.; Silva, H.; Parsajoo, C.; Dejaeghere, B.; Martinez, J.A.; Kauffmann, J.-M. Sens. Actuators, B. 2017, 248, 385–394.
- Zhou, Y.L.; Tian, R.H.; Zhi, J.F., Biosens. Bioelectron. 2007, 22, 822–828.
- Saini, A.S.; Kumar, J.; Melo, J.S. Anal. Chim. Acta. 2014, 849, 50–56.
- Abdollahi, K.; Yazdani, F.; Panahi, R. Int. J. Biol. Macromol. 2017, 94, 396–405.