ABSTRACT
This paper provides the first benchmarking study of green chemistry (GC) adoption by the Indian pharmaceutical supply chain based on information from industry representatives leading such efforts. Results demonstrate that generic drug pharma and Active Pharmaceutical Ingredients (API) manufacturers in India exhibit significant interest and some advances in using GC principles. At the same time, majority (65%) of Indian companies rely on treatment and disposal of waste water instead of source reduction and one in five (20%) does not use any GC metrics. The study found that generic pharma is more advanced in adopting GC principles than API manufacturers. Regulatory risk and time pressures to deliver drugs were reported as the two most significant barriers for greater adoption of GC in India, while cost savings and environmental regulations were cited as the top two drivers. The paper concludes with some recommendations for advancing GC adoption in India.
GRAPHICAL ABSTRACT
1. Introduction
The global pharmaceutical industry presently relies on the pharmaceutical supply chain in India and China for the manufacture of much of the active pharmaceutical ingredients (APIs) for both generic and branded drugs. China provides almost two-thirds of the global APIs ( Citation1) but Indian pharmaceutical companies have been found to have better capabilities in formulation development, finished drug manufacturing, and product marketing in regulated markets such as United States and Europe ( Citation2). The Indian pharmaceutical industry has seen revenue growth of 12% per year and the country is producing about 20% of all generics globally, second only to China ( Citation3). With growing efforts to reduce healthcare costs, generics are projected to represent 92% of all drugs globally by 2022 ( Citation4). India is well positioned to benefit from this trend; in the first half of 2017 alone Indian pharma companies received 40% of U.S approvals for generic drugs, an increase from 35% in 2016 ( Citation5).
At the same time, both India and China’s pharmaceutical industry has come under scrutiny for the pollution resulting from drug manufacturing. In particular, the emergence of antibiotic resistant bacteria, or “superbugs,” has been attributed in part to insufficient attention to management of pharmaceutical manufacturing waste ( Citation6). As early as 2007, Larsson et al. ( Citation7) highlighted this problem in a study of pharmaceutical manufacturing effluents in Hyderabad, India, and called for the incorporation of environmental considerations in ICH good manufacturing practice (GMP) guidelines. Recently, Indian authorities closed four polluting companies in the Hyderabad area ( Citation8) and China has taken regulatory action on almost 40% of the factories in 30 industrial provinces, raising concern about the security of the pharmaceutical supply chain ( Citation9). Researchers, NGOs, and policy makers have called for action by the global pharmaceutical industry to address the environmental performance of its entire API supply chain. This is a critical issue for companies as failure to comply with existing standards could disrupt the manufacturing and sales of pharmaceuticals to U.S. and EU-based companies and healthcare systems.
While better management of waste streams is an obvious corrective approach, the authors believe that green chemistry (GC) represents a better option to address the problem for its focus on source reduction and significant cost savings. GC is defined as “the design of chemical products and processes that reduce or eliminate the generation of hazardous substances” ( Citation10). The pharmaceutical industry was among the first to embrace GC for its significant potential to reduce costs and risks ( Citation11–13). Researchers have reported large declines in Toxics Release Inventory (TRI) releases from the pharmaceutical industry in the U.S. which correlate with the adoption of GC ( Citation14). At the same time, little is known about its current adoption in India and China as well as the existing barriers, drivers and future opportunities. A recent benchmarking study of the global pharmaceutical supply chain revealed that despite a lack of public disclosure, generic drug companies, API manufacturers and smaller R&D pharma companies’ exhibit interest and some advances in using GC principles ( Citation13). The study, however, included only six companies from India, therefore it could not provide in-depth insights into this important pharma market. The current paper aims to fill this gap and has three main objectives: (a) benchmark current adoption of GC by Indian pharmaceutical companies and API manufacturers; (b) examine the drivers and barriers to greater adoption of GC by the industry, and (c) identify opportunities to advance wider adoption of GC by the pharmaceutical industry and API manufacturers in India.
The main contribution of the paper is that it is the first effort to benchmark GC adoption by the pharmaceutical supply chain in India based on information from industry representatives leading such efforts. The paper begins with an overview of green chemistry and its adoption by the pharmaceutical industry. Next it focuses on India and provides an overview of environmental pollution, current regulations, and emerging GC initiatives. The authors then present the study goals and methods, followed by research results and discussion. The paper concludes with a summary of key findings and recommendations for future policy, practice and research.
2. Literature review
Introduced in the early 1990s, GC has seen significant growth over the past 25 years driven by both external and internal factors, such as stakeholder pressures to reduce environmental releases from manufacturing, cost savings, improved reputation, market positioning, and ability to attract and retain talent. It is defined by the 12 Principles of Green Chemistry introduced by Anastas and Warner ( Citation15) (see ). The market for GC is expected to outpace the overall global chemical market in the coming decades as companies respond to consumer demand for more sustainable products and tightening regulations on the use and generation of hazardous substances. For instance, the total alternative U.S. chemical end-use product market is projected to increase from $149.9 billion in 2016 to $884.1 billion in 2026, reflecting a 10-year compound annual growth rate (CAGR) of 19.4%. Green pharmaceuticals as a segment are projected to grow from $27 billion in 2016 to $96.2 billion in 2026 ( Citation16).
Text Box 1. The 12 principles of green chemistry ( Citation15).
2.1. Pharmaceutical industry overview
The global pharmaceutical industry had $996 billion in sales in 2017 ( Citation17) and is projected to reach $1.12 trillion by 2022 ( Citation18). Sales of pharmaceuticals are expected to grow at 6.3% annually over the next several years as result of aging population and increasing healthcare spending globally. United States remains the world’s largest market for prescription medicines with 45% of the global market. Six of the top 10 largest pharmaceutical companies globally are U.S. based research and development (R&D) pharma ( Citation19). The sector comprises four main segments: innovative pharmaceutical industry (also known as R&D pharma), biopharmaceutical industry, biologics, and generic pharmaceutical industry ( Citation20). Generic drug companies make copies of brand name drugs after expiration of their patents, which are identical in terms of API, strength dosage and route of administration ( Citation20). The global market for generic drugs reached $200 billion in 2015 and is projected to grow at 10.8% over the next several years, reaching $380 billion by 2021 ( Citation21). China and India have emerged as the leading markets for manufacturing API and R&D outsourcing due to their cost advantage and highly educated workforce. Manufacturing costs in India are estimated to be 30–40% lower than those in the United States and Western Europe, and labor costs – one seventh of those in the U.S. ( Citation22). In addition, the costs of running an FDA-inspected manufacturing plant in India have been found to be 50% lower than in developed countries ( Citation22).
As of 2018 India had one of the fastest growing economies globally with GDP projected to reach 7.4% in 2018 and 7.6% in 2019 ( Citation23). The pharmaceutical industry is one of its fastest growing sectors with revenues projected to expand at a rate of 12% annually and reach $100 billion by 2025 ( Citation24). In 2016 generics accounted for about 75% of its market by volume and generated $15 billion in revenue. The largest segments include anti-infective (including antibiotics), antiviral and antifungal, and the country is credited for helping provide more affordable medicines worldwide. The sector is highly fragmented with about 1,300 companies, where the top 10 represent approximately 34% of the market share ( Citation25). Some of its largest pharmaceutical companies include Sun Pharma, Dr. Reddy’s Labs, Cipla, and Ipca Laboratories. The government is committed to support the industry in order to surpass China and become the world’s top supplier of API and generic drugs. Yet, environmental pollution from drug manufacturing remains a major problem and significant risk for both the Indian pharmaceutical industry as well as “big pharma” outsourcing to India.
2.2. Environmental pollution and regulations in India
India has had a long history of environmental pollution. It is home to one of the world’s worst industrial accidents – the Bhopal explosion at the Union Carbide plant in 1984, which resulted in the death toll of between 3,800 and 16,000 people ( Citation26). Delhi, India’s capital, has been ranked as the most polluted city in the world, and a recent report found that within five years the number of polluted rivers in India increased from 121 to 275 ( Citation27). About 80% of India’s surface waters are contaminated due to the release of untreated sewage and industrial waste ( Citation28). The global chemical industry has long seen India as manufacturing hub and has been outsourcing their early stage intermediates, advanced intermediates as well as finished products ( Citation29). Most of these early stage and advanced intermediates involve inherently polluting and hazardous chemical processes like sulphonation, nitration, chlorosulphonation, reduction, fusion, diazotization, and Friedel–Crafts, among others. These processes generate highly acidic/alkaline waste streams in substantial quantities making it very challenging to store, handle and treat them. Treating and disposing of wastes in a responsible manner requires investment and adds significant cost to the product. Since a very large percentage of India’s manufacturing units are small and medium size enterprises (SMEs), they face many constraints, when it comes to addressing environmental challenges, such as small space, limited access to innovations and knowledge, limited human and financial resources to invest in new technologies. Competition from other countries supplying products at very low cost and continuous pressures from global customers to reduce the price of intermediates and finished products have led Indian companies to look for ways to reduce effluent treatment costs and do what’s bare minimum to manage their wastes ( Citation24).
Pharmaceutical manufacturing is classified as “red category” by India’s Environment Ministry due to the hazardous waste it produces. Recent studies have shown the escalating environmental pollution crisis in India’s pharmaceutical hubs Hyderabad and Visakhaptanam. Of particular concern is the release of untreated wastewater from antibiotics manufacturing which contributes to the growing problem of antibiotic resistance, considered one of the biggest human health threats of the 21st century by the World Health Organization ( Citation30). A 2007 Swedish study of the effluent from a wastewater treatment plant serving 90 bulk drug manufacturers near Hyderabad showed concentrations of antibiotics such as ciprofloxacin exceeding levels toxic to some bacteria by over 1,000-fold ( Citation7). The researchers also found that the releases were equivalent to a loss of €100,000 each day. More recently Lubbert et al. ( Citation31) tested for 25 anti-fungal pharmaceuticals in the direct environment of bulk drug manufacturers near Hyderabad. The researchers found that environmental specimens from all 28 different sampling sites were contaminated with antimicrobials and drug-resistant bacteria was present in 95% of the samples. A 2010 study by Swedish researchers showed that seven out of eight travelers to India returned to Sweden carrying drug-resistant bacteria in their gut ( Citation22). In addition to the problem with antibiotics releases, the groundwater near pharmaceutical manufacturing facilities has been found to contain toxics including lead, cadmium, vanadium and arsenic in concentrations thousand times higher than the maximum permissible levels for drinking water quality established by WHO and the Bureau of Indian Standards (BIS) ( Citation32).
Indian environmental laws date back to the 1970s and include over 200 separate regulations, including The Water Act of 1974, the Air Act of 1981, and the Environmental Protection Act of 1986. The country, however, has struggled with effective enforcement due to several main reasons outlined by Shroff and Jejurkar ( Citation33):
Problems with implementing agencies and pollution control boards. These agencies act as autonomous bodies having absolute authority but lack central coordination, human, technological and financial capacity to ensure enforcement. For instance, according to the Water Act if a local agency does not approve or reject industry proposal within four months, then the “consent is deemed to have been granted.” As a result, many facilities have been allowed to operate without proper evaluation of their environmental impacts.
Political conflicts, interference and inconsistency due to corruption. Corruption is widely spread in India’s political and administrative system, and especially prevalent in environmental cases.
Economic growth versus environment pollution. As developing country India is putting economic growth before environmental protection as attracting investments is critical for creating jobs and raising the standard of living.
Lack of implementation of the Polluter Pays Principle (PPP). This principle imposes financial and physical responsibility on the polluter to address the environmental contamination of its manufacturing. India, however, does not have clear guidelines and criteria for determining compensation and damages payable by companies causing environmental damage; these are currently left at the discretion of the Supreme Court.
Despite the growing pollution crisis, concerns have been raised recently that pharmaceutical industry regulation “is actually becoming more lax, and pollution levels are set to rise even further as the government lifts restrictions on plant expansion and weakens national pollution index” ( Citation22). One positive recent development is the proposal for establishment of an independent environment regulator – the National Environmental Appraisal and Monitoring Committee – to be in charge of reviewing and granting permissions to industrial facilities and thus helping reduce litigation. It remains, however to be seen how this will be implemented in practice ( Citation33).
In addition to the environmental problems, Indian companies have been found to violate quality standards such as the Good Manufacturing Practices (GMP) guidelines. With the increasing outsourcing of drug manufacturing, the U.S. Food and Drug Administration (FDA) has expanded its inspections in countries making generic drugs, in particular India and China. For example, FDA inspections in India increased from 108 in 2009 to 290 in 2015, and the agency found numerous instances of noncompliance, stating that “quality issues are an ongoing challenge for the Indian pharmaceutical industry.” In the second half of 2015, many Indian pharma firms including Dr. Reddy’s, Sun Pharma, Zydus Cadila, Wockhardt Ltd and IPCA Lab, received warning letters by the FDA, and the EU introduced a ban on 700 generic drugs supplied by Indian companies. The GMP guidelines, however do not presently include provisions for regulating environmental releases, thus authorities in the regulated markets have no formal power to address the environmental impact of pharmaceutical manufacturing there ( Citation22). This however can change in the future as countries such as Sweden and Germany have called for changes in the GMP guidelines to incorporate environmental considerations ( Citation34, Citation35).
The global pharmaceutical industry has taken some steps to address the environmental pollution crises, with the two most notable developments including the establishment of the Pharmaceutical Supply Chain Initiative (PSCI) and the Davos Declaration on Combating Antimicrobial Resistance. PSCI was launched in 2006 by a group of pharmaceutical and healthcare companies to promote better environmental, health and safety practices by the entire supply chain ( Citation36). As of June 2018 it had 30 members including most “big pharma” companies such as Pfizer, Allergan, Novartis, Bristol-Myers Squibb, and Sanofi ( Citation37). Yet, member companies do not publicly report their supplier performance and to date little research has been done to benchmark progress toward its mission. The Davos Declaration on Combating Antimicrobial Resistance was signed by 100 pharmaceutical companies in January 2016 ( Citation35, Citation38). It acknowledges the responsibility of pharmaceutical companies and creates a system for collective action to address the issue of the growing antibiotic resistance globally. Some industry leaders such as the CEO of DSM Sinochem Pharmaceuticals have called for industry “to go further by buying APIs only from manufacturers that do not pollute the environment and to introduce more transparency in the supply chain” ( Citation39).
2.3. Pharmaceutical industry and green chemistry
Sheldon ( Citation40) was the first to report that the pharmaceutical industry generated the most waste per unit of product compared to other chemical industry sectors – between 25 and 100 kg of waste per kilogram of API, compared to bulk chemicals which generated less than 15 kg of waste per kg of product and fine chemicals which generated between 5 and 50 kg of waste per kg of product ( Citation4). This metric called E-factor (or environmental factor), has since become a key indicator for tracking environmental and efficiency improvements by the pharmaceutical industry. Recent research has found that on average pharmaceutical companies use about 120 kg of material for making 1 kg of API and the majority of waste generated (∼80%) is solvent ( Citation11, Citation41, Citation42).
Tucker defines pharmaceutical green chemistry as “the quest for benign synthetic processes that reduce the environmental burden … within the context of enabling the delivery of our current standard of living” ( Citation43). He believes GC is driven by efficiency coupled with environmental responsibility. GC calls for the use of renewable chemicals as building blocks and reagents (Principles 7 and 9; see ). A recent practice becoming increasingly popular among big companies is biocatalysis, or the process of using enzymes as catalysts in chemical reactions. Enzymes are naturally occurring living organisms often referred to as “nature’s catalysts,” which have been found to reduce costs and risksFootnote1. Furthermore, enzymes can help reduce the number of steps and increase reaction throughput leading to a significant reduction in the time to manufacture – in some cases by 80% ( Citation44, Citation45). GC calls for using safer chemicals to minimize accidents (Principle 12) and a common practice in the pharmaceutical industry is the shift to less toxic solvents, which make up more than 80% of the material used for API manufacture and are associated with about 60% of the overall energy use and 50% of greenhouse gas (GHG) emissions ( Citation46). Green chemistry also requires energy-efficient design (Principle 6, ) and a growing number of pharmaceutical companies have begun to use their carbon footprint as a new green chemistry indicator ( Citation13).
Text Box 2. Companies participating in the green chemistry survey, February 21, 2017.
Pharmaceutical companies practicing GC design for their API processes have reported impressive decreases in manufacturing waste (see ). Such waste reductions are also associated with significant cost savings ( Citation11, Citation12). Yet, a small percent of drugs on the market presently are designed using GC principles. The API manufacturing processes for most generic drugs were designed before GC became a popular approach among pharmaceutical scientists about 15 years ago. Even for new drugs reaching the market today, it is estimated that less than half have been manufactured using GC design principles ( Citation20).
Table 1. Percent waste reduction of drug API’s using GC redesign.
To encourage greater use of GC and engineering principles the American Chemical Society (ACS) established in 2005 the ACS Green Chemistry Institute Pharmaceutical Roundtable (ACS GCIPR). The roundtable has grown from the three founding members (Pfizer, Merck and Eli Lilly) to 20 members and five associate members as of June 2018 ( Citation56). It has supported the development of tools such as Reagent Selection Guide, Solvent Selection Guide, Process Mass Intensity Calculation Tool, and Product Mass Intensity-Life Cycle Analysis Tool. It also provides grants to universities and research centers for green chemistry research and is actively involved in policy discussions in the U.S. and the EU. Generic drug companies and API manufacturers, however, have been, for the most part, absent from the roundtable membership and meetings.
Another group focused on advancing GC in the pharmaceutical industry is the Green Chemistry Working Group of the International Consortium for Innovation and Quality in Pharmaceutical Development (IQ) which was established in 2010 and includes 32 members ( Citation57). IQ analyzed the factors behind establishing an effective GC program and developed a framework of seven key elements required for implementing such a program: 1) Empowered green chemistry teams with management support; 2) Metrics and targets; 3) Resources and tools; 4) Education; 5) Awareness and recognition; 6) Investment in green technology; and 7) External collaborations. This framework has been used to establish effective GC programs as well as benchmark industry practices using publicly available data ( Citation13, Citation20).
The industry has adopted several key metrics to track progress in GC, including E-factor, Process Mass Intensity (PMI), atom economy, number of steps, and carbon footprint, among others. While E-factor was the first metric used by the industry, recent studies have pointed out PMI as the most preferred metric among “big pharma” ( Citation13). PMI measures the ratio between the mass of all materials used to make a product and the mass of the product. It is a leading indicator (compared to E-factor which is lagging), which is easy to generate and compare, and easy to apply by chemists and engineers, although it does not address concerns about environmental, health and safety of used materials. A new metric that is gaining popularity is the Green Aspiration LevelTM (GAL), the goal of which is to unify mass-based metrics ( Citation58).
Research has found that both internal (top management commitment, cost savings) and external (regulatory requirements, stakeholder pressures) factors are driving greater adoption of GC by the pharmaceutical industry ( Citation59). With the pressures to reduce prices, cost savings from GC are becoming increasingly important ( Citation11, Citation12). Such savings stem from more efficient production, reduced energy and material costs, waste disposal fees, improved employee health and safety, and lower insurance premiums. The increasing environmental legislation globally has been reported as another important driver for the adoption of GC by the pharmaceutical industry ( Citation20, Citation60). This includes European Union REACH directive, U.S. TSCA and NEPA, and incentive schemes such as the Swedish National Pharmaceutical Strategy (NPS), which calls for reducing the environmental impacts of pharmaceuticals and reporting materiality analysis, carbon footprint and other environmental metrics ( Citation13). Additional drivers reported in the literature include large customers, Sustainable, Responsible and Impact (SRI) investors, reputation and ability to attract and retain talent ( Citation13).
Researchers have identified several main types of barriers to greater adoption of green chemistry such as economic, financial, regulatory, technical, organizational, and cultural ( Citation58, Citation61). Among these, the pressures to deliver drugs faster and regulatory risk (real or perceived) have been reported as the top two barriers to greater adoption of GC by the industry ( Citation13). The perceived risk relates to the possibility (at least in theory) for a company to lose the approved status of its drug when it refiles for FDA approval after redesigning with GC considerations ( Citation20, Citation57). Additional barriers to GC identified in the literature include the upfront investment in green technology, lack of expertise and awareness of the business benefits of GC ( Citation13). Mehta reports six main barriers to implementation of GC in India ( Citation62): a) lack of available green technologies, b) scale-up and commercialization, c) lack of connection between GC solutions providers and industry, d) limited knowledge of the basic principles of GC and engineering, e) the myths that GC is difficult and complex and thus not viable for small and medium size companies, and f) regulatory hurdles, or the time and cost involved in filing a new Drug Master File (DMF) application when a change in the manufacturing process is made.
2.4. Indian pharmaceutical industry and green chemistry
Green chemistry in India started almost 20 years ago with a few small GC academic conferences. As part of the environmental movement the Department of Chemistry at the University of Delhi organized a National Symposium on Green Chemistry in January 1999 to bring together for a first time scientists who were practicing GC. Inspired by the significant attendance and interest, the university launched a course in green chemistry and the first Green Chemistry Chapter of India ( Citation63). In late 2000, GC began to gain some initial recognition by the Indian chemical industry, with the launch of industry focused green chemistry initiatives such as the Industrial Green Chemistry World ( Citation64). Between 2011 and 2015 the awareness continued to expand at different levels in the chemical industry, from chemists and chemical engineers working in laboratories and manufacturing to senior management. Various stakeholders in the industry began to examine the relevance of GC to their business and its impact. In the last couple of years GC has gained momentum in India at government level, industry, academia and media, and the number of initiatives has grown significantly ( Citation65).
According to Newreka, industry in India is transitioning from awareness to implementation phase and beginning to explore some green chemistry-based technologies and solutions. Companies are expressing the need to train their teams on GC&E principles and tools. Senior decision makers in the chemical industry are beginning to see the value that GC can add to their business and are willing to invest time, efforts and resources in exploring GC&E-based innovations ( Citation66, Citation67). Some companies in India now have a dedicated GC team and a few of them have even connected their chemist’s Key Performance Indicators (KPI) to E-Factor of the process developed by him/her ( Citation67, Citation68). In addition, there has been an increase in the number of companies nominated for the Industrial Green Chemistry Awards at the IGCW conventions and a shift from being process-centric to demonstrating a more integrated and long-term approach to GC&E implementation ( Citation69).
Indian government bodies such as the Department of Science and Technology, the Ministry of Chemicals and Fertilizers, and the Department of Pharmaceuticals, are beginning to organize various green chemistry initiatives and in some cases to partner with SMEs to partially fund investments in green technology ( Citation65). Regulatory bodies such as the State Pollution Control Boards (SPCBs) have in recent years moved from “being a pollution control board” to “being a facilitator.” These SPCBs have begun to send their environmental engineers to various GC training programs to make them aware of the various solutions available in the market place. The goal is to support and partner with industry in solving environmental problems ( Citation70).
Various national research institutes, particularly those in the domain of chemicals and applied sciences are increasingly working on GC&E related projects. For instance, India’s leading national research laboratories’ cluster – The Council of Scientific and Research Institute (CSIR) – received responses from over 10 CSIR Labs, listing their GC&E related research projects across India ( Citation71). While there is no academic emphasis on teaching GC as a subject, there has been an increase in the number of events and workshops in the area of GC as well as the number of academic institutes conducting such research. The Green Chemistry Network Centre (GCNC) at Delhi University for example, has been coordinating many educational and networking events in India ( Citation72). In addition, the Institute of Chemical Technology in Mumbai, India, recently launched a Master’s program in GC ( Citation73).
3. Study design and method
To conduct the study the researchers designed a 15-question survey to benchmark current practices, main barriers and future opportunities for greater adoption of GC by the Indian pharmaceutical industry. The survey closely matchеd the benchmarking survey used by Veleva et al. ( Citation13) in order to allow for comparisons. It was based on the IQ Green Chemistry working group framework for effective GC program and included questions about familiarity with GC, use of metrics, green technology investments, external collaborations, support for GC education, main barriers, and key drivers, among others. A few additional questions were included to gauge issues specific to the Indian pharmaceutical industry and API manufacturers, such as stage of the waste management hierarchy and chemical characterization of wastewater. The research team used a non-probability convenience sample survey. All questions allowed for quantitative analysis (% of sample population) to be used for actionable recommendations.
The paper survey was conducted in February 2017, during the second day of a two-day green chemistry conference in Vishakhapatnam, organized by Green ChemisTree Foundation and NewrekaFootnote2 in partnership with PSCI and the ACS GCIPR ( Citation74). All 120 participants that day received a copy of the survey and 89 completed it (response rate of 74%). No incentives were provided for participating in the survey; the participants were encouraged to do so in order to support the research. The data from all 89 surveys were entered into an Excel spreadsheet for cleanup and analysis. The research team worked to eliminate duplicate responses and keep only one response per company using two main criteria: a) familiarity with company GC practices, and b) seniority of the person completing the survey. Multiple responses were evaluated and integrated into one single response per company. This resulted in 56 qualified responses which were classified into four separate categories: (1) large R&D pharma, (2) smaller R&D based pharma, (3) generic pharma, and (4) API manufacturers and pharma intermediates manufacturers (called herein “API manufacturers”), and (5) anonymous companies. The last category was created as many participants did not include the name of their company (they were given this option when completing the survey). The research team did not want to lose this important data and therefore included these responses in a separate category, recognizing that there are likely multiple responses from some companies. While many companies had subsidiaries representing more than one of the above categories, they were assigned into a category based on their predominant business model. Major part of API manufacturers’ business is to design, manufactures and sell API to R&D-based pharma (groups 1 and 2) and generic pharma companies anywhere in the world (group 3). Pharma intermediates manufacturers were also included in group 4 as they are considered similar to API manufacturers (they design, manufacture and sell API intermediates to companies in all four groups located anywhere in the world). Two companies classified themselves as CRO (contract research manufacturers) and were also added to group 4. CRO's are companies manufacturing advanced intermediates and APIs for large and small pharmaceutical companies worldwide, therefore they were also included in category 4. lists all companies participating in the study. Data were analyzed with SPSS Version 20. In addition to analyzing aggregate results for each question, the authors conducted analysis for the two largest and most important categories in India: generic pharma (category 3; total of 12 companies) and API manufacturers and pharma intermediary manufacturers (category 4; total of 23 companies) to compare and contrast these two important pharmaceutical segments in India.
4. Study results and discussion
Of the 56 study participants, only 6% (3 companies) were not familiar with their company green chemistry and engineering (GC&E) activities and 38% (20 companies) were somewhat familiar. Majority of survey participants were either familiar or very familiar, and 11% (6 companies) were in charge of leading their companies’ GC&E initiatives. The study revealed that majority of the companies (73%) use a cross-functional team to lead their GC initiatives (100% of generic pharma and 59% of API manufacturers). Only two companies (4%) reported having full-time GC leader. Twenty-three percent of API manufacturers reported using other system of governance (mostly raising awareness among chemists/researchers or in a process of forming a team.)
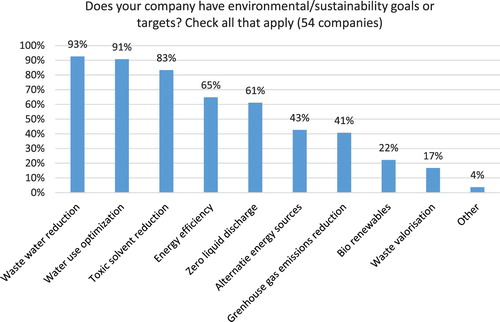
All companies in the study reported having environmental/sustainability goals and targets, with the top three related to waste water reduction (93% of companies), water use optimization (91% of companies) and toxic solvent reduction (83%) (see ). No significant differences were uncovered when comparing generic pharma and API manufacturers. These results demonstrate that Indian companies recognize water pollution as top business risk and have set goals to track improvements.
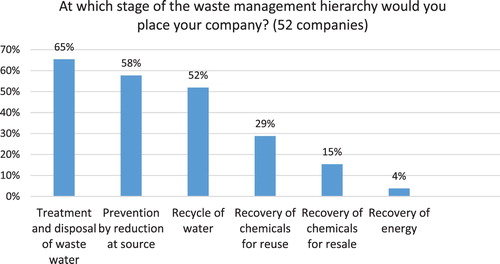
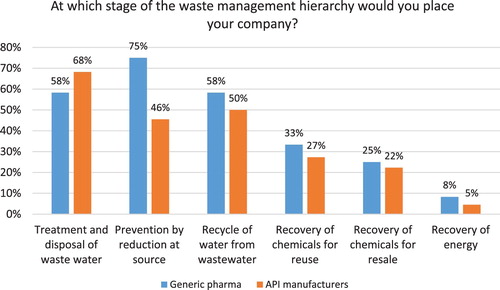
The study aimed to assess how Indian pharmaceutical companies apply the hazardous waste management hierarchy established by the European Union Waste Framework Directive (2008/98/EC), which includes the following options (from most preferred to least preferred): (a) prevention; (b) preparing for re-use; (c) recycling; (d) other recovery (e.g. energy recovery); (e) disposal ( Citation75). Each company was able to select more than one option. The results reveal that majority of Indian companies use the least preferred method of treatment and disposal (see ). However, a large number of companies (57% of respondents) reported that they also implement source reduction. Recycling of water (52%) and recovery of chemicals for reuse (29%) where the next most preferred strategies which can lead to cost savings. Interestingly, when comparing generics with API manufacturers some clear differences emerge. For instance, 75% of generic pharma reports prevention by source reduction as the number one strategy versus 46% of API manufacturers, which primarily implement treatment and disposal of waste water (68% of respondents). Both generic pharma and API manufacturers report recycling of water as the second most frequently used option (58% and 50%, respectively). Fifty-eight percent of generic pharma also reports using treatment and disposal of waste water (see ).
Forty-three percent of respondents (22 companies) reported that they do not follow comprehensive chemical characterization of their wastewater but instead characterize in the form of lumped parameters such as chemical oxygen demand (COD), biological oxygen demand (BOD), total organic carbon (TOC), total dissolved solids (TDS), and pH. Twenty-two percent (11 companies) reported that they do comprehensive chemical characterization of over 90% of their wastewater streams, and equal numbers (14% or seven companies) reported they do that for 50–70% and 70–90% of their wastewater. There were no significant differences between generic pharma and API manufacturers.
Ninety-two percent of respondents reported using GC principles, which is not surprising considering that the survey was administered at a GC conference in India. These principles were most often used in manufacturing (69% of respondents), followed by chemical development (39%) and synthetic API alone (35%).
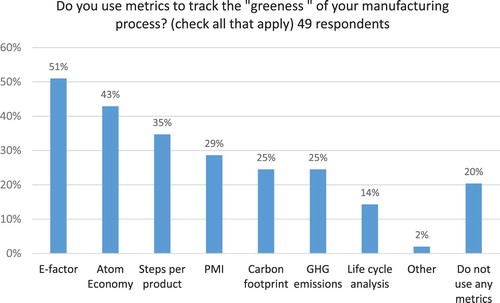
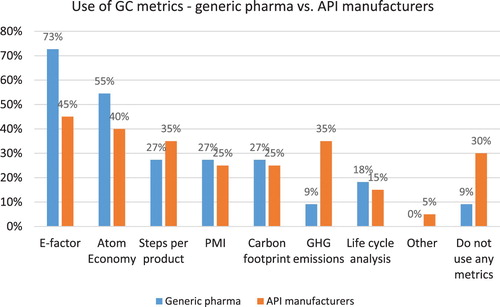
Metrics are critical for tracking performance and making improvements, yet 20% of survey respondents reported that their companies do not use any GC metrics (see ). The top three metrics used by study participants included E-factor (51% of participants), atom economy (43%), and steps per product (35%). Among generic pharma companies only 9% (1 company) reported not using any metrics while 73% reported using E-factor, 55% – atom economy and 27% – PMI, carbon footprint and steps per product (see ). Thirty percent of API manufacturers (6 companies) reported not using any GC metrics, 45% are using E-factor, 40% – atom economy and equal number (35%) are using steps per product and GHG emissions. This finding reveals that in contrast to “big pharma” which has embraced PMI as the most preferred indicator ( Citation13), Indian pharmaceutical companies continue to use E-factor as their top metric. In addition, generic pharma has adopted GC metrics to a larger extent, compared to API manufacturers.
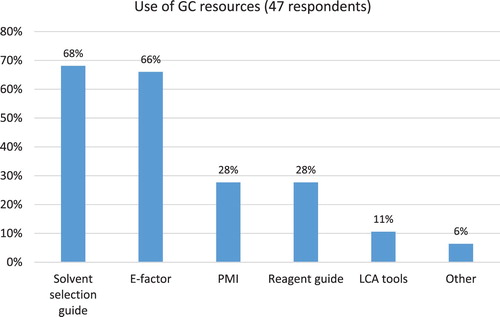
The most commonly used GC resources by study participants included Solvent Selection Guide (68% of respondents) and E-factor (66% of respondents). Less than a third of companies use PMI and Reagent Guide (see ). Additional analysis revealed that generic pharma uses to a larger extent Reagent Guide and PMI, compared to API manufacturers (46% vs. 11% and 36% vs. 17%, respectively).
Sixty percent of study participants stated that their company supports GC education and awareness. They do so primarily by supporting scientists/researchers’ training at local colleges or conferences (47%), offering internal lectures (30%), and offering eLearning opportunities in GC (26%). One in five companies reported having an internal GC award or recognition program. No significant differences were found between generic pharma and API manufacturers in the ways and extent they support GC education and awareness.
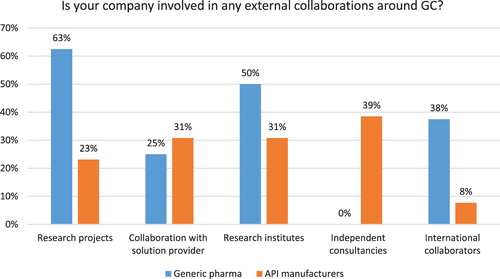
Sixty-four percent of respondents reported their company is involved in some external GC collaborations, such as research projects (33%), collaborations with solution providers (31%), research institutes and independent consultancies (each at 28%). Generic pharma reports much greater level of external collaborations with 63% of companies involved in research projects, 50% working with research institutes and 38% involved in international collaborations (see ). API manufacturers seem to outsource much of the GC work as 39% of study participants reported working with independent consultancies, while none of the generic pharma reported such practices.
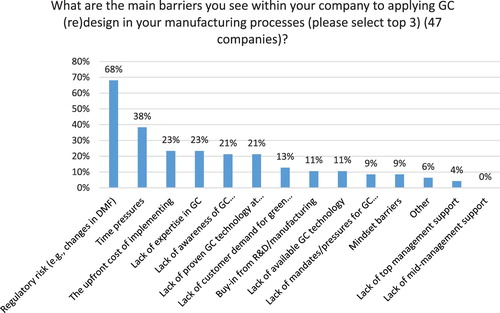
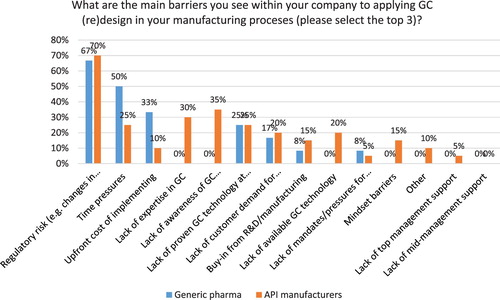
The top three barriers to greater adoption of GC in India according to study participants include: regulatory risk (68%), time pressures for delivering new drugs (38%) and the upfront cost of investing GC and lack of GC expertise (each at 23%) (see ), which confirms previous research by Veleva et al. ( Citation13). There are, however, some notable differences between generic pharma and API manufacturers. While both segments identify regulatory risk as the top barrier to greater adoption of GC (see ), for generic pharma other important barriers include time pressures (50%) and the upfront cost of GC (33%). For API manufacturers the lack of awareness of GC technology/solution providers (35%) and lack of expertise in GC (30%) demonstrate the need for more education and training for this segment. One in four companies (both generic and API manufacturers) reports lack of green technology as a key barrier. Interestingly, lack of top and middle management support and buy-in were not reported as significant barriers.
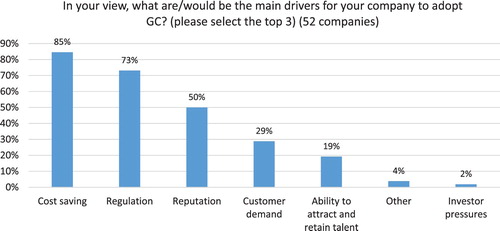
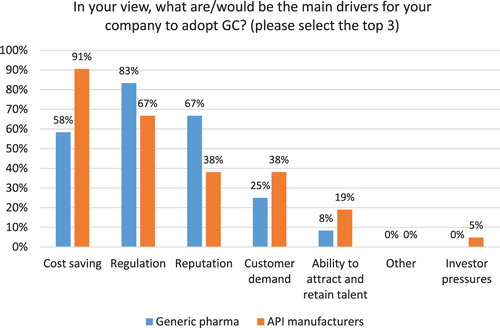
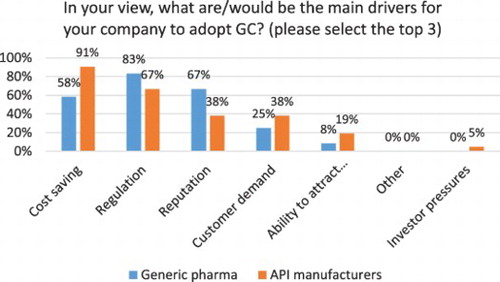
Cost savings (85%), regulation (73%) and reputation (50%) are reported as the top drivers for adoption of GC by Indian pharmaceutical companies (see ), which confirms previous research findings ( Citation13, Citation20). Generic pharma, however reports regulation as the top driver (83%), followed by reputation (67%) and cost savings (58%) (see ). For Indian API manufacturers, cost savings are the main driver reported by 91% of participants, followed by regulation (67%) and reputation and customer demand (each at 38%). This confirms previous studies outlining the importance of regulatory mandates for generic drug companies and API manufacturers that are almost entirely compliance driven and experience few pressures form investors and NGOs ( Citation20, Citation23, Citation62, Citation65).
When asked how relevant and critical it is for the pharma industry to adopt GC practices, 83% of participants reported that it as “very relevant and critical,” 11% see it as “moderately relevant,” and only 6% (3 companies) as “not so relevant.”
5. Conclusion and recommendations
The main contribution of this paper is that it provides the first benchmarking study of GC adoption by the Indian pharmaceutical supply chain based on information from industry representatives leading such efforts. The findings confirm previous research that generic drug pharma and API manufacturers in India exhibit significant interest and some advances in using GC principles (e.g. 92% use GC principles, 80% use metrics, over two thirds use GC resources such as the Solvent Selection Guide, 60% support GC education and awareness, and 64% are involved in external GC collaborations). At the same time, majority (65%) of Indian companies rely on treatment and disposal of waste water versus source reduction. E-factor remains the most popular GC metric used by Indian pharmaceutical companies and API manufacturers, compared to “big pharma” which recently has shifted toward using PMI to a larger extent ( Citation13).
There are also some notable differences between generic pharma and API manufacturers, demonstrating that generic pharma is more advanced in the adoption of GC principles, possibly a result of having more resources and selling directly to regulated markets like U.S. and Europe. For instance, 75% of generic pharma report using source reduction as part of their waste management, compared to 46% of API manufacturers who rely primarily on treatment and disposal (68%). Only 9% of generic pharma reported not using any GC metrics, compared to 30% of API manufacturers. Generic pharma is also involved in external collaborations to a larger extent than API manufacturers, which seem to outsource GC work to solution providers (39%).
The study confirmed previous findings that regulatory risk and time pressures to deliver drugs are the two most significant barriers for greater adoption of GC ( Citation13). In addition, it found that for API manufacturers in particular the lack of awareness and expertise in GC are also a significant barrier, demonstrating the need for more training and education. Cost savings and environmental regulations were cited as the top two drivers for adopting GC in India, confirming previous findings by Veleva et al. ( Citation13). For generic pharma, however, reputation was reported as a top driver cited by 67% of participants (regulation was also cited by 67% and cost savings by 58% of study participants). This finding demonstrates that external drivers are becoming increasingly important for generic drug companies due to growing pressures from various stakeholders to address their environmental impacts. In addition, the findings demonstrate the need for stricter regulation to advance GC as Indian pharmaceutical companies and API manufacturers are to a large extent compliance driven. Overcoming the main barriers requires addressing the issue of regulatory risk (real or perceived) by implementing fast track or other more streamlined approval for green drugs. Advancing GC adoption in India requires improving the supply chain management by increasing transparency and reporting of supplier practices by PSCI members and other large pharmaceutical companies, similar to the steps taken by other sectors (e.g. electronics and footwear).
The research has several limitations. First, because of limited time and resources the study involved only 56 companies; future research should aim to include a larger number of companies to allow for additional analysis and comparisons. Second, because of the low number of survey participants from large and small R&D pharma, it was not possible to do separate analysis for these segments. Third, the survey allowed participants to avoid listing their company name, thus it is possible that there are multiple responses for the same company among the 17 anonymous respondents. Finally, using a survey for data collection presents some inherent limitations such as data reliability and validity, potential bias of respondents who choose to participate in the survey, and incorrect answers due to limited knowledge on a particular issue. Although the research team carefully designed the questions to be specific and non-subjective, it is recommended that future research includes additional data collection methods such as interviews and publicly available data.
On the basis of the literature review, their previous research, and the findings from the current study, the authors see the need for the following actions to advance GC adoption by the Indian pharmaceutical supply chain:
Pharmaceutical companies which purchase formulated drug, API or a regulatory starting material/advanced API intermediates should include green chemistry performance indicators as part of their supplier audit.
To address the persistent references to regulations being a barrier to adopting green chemistry, there is a need to raise awareness among global regulatory agencies like the FDA, EMEA and WHO, about the opportunities that GC process redesign provides to substantially reduce the environmental impact of drug manufacturing. Ideally, regulatory agencies should implement a “fast track” or other more streamlined approval for drugs with GC to eliminate the regulatory risk (perceived or real) as proposed previously (Citation20).
Convene an ICH-like workgroup comprised of companies, regulatory agencies and GC experts to design a guidance based on application of GC principles. The goal of this effort should be to incorporate environmental considerations in the GMP’s. Such guidance should incorporate metrics to demonstrate the effectiveness of the GC design effort, including a comparison of waste from the existing process and the greener alternative.
Develop a pharmaceutical-centric GC education program to train employees who work in the generic drug and API manufacturing sectors. This program should be part of an employee’s professional certification.
Finally, the authors see the need to conduct a similar benchmarking study for generic drug companies, API and advanced intermediate manufacturers in China, in order to compare and contrast these two important markets. The authors have already begun to plan such a study and are currently seeking funding and a local partner.
This study demonstrates that almost 20 years after the emergence of GC in India, only a small number of pharmaceutical companies have implemented it in a strategic way. While generic pharma appears more advanced than API manufacturers, both segments need more education, technical and financial assistance, regulatory mandates and customer demand for “greener” drugs, to make further progress. With increasing outsourcing to India and China, and the shift to generic drugs, addressing the environmental impacts of drug manufacturing is crucial for both Indian companies (which could lose their competitive position to China) as well as “big pharma” sourcing from there (which could experience supply chain disruptions due to regulatory or stakeholder actions).
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr. Vesela R. Veleva is a faculty member in the Department of Management and the Center for Sustainable Enterprise and Regional Competitiveness (SERC) at the University of Massachusetts Boston. She also serves as Director of the MBA Program and Interim Director of the Healthcare Management Program at the College of Management, UMass Boston. Her research focuses on the circular economy, green chemistry, industrial ecology, environmental health and corporate social responsibility. She has published in peer reviewed journals including the Journal of Cleaner Production, Sustainable Chemistry & Engineering, Greener Management International, Corporate Environmental Strategy, and Benchmarking: An International Journal. She has published several teaching cases and a book - “Business, Environment and Society: Themes and Cases” (Baywood Publishing Co., 2014). Dr. Veleva has a doctorate in Cleaner Production and Pollution Prevention from the University of Massachusetts Lowell, an M.S. in Pollution & Environmental Control from the University of Manchester UK, and a BS in Electrical Engineering from the Technical University of Varna, Bulgaria.
Dr. Berkeley W. Cue consults for pharmaceutical and technology companies through BWC Pharma Consulting, LLC. While he was at Pfizer (1975-2004) he was responsible for Pharmaceutical Sciences at the Groton, Connecticut R&D center. He was a member of the site leadership team and the Global Pharmaceutical Sciences Executive Team. He created and led Pfizer’s worldwide green chemistry efforts until he retired, after almost 29 years. From 2004, he was first a governing board member then the chair of the governing board of the ACS Green Chemistry Institute, where he helped found and led their Pharmaceutical Roundtable. He also was an advisor and founding member of the Green Chemistry and Commerce Council (GC3). He has given over 150 presentations on green chemistry and sustainability, published nearly two dozen peer reviewed articles and holds almost twenty patents. With Professor Wei Zhang at UMass-Boston he edited two green chemistry texts, “Green Techniques for Organic Synthesis and Medicinal Chemistry,” published by Wiley in June 2012, and the second edition in 2018. At UMass Boston he is an adjunct professor in the chemistry department, a member of advisory boards for their College of Science and Mathematics and the College of Management’s Center for Sustainable Enterprise and Regional Competiveness (SERC). In 2013 he received EPA Region 1’s Lifetime Environmental Merit Award. Dr. Cue graduated with a BA in Chemistry from UMass Boston in 1969 and earned his PhD from the University of Alabama.
Dr. Svetlana Todorova is a visiting lecturer in Business Statistics at the D’Amore-McKim School of Business, Northeastern University, Boston, Massachusetts. In addition, she is a chief assistant professor in Statistics at Varna University of Economics, Bulgaria. Dr. Todorova’s research interests are highly interdisciplinary. Her research focuses on the applications of statistics in the fields of business, economics, education, and most recently sustainability, “zero waste”, and green chemistry. She is an author and coauthor of over 30 publications, including monographs, book chapters, peer-reviewed journal articles, and papers presented at various international conferences. She holds a B.S. in Socio-Economic Information and an M.S. and Ph.D. in Statistics, all from Varna University of Economics, Bulgaria.
Mr. Harshrajsinh Thakor is an Environmental Engineer for the State of Massachusetts in the Department of Environmental Protection. Presently he is pursuing his MBA at the University of Massachusetts Boston with specialization in Environmental Management. He has published a book chapter in ACS Emerging Micro-Pollutants in the Environment: Occurrence, Fate, and Distribution and has co-authored an article in peer reviewed journal, Journal of Nanoparticle Research. He holds a B.E in Civil Engineering from the University of Pune, India, and a M.S in Environmental Engineering from University of Massachusetts Lowell, USA.
Nitesh H. Mehta is founding director of Newreka Green Synth Technologies Pvt. Ltd., based in India. He holds a master’s degree in chemical engineering from the Indian Institute of Technology, IIT-Bombay, and a bachelor’s degree in chemical engineering from the University of Pune. His expertise is in process development, scale-up and technology implementation at commercial scales. His professional experience includes implementing more than 500 commercial batches with Newreka Reduction Technology, in more than 25 different products. Currently, his key focus involves marketing Newreka’s knowledge-based Green Chemistry technologies in Indian and international markets. Nitesh Mehta is also the co-founder of Green ChemisTree Foundation and convener of Industrial Green Chemistry World (IGCW), a not-for-profit initiative launched in 2009, with the commitment to promote and advance the implementation of Green Chemistry in India.
Krishna B. Padia is Executive Director of Green ChemisTree Foundation. She has a Master’s degree in Environment & Ecology and has extensive experience working in the social sector, specifically in environmental conservation and related activities. Ms. Padia is associated with Green ChemisTree Foundation since its inception in 2008. She is responsible for the overall execution of Foundation’s activities and expanding the awareness of Green Chemistry & Green Engineering amongst chemical communities (both industry and non-industry). She is also on the Advisory Board of an NGO working on environmental rehabilitation project in the Tribal villages of Palghar District, Maharashtra.
Notes
1 Typical catalysis often includes rare earth metals such as palladium which are not only costly but present a range of sociopolitical risks as supply is controlled by South Africa, Russia and China.
2 Newreka is a knowledge-based company involved in developing, customizing and commercializing green chemistry-based technologies for the Indian chemical industry. It is headquartered in Mumbai and has three R&D laboratories in Western India. Green ChemisTree Foundation is the philanthropic arm of Newreka, dedicated to promoting and advancing green chemistry in India. Its flagship initiative is the Industrial Green Chemistry World (IGCW), which brings together a variety of stakeholders from industry, government, academia and consultancies to expand the awareness and technical capabilities in the area of green chemistry and engineering (GC&E). For more information see: www.industrialgreenchem.com.
References
- Macdonald, G. China Still the Biggest Source of Drug Ingredients Says Indian Health Ministry. In-Pharma, January 9, 2018, https://www.in-pharmatechnologist.com/Article/2018/01/09/China-still-biggest-source-of-drug-ingredients-says-Indian-Health-Minister?utm_source=copyright&utm_medium=OnSite&utm_campaign=copyright (accessed June 17, 2018).
- Zhang, J. Comparing China’s and India’s Pharmaceutical Manufacturing. Pharmaceutical Online, August 30, 2012, https://www.pharmaceuticalonline.com/doc/comparing-china-s-and-india-s-pharmaceutical-manufacturing-0001 (accessed June 17, 2018).
- Chidambaram, A. Indian Generic Pharmaceutical Market: A Snapshot. Frost & Sullivan, July 27, 2012, http://www.frost.com/sublib/display-market-insight-top.do?id=264038078 (accessed June 20, 2018).
- Deloitte. 2017 Global Life Sciences Outlook. 2016. https://www2.deloitte.com/global/en/pages/life-sciences-and-healthcare/articles/global-life-sciences-sector-outlook.html (accessed June 17, 2018).
- Altestedter, A.; Hopkins, J.; Modi, M. With US Generic Drug Market in Chaos, Indian Upstarts Rise. August 31, 2017, https://www.bloomberg.com/news/articles/2017-08-30/family-owned-drug-factories-in-india-erode-u-s-generic-prices (accessed July 6, 2018).
- Guttager, S. Pollution from Pharmaceutical Manufacturing an Unexpected Source of Superbugs. The Conversation, June 7, 2017, https://phys.org/news/2017-06-pollution-pharmaceutical-unexpected-source-superbugs.html (accessed June 20, 2018).
- Larsson, D.; de Pedro, C.; Paxeus, N. Effluent From Drug Manufactures Contain Extremely High Levels of Pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755.
- Reddy, U. PCB Cracks Down, Shuts Four Polluting Industries. The Times of India, March 22, 2018, https://timesofindia.indiatimes.com/city/hyderabad/pcb-cracks-down-shuts-four-polluting-industries/articleshow/63408285.cms (accessed June 20, 2018).
- Mullin, R. Drug Chemical Makers Brace as China Cracks Down on Pollution. Chem. Eng. News 2018, 96 (7), 23–25.
- U.S. EPA, Green Chemistry. 2015, http://www2.epa.gov/green-chemistry (accessed June 21, 2018).
- Cue, B.; Berridge, J.; Manley, J. PAT and Green Chemistry: The Intersection of Benign by Design and Quality by Design. Pharma. Eng. 2009, March/April, 29 (2), 8-18. http://honors490-2014-penner.wikispaces.umb.edu/file/view/PAT_final_print_ISPE_March_April_2009.pdf (accessed June 20, 2018).
- Veleva V.; Sarkar, M. Pfizer: Environmental and Business Benefits of Green Chemistry. Case # 9B15M043, Richard Ivey School of Business, The University of Western Ontario, Canada, 2015, http://www.iesep.com/en/pfizer-environmental-and-business-benefits-of-green-chemistry-117504 (accessed June 20, 2018).
- Veleva, V.; Cue Jr, B.; Todorova, S. Benchmarking Green Chemistry Adoption by the Global Pharmaceutical Supply Chain. ACS Sustain. Chem. Eng. 2018, 6 (1), 2–14.
- DeVito, S.; Keenan, C.; Lazarus, D. Can Pollutant Release and Transfer Registers (PRTRs) be Used to Assess Implementation and Effectiveness of Green Chemistry Practices? A Case Study Involving the Toxics Release Inventory (TRI) and Pharmaceutical Manufacturers. Green Chem. 2015, 5, 2679–2692.
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998, 1998.
- BCC Research. Market Opportunities Aplenty as Adoption of “Green Chemistry” Grows. 2016, https://www.bccresearch.com/pressroom/chm/market-opportunities-aplenty-as-adoption-of-%E2%80%9Cgreen-chemistry%E2%80%9D-grows (accessed June 20, 2018).
- Statista. Global Pharmaceutical Sales from 2015 to 2017 by Region (in Billion U.S. Dollars). 2018, https://www.statista.com/statistics/272181/world-pharmaceutical-sales-by-region/
- Pharmaceutical Commerce. Global Pharma Market Will Reach $1.12 Trillion in 2022. 2016, http://pharmaceuticalcommerce.com/business-and-finance/global-pharma-market-will-reach-1-12-trillion-2022/ (accessed June 20, 2018).
- Statista. U.S. Pharmaceutical Industry: Statistics & Facts. 2018, https://www.statista.com/topics/1719/pharmaceutical-industry/ (accessed June 20, 2018).
- Veleva, V.; Cue Jr., B. Benchmarking Green Chemistry Adoption by “big Pharma” and Generics Manufacturers. Benchmarking Int. J. 2017, 24 (5), 1414–1436.
- Zion Market Research. Global Generic Drug Market Will Reach $380.6 Billion by 2021. 2017, https://globenewswire.com/news-release/2017/05/04/978298/0/en/Global-Generic-Drug-Market-will-reach-USD-380-60-Billion-by-2021-Zion-Market-Research.html (accessed June 18, 2018).
- Nordea. Hyderabad’s Pharmaceutical Pollution Crisis. 2018, http://changingmarkets.org/wp-content/uploads/2018/01/CM-HYDERABAD-s-PHARMACEUTICAL-POLLUTION-CRISIS-FINAL-WEB-SPREAD.pdf (accessed June 20, 2018).
- Mourdoukoutas, P. India’s Economy on Track to Beat China. Forbes, April 21, 2018, https://www.forbes.com/sites/panosmourdoukoutas/2018/04/21/indias-economy-on-track-to-beat-china/#3618edbf5136 (accessed July 6, 2018).
- Nordea. Impacts of Pharmaceutical Pollution on Communities and Environment in India. Report Funded by Changing Market Foundation, 2016, http://changingmarkets.org/wp-content/uploads/2016/12/Impacts-of-pharmaceutical-pollution-on-communities-and-environment-in-India-WEB-light.pdf (accessed June 20, 2018).
- Mathew, G.; Unnikrishnan, M. The Emerging Environmental Burden from Pharmaceuticals. Economic & Political Weekly, Vol. XLVII, No.18, May 5th, 2012, http://www.indiaenvironmentportal.org.in/files/file/Burden%20from%20Pharmaceuticals.pdf (accessed June 19, 2018).
- Taylor, A. Bhopal: The World’s Worst Industrial Disaster, 30 Years Later. The Atlantic, December 2, 2014, https://www.theatlantic.com/photo/2014/12/bhopal-the-worlds-worst-industrial-disaster-30-years-later/100864/ (accessed June 20, 2018).
- Burke, J. Half of India’s Rivers Are Polluted, Says a Government Report. The Guardian, April 7, 2015, https://www.theguardian.com/world/2015/apr/07/half-india-rivers-polluted-new-government-report (accessed June 20, 2018).
- Dey, S. 80% of India’s Surface Water May Be Polluted, Report by International Body Says. The Times of India, June 28, 2015, https://timesofindia.indiatimes.com/home/environment/pollution/80-of-Indias-surface-water-may-be-polluted-report-by-international-body-says/articleshow/47848532.cms (accessed June 20, 2018).
- PriceWaterhouseCoopers (PWC). Global Pharma Looks to India: Prospects for Growth. 2010, https://www.pwc.com/gx/en/pharma-life-sciences/pdf/global-pharma-looks-to-india.pdf (accessed June 21, 2018).
- World Health Organization (WHO). WHO’s First Global Report on Antibiotic Resistance Reveals Serious, Worldwide Threat to Public Health. 2014, http://www.who.int/mediacentre/news/releases/2014/amr-report/en/ (accessed June 9, 2018).
- Lubbert, C.; Baars, C.; Dayakar, A.; Lippmann, N.; Rodloff, A.; Kinzig, M.; Sorgel, F. Environmental Pollution with Antimicrobial Agents From Bulk Drug Manufacturing Industries in Hyderabad, South India, is Associated with Dissemination of Extended-Spectrum Beta-Lactamase and Carbapenemase-Producing Pathogens. Infection 2017, 45 (4), 479–491.
- Purushotham, D.; Linga, D.; Sagar, N.; Mishra, S.; Naga Vinod, G.; Venkatesham, K.; Saikrishna, K. Groundwater Contamination in Parts of Nalgonda District, Telangana, India as Revealed by Trace Elemental Studies. J. Geol. Soc. India 2017, 90 (4), 447–458.
- Shroff V.; Jejurkar, A. Environmental Law in India – Does it lack teeth? India Law News, September 1, 2011, https://indialawnews.org/2011/09/01/environmental-law-in-india-does-it-lack-teeth/ (accessed June 21, 2018).
- Pruden, A.; Larsson, J.; Amezquita, A.; Collignon, P.; Brandt, K.; Graham, D.; Lazorchak, J.; Suzuki, S.; Silley, P.; Snape, J.; Topp, E.; Zhang, T.; Zhu, Y. Management Options for Reducing the Release of Antibiotics and Antibiotic Resistance Genes to the Environment. Environ. Health Perspect. 2013, 121 (8), 878–885.
- Milmo, S. Pharmaceutical Environmental Pollution and Antimicrobial Resistance. PharmaTech.com, October 2, 2017, http://www.pharmtech.com/pharmaceutical-environmental-pollution-and-antimicrobial-resistance (accessed June 21, 2018).
- Pharmaceutical Supply Chain Initiatives (PSCI). About Us: Helping Suppliers Meet Industry Expectations. 2018, https://pscinitiative.org/home (accessed June 21, 2018).
- Pharmaceutical Supply Chain Initiatives (PSCI). Our Members. 2018, https://pscinitiative.org/members (accessed June 21, 2018).
- IFPMA. Industry Roadmap for Progress on Combating Antimicrobial Resistance. September 2016, 2016, https://www.ifpma.org/wp-content/uploads/2018/06/Roadmap-for-Progress-on-AMR-FINAL.pdf (accessed June 21, 2018).
- Macdonald, G. DSP: Pharmas Must Be Open about API Suppliers to Fight Antibiotic Resistance. In-PharmaTechnologist.com, September 25, 2016, https://www.in-pharmatechnologist.com/Article/2016/09/26/DSP-Pharmas-must-be-open-about-API-suppliers-to-fight-antibiotic-resistance.
- Sheldon, R.A. Green Chemistry Performance Metrics: E-Factor. Chem. Tech. 1994, 24, 38–47.
- Jimenez-Gonzalez, C.; Curzons, A.; Constable, D.; Cunningham, V. Cradle-to-Gate Life Cycle Inventory and Assessment of Pharmaceutical Compound. Int. J. Life Cycle Assess. 2004, 9 (2), 114–121.
- Henderson, R.; Kindervater, J.; J. Manley. Lessons Learned Through Measuring Green Chemistry Performance – The Pharmaceutical Experience. Presented at the 11th Annual Green Chemistry and Engineering Conference, Washington DC, June 2007.
- Tucker, J. Green Chemistry, a Pharmaceutical Perspective. Org. Process Res. Dev. 2006, 10 (2), 315–319.
- Sharma, V. Applicability of Green Chemistry in Pharmaceutical Processes. Pharma. BioWorld. 2015, March, pp. 34-36, http://www.piramal.com/pharmasolutions/wp-content/uploads/Applicability-of-Green-Chemistry-in-Pharmaceutical-Processes.pdf (accessed June 21, 2018).
- Tucker, J.; Faul, M. Drug Companies Must Adopt Green Chemistry. Nature 2016, 534, 27–29.
- Dunn, P. The Importance of Green Chemistry in Process Research and Development. Chem. Soc. Rev. 2012, 41, 1452–1461.
- Taber, G.; Pfisterer, D.; Colberg, J. A New and Simplified Process for Preparing N-[4-(3,4-Dichlorophenyl)-3,4-Dihydro-1(2H)-Naphthalenylidene]Methanamine and a Telescoped Process for the Synthesis of (1S-cis)-4-(3,4-Dichlorophenol)-1,2,3,4-Tetrahydro-N-Methyl-1-Naphthalenamine Mandelate: Key Intermediates in the Synthesis of Sertraline Hydrochloride. Org. Proc. Res. Dev. 2004, 8 (3), 385–388.
- Dunn, P.; Galvin, S.; Hettenbach, K. The Development of an Environmentally Benign Synthesis of Sildenafil Citrate (Viagra™) and Its Assessment by Green Chemistry Metrics. Green Chem. 2004, 6, 43–48.
- Letendre, L.; Snoddy, C.; Klemm, G.; McGhee, W. Green Chemistry in the Redesign of the Celecoxib Manufacturing Process. AIChE Annual Meeting, Abstract #223, 2008, https://www.aiche.org/conferences/aiche-annual-meeting/2008/proceeding/paper/223a-green-chemistry-redesign-celecoxib-manufacturing-process (accessed June 22, 2018).
- Martinez, C.; Hu, S.; Dumond, Y.; Tao, J.; Kelleher, P.; Tully, L. Development of a Chemoenzymatic Manufacturing Process for Pregabalin. Org. Process Res. Dev. 2008, 12 (3), 392–398.
- Jennings, S. A Green Process for the Synthesis of Quinapril Hydrochloride: Summary for the Presidential Green Chemistry Challenge Awards Program. 2005, www.epa.gov/greenchemistry (accessed June 22, 2018).
- Desai, A. Sitagliptin Manufacture: A Compelling Tale of Green Chemistry, Process Intensification, and Industrial Asymmetric Catalysis. Angew. Chem. 2011, 50 (9), 1974–1976.
- Dunn, P.; Wells, A.; Williams, M. The Taxol® Story – Development of a Green Synthesis via Plant Cell Fermentation. In Green Chemistry in the Pharmaceutical Industry, Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp 145–160. Chapter 7.
- Verghese, J.; Kong, C.J.; Rivalti, D.; Yu, E.C.; Krack, R.; Alcazar, J.; Manley, J.C.; McQuade, D.T.; Ahmad, S.; Belecki, K.; Gupton, B.F. Increasing Global Access to the High-Volume HIV Drug Nevirapine Through Process Intensification. J. Green Chem. 2017, 19, 2986–2991.
- Roche Colorado Corporation. An Efficient Process for the Production of Cytovene®, a Potent Antiviral Agent. Presidential Green Chemistry Challenge, A Greener Synthetic Pathways Award, 2000, https://www.epa.gov/greenchemistry/presidential-green-chemistry-challenge-2000-greener-synthetic-pathways-award (accessed June 22, 2018).
- American Chemical Society (ACS). ACS GCI Pharmaceutical Roundtable. https://www.acs.org/content/acs/en/greenchemistry/industry-business/pharmaceutical.html (accessed June 21, 2018).
- Leahy, D.; Tucker, J.; Mergelsberg, I.; Dunn, P.; Kopach, M.; Purohit, V. Seven Important Elements for an Effective Green Chemistry Program: An IQ Consortium Perspective. Org. Process Res. Dev. 2013, 17, 1099–1109.
- Roschangar, F.; Sheldon, R.; Senanayake, C. Overcoming Barriers to Green Chemistry in the Pharmaceutical Industry – the Green Aspiration LevelTM Concept. Green Chem. 2015, 17, 752–768.
- Sharma, V. Applicability of green chemistry in pharmaceutical processes. Pharma Bio World, March 2015, pp. 34-36, http://www.piramal.com/pharmasolutions/wp-content/uploads/Applicability-of-Green-Chemistry-in-Pharmaceutical-Processes.pdf.
- Pinto, R.; Silvestre, S. Pharmaceutical Green Chemistry: Is Just Green Chemistry or is Something Else More? J. Chem. Eng. Chem. Res. 2014, 1 (5), 290–301. http://www.ethanpublishing.com/uploadfile/2014/1202/20141202102937656.pdf.
- Matus, K.; Clark, W.; Anastas, P.; Zimmerman, J. Barriers to Implementation of Green Chemistry in the United States. Environ. Sci. Eng. 2012, 46 (20), 10892–10899.
- Mehta, N. Six barriers to implementation of green chemistry in India. Business Standard, January 6, 2014, http://www.business-standard.com/content/b2b-chemicals/six-barriers-to-implementation-of-green-chemistry-in-india-114010600785_1.html (accessed July 2, 2018).
- Kidwai, M. Green Chemistry in India. Pure Appl. Chem. 2001, 73 (8), 1261–1263.
- Industrial Green Chemistry World (IGCW). 2018, https://www.revolvy.com/topic/Industrial%20Green%20Chemistry%20World (accessed June 21, 2018).
- Office of the Principal Scientific Adviser to the Government of India (OPSAGI). Green Chemistry. 2018, http://psa.gov.in/green-chemistry (accessed June 21, 2018).
- Indian Chemical Industry (ICI). Five Year Plan – 2012-2017. 2012, http://planningcommission.gov.in/aboutus/committee/wrkgrp12/wg_chem0203.pdf (accessed June 21, 2018).
- Doshi, M. IGCW -2017 Advances Green Chemistry Innovation in India. August 17, 2017, https://communities.acs.org/community/science/sustainability/green-chemistry-nexus-blog/blog/2017/08/17/igcw-2017-advances-green-chemistry-innovation-in-india (accessed June 21, 2018).
- Green ChemisTree Foundation. 5th IGCW – 2-17 Convention & Ecosystem Outcome Report. 2017, http://www.industrialgreenchem.com/report.compressed.pdf (accessed July 3, 2018).
- Industrial Green Chemistry World (IGCW). SERB – IGCW 2017 Awards. 2017, http://industrialgreenchem.com/SERB.html (accessed June 21, 2018).
- Industrial Green Chemistry World (IGCW). IGCW 2017: GC&E conference for State Pollution Control Boards. Chemical Weekly, August 15, 2017, http://www.industrialgreenchem.com/images/pdf-docs/IGCW2017/August%2015.pdf.
- CSIR. CSIR – Indian Institute of Petroleum. 2018, http://www.iip.res.in/divisions.php?pgID=div_34&type=34 (accessed June 21, 2018).
- Sharma, V. Green Chemistry Education: Facilitating Transition to Green Technologies and Processes in India. 13th Annual Green Chemistry and Engineering Conference, 2009, http://acs.confex.com/acs/green09/webprogram/Paper69426.html (accessed June 19, 2018).
- Institute of Chemical Technology Mumbai (ICTM). Centre for Green Chemistry, 2018, http://ictmumbai.edu.in/DisplayPage.aspx?page=i&ItemID=12 (accessed June 21, 2018).
- Green ChemisTree Foundation. Two-day Conference & Exhibition on Green Chemistry & Engineering enabling greater business value for an environmentally responsible Pharma supply chain. February 20-21, 2017, Vishakhapatnam, India, http://www.greenchemistree.co.in/pdf-docs/Vizag%20Event/Vizag%20Conference%20Flyer.pdf (accessed July 21, 2018).
- European Commission. Directive 2008/98/EC on Waste (Waste Framework Directive). 2016, http://ec.europa.eu/environment/waste/framework/ (accessed July 6, 2018).