ABSTRACT
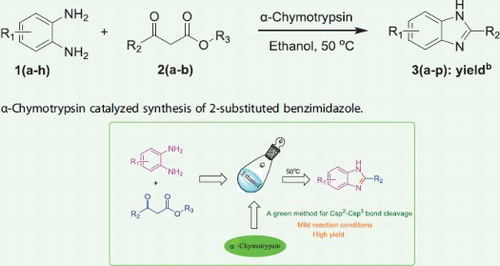
Benzimidazoles are important components of pharmaceutical compounds owing to their promising bioactivities. The green, safe, and efficient synthesis of benzimidazoles has always been one of the hot topics for chemical workers. α-Chymotrypsin-catalyzed synthesis of benzimidazoles was developed by a reaction between β-keotester and o-phenylenediamine via retro-Claisen reaction. In this eco-friendly medium, a variety of benzimidazole derivatives were obtained in good to excellent yields, with just 10 mg/mL α-Chymotrypsin as catalyst.
GRAPHICAL ABSTRACT
KEYWORDS:
Introduction
Benzimidazoles are abundant in a broad number of pharmaceutical compounds, and are widely applied as bactericides ( Citation1), anticarcinogens ( Citation2), and peptic ulcer agents ( Citation3) owing to their promising bioactivities ( Citation4–8). Therefore, tremendous efforts have been devoted to develop a methodology to construct benzimidazoles during the past decades. Conventional protocols intensively focused on the condensation of o-phenylenediamine with aldehydes using oxidants or carboxylic acid derivatives under harsh conditions ( Citation9–18). With the gradual development of organic synthesis, oxidative condensation of alcohols or amines with aromatic 1,2-diamines has also been developed ( Citation19,Citation20). In particular, benzimidazoles were readily accessed by the copper(I)-catalyzed coupling of o-haloacetanilide with amine/amidine ( Citation21–24), in which transition metals and a long reaction time were required to obtain satisfactory yields. In addition, Bronsted acids have been applied as good accelerators for the synthesis of benzimidazoles from 2-amino anilines with β-dicarbonyl compounds ( Citation25,Citation26). Although significant achievements have been made, there are several drawbacks in the reported methodologies, including harsh reaction conditions, long reaction times, excess strong oxidants, high loading of expensive catalysts (additives), and difficulty in experiment handling. Therefore, effective, eco-friendly, and sustainable biocatalytic methods are still in high demand.
Compared with chemical catalysts, biocatalysts have attracted greater attention owing to their low toxicity, high efficiency, and good selectivity (chemical, regional, and optical selectivities), which make them suitable for application in agrochemical products, drugs, and refined chemicals ( Citation27,Citation28). As efficient biocatalysts, enzymes facilitate multifunctional non-natural reactions ( Citation29) because of their unique catalytic promiscuity, which strongly favors their application in the realm of organic synthesis, e.g. aldol reactions ( Citation30–32), Michael additions ( Citation33–35), Mannich reactions ( Citation36–37), and Henry reactions ( Citation38). As a proteolytic enzyme, α-chymotrypsin exhibits higher catalytic activity and selectivity in the synthesis of heterocyclic compounds ( Citation39–41). In order to extend the application of enzymes to more challenging organic synthesis, we envisioned that α-chymotrypsin catalysis may provide a practical approach to directly accessing various functional benzimidazoles.
Here, α-chymotrypsin was selected as the biocatalyst, and a series of benzimidazoles were readily obtained from substituted o-phenylenediamines and β-ketoesters via retro-Claisen reaction under mild reaction conditions. In contrast to previous reports, our protocol provides an oxidant-free route to various functional-group-substituted benzimidazoles in excellent yields.
Results and discussion
To confirm our hypothesis, the catalytic activities of various enzymes were explored using o-phenylenediamine 1a and ethyl acetoacetate 2a in a model reaction (). No product was observed in the absence of the enzyme (, entry 1). After detailed optimization of various reaction conditions, 93% yield of 2-methyl benzimidazole 3a was obtained in the presence of α-chymotrypsin and ethanol as the solvent at 50°C for 18 h (, entry 2). When pepsin and lipase were applied under standard conditions, the yields were decreased (45% and 48%, respectively, entries 3 and 4). However, other hydrolases, such as protease from Aspergillus saitoi, amano lipase from Pseudomonas fluorescens, amano lipase M from Mucor javanicus, and protease from Bacillus licheniformis only provided low yields (, entries 5–8). In addition, no reaction occurred when other enzymes were applied directly, which included albumin from bovine serum, papain from papaya latex, amano lipase A from Aspergillus niger, amano lipase PS from Burkholderiacepacia, and amano acylase, lipase B from Candida antarctica (, entries 9–14). No product was detected when the reaction was incubated with denatured α-chymotrypsin, which suggested that the specific structure of the enzyme was necessary for this biocatalytic reaction (, entry 15).
Table 1. Catalytic activities of different enzymes.a
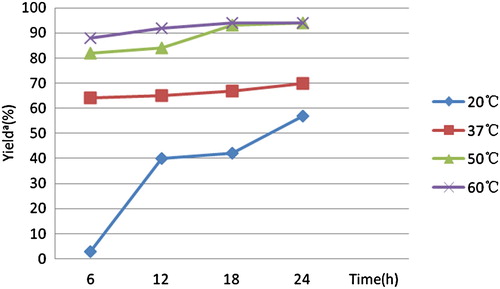
Due to the key influencing factors in most biocatalytic reactions, the effect of temperature was studied under α-chymotrypsin catalysis. As can be seen from , compared to the yields at 20°C and 37°C, a higher yield of 93% was obtained at 50°C in 18 h. Though a higher yield was obtained at 60°C in a short time, 50°C was chosen as the optimal temperature in terms of energy consumption and possible deactivation of the enzyme upon prolonged exposure to high temperatures.
Figure 1. Influence of temperature on the model reactiona. Notes: aConditions: o-phenylenediamine (0.25 mmol), β-ketoester (0.25 mmol), α-Chymotrypsin (10 mg), ethanol (2 mL). bYield was determined by HPLC.
To further explore the activity of α-chymotrypsin in this novel biocatalytic protocol, a series of o-phenylenediamines and β-ketoesters were investigated (). A mixture of o-phenylenediamine 1 (0.25 mmol), β-ketoester 2 (0.25 mmol), and α-chymotrypsin (10 mg) in 2 mL ethanol was incubated at 50°C and 200 rpm. for 18 h. After completion of the reaction (TLC), the mixture was concentrated under reduced pressure to afford the crude product, which was purified by column chromatography on silica gel (PE: EtOAc = 1: 2) to give pure product 3. All substrates with various groups showed good compatibility with the reaction conditions, and good to excellent yields were obtained. In addition, the steric effect hardly influenced the biocatalysis efficiency. Identical results were obtained with 3,4-diaminotoluene and 2,3-diaminotoluene (91% and 83%, , 3b and 3c). Obviously, strong electron-withdrawing groups had no effect on this biocatalysis, and the corresponding benzimidazoles were formed in good yields (, 3d–3g, 3l–3o).
Table 2. α-Chymotrypsin catalyzed synthesis of 2-substituted benzimidazole.a
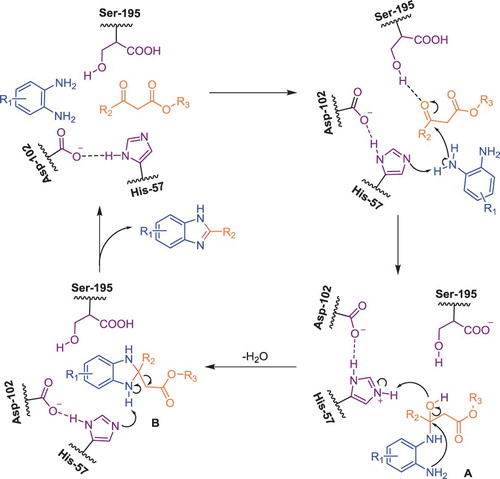
α-Chymotrypsin belongs to the family of serine proteases and is a polypeptide chain consisting of 245 amino acids; His-57, Asp-102, and Ser-195 constitute the catalytic triad ( Citation42–44). Combining this information and our part work ( Citation45), we purpose a plausible mechanism, as illustrated in . Initially, nucleophilic addition occurs to form intermediate imino ester A, in which the proton from the amido group of o-phenylenediamine may be abstracted by His 57 and the carbonyl of the ketone is effectively activated by Ser-195 ( Citation46,Citation47). Subsequently, A undergoes cyclization to form aminal B along with the removal of water. In the presence of a His-57, the corresponding benzimidazole is produced by retro-Claisen reaction, along with regeneration of α-chymotrypsin to complete the catalytic cycle.
Conclusion
In summary, α-chymotrypsin exhibits high activity in the synthesis of 2-substituted benzimidazoles from o-phenylenediamines and diverse β-ketoesters. This protocol is the first example of the synthesis of various benzimidazole derivatives with good functional group tolerance via an effective, eco-friendly, and mild reaction. Further investigation for exploiting high-activity enzymes toward non-natural reactions toward the direct syntheses of structurally heterocyclic compounds is ongoing in our laboratories.
Supplemental_Material.doc
Download MS Word (5.7 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Lian-Sheng Liu received the master's degree from East China University of Technology in 2018. He was mainly engaged in synthesis of N-heterocyclic compounds catalyzed by protease during his graduate studies.
Zong-Bo Xie received the Ph.D. degree from Sichuan University, China, in 2013. In 2009, he was promoted to associate professor. In 2010, he was appointed as a master's tutor. In 2015, he was promoted to professor. In recent years, he has been mainly engaged in green synthesis methodologies and promiscuity studies of enzymes.
Can Zhang received the bachelor's degree from East China University of Technology in 2018.
Lei-Han Fu, master’s degree candidate of East China University of Technology.
Hai-Bo Zhu received the Ph.D. degree from Fudan University, China, in 2017. Sine then, he worked in East China University of Technology, China.
Zhang-Gao Le received the Ph.D. degree from Zhejiang University, China, in 2005. From 2003 to 2018, he was hired as a professor and is currently the director of the personnel department of East China University of Technology.
Additional information
Funding
References
- Charifson, P.S.; Grillot, A.L.; Grossman, T.H.; Parsons, J.D.; Badia, M.; Bellon, S.; Deininger, D.D.; Drumm, J.E.; Gross, C.H.; LeTiran, A.; Liao, Y.; Mani, N.; Nicolau, D.P.; Perola, E.; Ronkin, S.; Shannon, D.; Swenson, L.L.; Tang, Q.; Tessier, P.R.; Tian, S.K.; Trudeau, M.; Wang, T.; Wei, Y.; Zhang, H.; Stamos, D. J. Med. Chem. 2008, 51, 5243–5263. doi: 10.1021/jm800318d
- White, A.W.; Almassy, R.; Calvert, A.H.; Curtin, N.J.; Griffin, R.J.; Hostomsky, Z.; Maegley, K.; Newell, D.R.; Srinivasan, S.; Golding, B.T. J. Med. Chem. 2000, 43, 4084–4097. doi: 10.1021/jm000950v
- Shiraishi, Y.; Sugano, Y.; Tanaka, S.; Hirai, T. Angew. Chem. Int. Ed. 2010, 49, 1656–1660. doi: 10.1002/anie.200906573
- Hutchinson, I.; Bradshaw, T.D.; Matthews, C.S.; Stevens, M.F.G.; Westwell, A.D. Bioorg. Med. Chem. Lett. 2003, 13, 471–474. doi: 10.1016/S0960-894X(02)00930-7
- Huang, S.T.; Hsei, I.J.; Chen, C. Bioorg. Med. Chem. 2006, 14, 6106–6119. doi: 10.1016/j.bmc.2006.05.007
- Janupally, R.; Jeankumar, V.U.; Bobesh, K.A.; Soni, V.; Devi, P.B.; Pulla, V.K.; Suryadevara, P.; Chennubhotla, K.S.; Kulkarni, P.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. 2014, 22, 5970–5987. doi: 10.1016/j.bmc.2014.09.008
- Tonelli, M.; Simone, M.; Tasso, B.; Novelli, F.; Boido, V.; Sparatore, F.; Paglietti, G.; Pricl, S.; Giliberti, G.; Blois, S.; Ibba, C.; Sanna, G.; Loddo, R.; La-Colla, P. Bioorg. Med. Chem. 2010, 18, 2937–2953. doi: 10.1016/j.bmc.2010.02.037
- Hu, F.; Szostak, M. Adv. Synth. Catal. 2015, 357, 2583–2614. doi: 10.1002/adsc.201500319
- Blacker, A.J.; Farah, M.M.; Hall, M.I.; Marsden, S.P.; Saidi, O.; Williams, J.M.J. Org. Lett. 2009, 11, 2039–2042. doi: 10.1021/ol900557u
- Mukhopadhyay, C.; Tapaswi, P.K. Tetrahedron Lett. 2008, 49, 6237–6240. doi: 10.1016/j.tetlet.2008.08.041
- Adharvana-Chari, M.; Shobha, D.; Sasaki, T. Tetrahedron Lett. 2011, 52, 5575–5580. doi: 10.1016/j.tetlet.2011.08.047
- Patil, S.S.; Bobade, V.D. Synth. Commun. 2009, 40, 206–212. doi: 10.1080/00397910902906602
- Inamdar, S.M.; More, V.K.; Mandal, S.K. Tetrahedron Lett. 2013, 54, 579–583. doi: 10.1016/j.tetlet.2012.11.091
- Sharghi, H.; Asemani, O. Synth. Commun. 2009, 39, 860–867. doi: 10.1080/00397910802431214
- Wen, X.; Bakali, J.E.; Deprez-Poulain, R.; Deprez, B. Tetrahedron Lett. 2012, 53, 2440–2443. doi: 10.1016/j.tetlet.2012.03.007
- Santra, S.; Majee, A.; Hajra, A. Tetrahedron Lett. 2012, 53, 1974–1977. doi: 10.1016/j.tetlet.2012.02.021
- Maiti, D.K.; Halder, S.; Pandit, P.; Chatterjee, N.; De-Joarder, D.; Pramanik, N.; Saima, Y.; Patra, A.; Maiti, P.K. J. Org. Chem. 2009, 74, 8086–8097. doi: 10.1021/jo901458k
- Tandon, V.K.; Kumar, M. Tetrahedron Lett. 2004, 45, 4185–4187. doi: 10.1016/j.tetlet.2004.03.117
- Ruiz, V.R.; Corma, A.; Sabater, M.J. Tetrahedron 2010, 66, 730–735. doi: 10.1016/j.tet.2009.11.048
- Nguyen, T.B.; Ermolenko, L.; Dean, W.A.; Al-Mourabit, A. Org. Lett. 2012, 14, 5948–5951. doi: 10.1021/ol302856w
- Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. J. Org. Chem. 2011, 76, 716–719. doi: 10.1021/jo1021426
- Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. J. Org. Chem. 2008, 73, 7841–7844. doi: 10.1021/jo8014984
- Zou, B.; Yuan, Q.; Ma, D. Angew. Chem. Int. Ed. 2007, 46, 2598–2601. doi: 10.1002/anie.200700071
- Jaseer, E.A.; Prasad, D.J.C.; Dandapat, A.; Sekar, G. Tetrahedron Lett. 2010, 51, 5009–5012. doi: 10.1016/j.tetlet.2010.07.079
- Li, Z.; Dong, J.; Chen, X.; Li, Q.; Zhou, Y.; Yin, S.F. J. Org. Chem. 2015, 80, 9392–9400. doi: 10.1021/acs.joc.5b00937
- Mayo, M.S.; Yu, X.; Zhou, X.; Feng, X.; Yamamoto, Y.; Bao, M. Org. Lett. 2014, 16, 764–767. doi: 10.1021/ol403475v
- Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301–312. doi: 10.1039/B918763B
- Gotor-Fernandez, V.; Brieva, R.; Gotor, V. J. Mol. Catal. B-Enzym. 2006, 40, 111–120. doi: 10.1016/j.molcatb.2006.02.010
- O’Brien, P.J.; Herschlag, D. Chem. Biol. 1999, 6, R91–R105. doi: 10.1016/S1074-5521(99)80033-7
- Li, H.H.; He, Y.H.; Yuan, Y.; Guan, Z. Green Chem. 2011, 13, 185–189. doi: 10.1039/C0GC00486C
- Xie, Z.B.; Wang, N.; Zhou, L.H.; Wan, F.; He, T.; Le, Z.G.; Yu, X.Q. Chemcatchem 2013, 5, 1935–1940. doi: 10.1002/cctc.201200890
- Li, H.H.; He, Y.H.; Guan, Z. Catal Commun. 2011, 12, 580–582. doi: 10.1016/j.catcom.2010.12.003
- Cai, J.F.; Guan, Z.; He, Y.H. J. Mol. Catal. B-Enzym. 2011, 68, 240–244. doi: 10.1016/j.molcatb.2010.11.011
- Zandvoort, E.; Geertsema, E.M.; Baas, B.J.; Quax, W.J.; Poelarends, G.J. Angew. Chem. Int. Edit. 2012, 51, 1240–1243. doi: 10.1002/anie.201107404
- Miao, Y.F.; Geertsema, E.M.; Tepper, P.G.; Poelarends, G.J. Chembiochem 2013, 14, 191–194. doi: 10.1002/cbic.201200676
- Li, K.; He, T.; Li, C.; Feng, X.W.; Wang, N.; Yu, X.Q. Green Chem. 2009, 11, 777–779. doi: 10.1039/b817524a
- He, T.; Li, K.; Wu, M.Y.; Feng, X.W.; Wang, N.; Wang, H.Y.; Li, C.; Yu, X.Q. J. M. Catal. B-Enzym. 2010, 67, 189–194. doi: 10.1016/j.molcatb.2010.08.004
- Purkarthofer, T.; Gruber, K.; Gruber-Khadjawi, M.; Waich, K.; Skranc, W.; Mink, D.; Griengl, H. Angew. Chem. Int. Ed. 2006, 45, 3454–3456. doi: 10.1002/anie.200504230
- Sun, D.Z.; Jiang, G.F.; Xie, Z.B.; Le, Z.G. Chin. J. Chem. 2015, 33, 409–412. doi: 10.1002/cjoc.201400892
- Liang, M.; Xie, Z.B.; Ai, F.; Le, Z.G. Chin. J. Org. Chem. 2016, 36, 2704–2708. doi: 10.6023/cjoc201604051
- Xie, Z.B.; Zhang, S.G.; Jiang, G.F.; Liang, M.; Le, Z.G. Chin. J. Org. Chem. 2017, 37, 514–519. doi: 10.6023/cjoc201606030
- Liu, Y.; Liu, R. Food Chem. Toxicol. 2012, 50, 3298–3305. doi: 10.1016/j.fct.2012.06.037
- Kumar, A.; Venkatesu, P. Chem. Rev. 2012, 112, 4283–4307. doi: 10.1021/cr2003773
- Blow, D.M.; Birktoft, J.J.; Hartley, B.S. Nature 1969, 221, 337–340. doi: 10.1038/221337a0
- Xie, Z.B.; Sun, D.Z.; Jiang, G.F.; Le, Z.G. Molecules 2014, 19, 19665–19677. doi: 10.3390/molecules191219665
- Martichonok, V.; Jones, J.B. J. Am. Chem. Soc. 1996, 118, 950–958. doi: 10.1021/ja952816j
- Svedendahl, M.; Hult, K.; Berglund, P. J. Am. Chem. Soc. 2005, 127, 17988–17989. doi: 10.1021/ja056660r