ABSTRACT
In this work, supercritical carbon dioxide was used as reaction media in the lipase-mediated epoxidation of alkenes for the first time. Under the optimal conditions (alkene (1 mmol), novozym 435 (15 mg), H2O2 (50% aqueous solution, 1.4 mmol), n-caprylic acid (0.05 mmol), 40°C, scCO2 (10 MPa, 25 mL)), high yields (83–98%) of epoxidation of alkenes could be obtained in a short reaction time. Furthermore, novozym 435 exhibited a satisfactory reusability in the epoxidations. Thus, this work not only presents a green and mild method of lipase-mediated epoxidation with higher yield and reaction rate than other lipase-mediated routes ever reported but also expands the application of supercritical carbon dioxide in enzyme catalytic promiscuity.
GRAPHICAL ABSTRACT
Introduction
Epoxidation of alkenes is one of the important reactions in organic synthesis as the epoxy compounds are versatile building blocks in the manufacture of bulk and fine chemicals/intermediates ( Citation1). Some expensive and environmentally unacceptable processes such as organic peracids or metal catalysts/O2 are still widely used in epoxidation reactions ( Citation2–5). At the forefront of existing challenges is the development of new mild oxidative methodologies that can be practical, efficient and minimize the environmental impact. Over the past few years, many reports have demonstrated that lipase could catalyze the in situ generation of peracids through a perhydrolysis of carboxylic acids or esters ( Citation6, Citation7). And the in situ formed peracids have been successfully utilized in many oxidations, such as epoxidation of alkenes, Baeyer–Villiger reaction, Dakin oxidation, oxidations of amines, alcohols and sulfanyl compounds ( Citation8–15). These oxidative processes are representative examples of enzyme catalytic promiscuity, which is a term used to describe an enzyme that can catalyze chemical reactions fully different than physiological ones ( Citation16). Recent success in this significant field suggests new potential applications in enzyme-catalyzed organic synthesis ( Citation17, Citation18). Compared with the chemical methods, lipase-mediated oxidations benefit from the mild reaction conditions, environmental friendliness and green reusable catalyst. However, these methods have also encountered some drawbacks, such as long reaction time and the using of volatile organic solvents.
It is known that supercritical carbon dioxide (scCO2) has a prodigious potential as an environmentally benign reaction medium for sustainable chemical synthesis due to its advantages, such as low cost, nontoxicity, non-flammability, inertness, full recovery and moderate critical properties (Pc= 7.38 MPa, Tc = 304.2 K) when compared to other green solvents ( Citation19–22). Many researches showed that lipases are active and stable in scCO2, which increased the potential use of scCO2 in lipase-catalyzed reactions ( Citation23–25). For example, isoamyl acetate could successfully be synthesized from isoamyl alcohol in scCO2 by novozym 435, and an esterification extent of 100% was obtained ( Citation26). Santos et al. reported that higher esterification rates could be obtained when the esterification of oleic acid with methanol was catalyzed by lipase in scCO2 ( Citation27). These findings encourage further studies to develop scCO2 as a reaction medium in enzymatic promiscuous reactions, such as lipase-mediated epoxidation.
As part of our program in environmentally friendly biocatalysis, we are interested in designing a “green” process to effectively oxidize a variety of alkenes. In this study, lipase-mediated epoxidation of alkenes in scCO2 is reported for the first time (Scheme 1). Furthermore, this work also provides the first example of scCO2 as reaction media in enzymatic promiscuous reactions, which broadens the application of scCO2 in biocatalysis.
Results and discussion
According to the previous reports ( Citation28, Citation29), novozym 435 (a commercial immobilized Candida antarctica lipase B) has always been used to be the efficient catalyst combined with H2O2 as an oxidant for the lipase-mediated epoxidation, and novozym 435 exhibits a good stability when in these reports. Furthermore, the use of H2O2 as an oxidant for the lipase-catalyzed perhydrolysis is much more promising from economic and environmental points of view as the only byproduct (H2O) is environmentally benign ( Citation30). Therefore, we selected H2O2 as the oxidant and novozym 435 as the catalyst for the perhydrolysis of n-caprylic acid to generate peracid.
In this study, a stainless-steel pressure-resistant vessel (internal volume: 25 mL) was used as the reactor. Initially, styrene was adopted as the model substrate for the epoxidation. Different reaction media were investigated and the results were shown in . The highest yield (92 ± 1.2%) could be obtained when scCO2 (10 MPa) was used as the reaction medium. To our knowledge, compared with other lipase-mediated epoxidation reported so far, novozym 435 presents the highest catalytic efficiency when scCO2 was used as the reaction medium. The higher diffusivity and lower viscosity and surface tension of scCO2 are responsible for the reduction of interphase transport limitations, thus increasing the reaction rate in this lipase-mediated epoxidation. And trace epoxidative product could be obtained in the control experiments (entry 5–6) which suggested that the active conformation of the enzyme was necessary for this enzymatic process. The above results were according to the literature which reported that hydrogen peroxide alone only presented very poor epoxidative ability in scCO2 ( Citation31). Furthermore, we also examined the high-pressure n-hexane as the reaction medium on this epoxidation (entry 7), and the yield was almost the same with that under atmospheric pressure due to the unchanged properties of n-hexane under different pressures.
Table 1. Lipase-mediated epoxidation of styrene in different solventsa.
As a function of results in supercritical media, the effect of different scCO2 conditions, determined by all the following temperatures (35, 40 and 45°C) and pressures (10–30 MPa), on the lipase-mediated epoxidation of styrene was investigated. As shown in , when the temperature was fixed, the yield of epoxidation decreased with the increasing of pressure from 10 to 30 MPa. The increase in pressure, which involves an increase of the scCO2 density, produced a decline in the enzyme performance. And also, a higher pressure could inactivate enzyme in an enzymatic reaction. The temperature obviously affected the lipase-mediated epoxidation at all the assayed pressures and the highest yield could be obtained at 40°C. High temperature could decrease in fluid density, which produced a reduction in internal diffusion limitations into the enzyme particle. However, higher temperature promoted the degradation of H2O2, such that the generation of peracid reduced dramatically.
Table 2. Effect of temperature and pressure on lipase-mediated epoxidation in scCO2 a
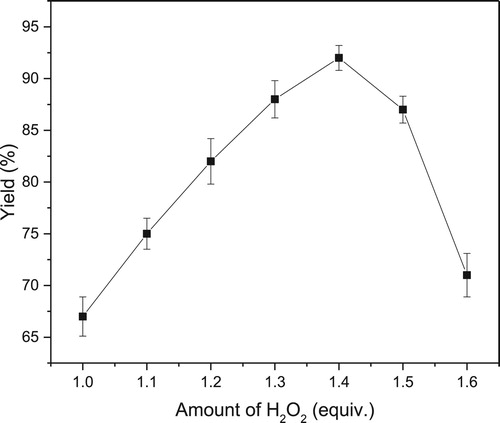
Generally, the amount of oxidant might affect the yield of epoxidation. In this study, the effect of the amount of H2O2 was investigated (). The yield of expoxidation increased when the amount of H2O2 was increased from 1 to 1.4 equiv. After that, the yield was decreased markedly. High oxidant loading in scCO2 induced the inactivation of lipase, and then the yield of epoxidation declined. Furthermore, higher dosage of H2O2 solution improved the water content of the reaction system, and made the enzyme conformation too flexible, which may also decrease the activity of the enzyme. Therefore, 1.4 equiv. of H2O2 was selected for this lipase-mediated epoxidation in scCO2.
Figure 1. Effect of amount of H2O2 on lipase-mediated epoxidation in scCO2. Reaction conditions: styrene (1 mmol), novozym 435 (15 mg), H2O2 (50% aqueous solution), n-caprylic acid (0.05 mmol), 40°C, scCO2 (10 MPa, 25 mL), 1 h.
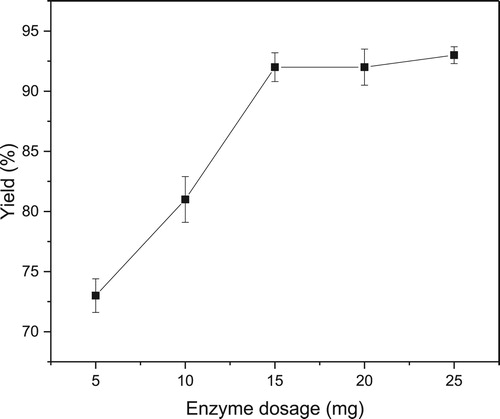
The epoxidation of styrene was carried out in scCO2 with different amounts of novozym 435 and the results were demonstrated in . The temperature and pressure were fixed at 40°C and 10 MPa, respectively. It could be observed that a clear increase in the yield of epoxidation with an increasing enzyme amount from 5 to 15 mg, and similar yields were obtained when the enzyme amount was higher than 15 mg. Therefore, 15 mg of novozym 435 was sufficient for this reaction.
Figure 2. Effect of enzyme dosage on lipase-mediated epoxidation in scCO2 Reaction conditions: styrene (1 mmol), novozym 435, H2O2 (50% aqueous solution, 1.4 mmol), n-caprylic acid (0.05 mmol), 40°C, scCO2 (10 MPa, 25 mL), 1 h.
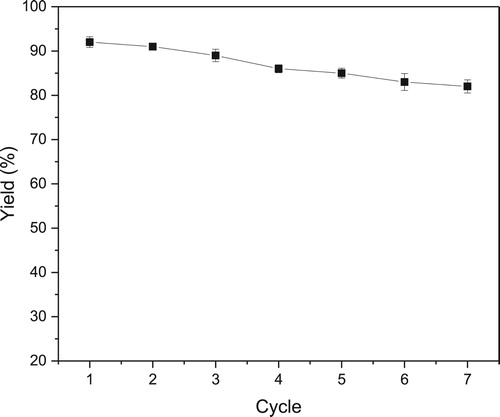
The main advantage of immobilized lipases over the free form is that the enzyme can be reused many times in the process, thus reducing the cost of catalyst ( Citation32, Citation33). Thus, the reusability of novozym 435 was investigated to assess the economic potential in this epoxidation (). The lipase was washed with n-hexane to remove styrene and epoxide after each cycle. The catalytic performance was retained in first three cycles. The yields of epoxides decreased to 82% after seven cycles. The gradual decrease of epoxidative yield might be attributed to the leakage of the enzyme from the support during the epoxidation. Besides, we scaled up the reaction system in a pressure-resistant vessel with internal volume of 1 L (styrene (40 mmol), novozym 435 (0.6 g), H2O2 (50% aqueous solution, 56 mmol), n-caprylic acid (2 mmol), scCO2 (10 MPa), 40°C). The yield of epoxide was up to 95% after 1 h. It is noteworthy that the hydrolysis of styrene oxide was not observed in this lipase-mediated epoxidation. These results indicate that this green method has a high potential for practical application.
Figure 3. Reusability of novozym 435 on lipase-mediated epoxidation in scCO2.Reaction conditions: styrene (1 mmol), novozym 435 (15 mg), H2O2 (50% aqueous solution, 1.4 mmol), n-caprylic acid (0.05 mmol), 40oC, scCO2 (10 MPa, 25 mL), 1 h.
This new system was used for the epoxidation of other alkenes. As shown in , oxidation of aromatic alkenes gave the satisfactory yields of epoxides (entry 1–4). And the epoxidations of aliphatic alkenes (entry 5–6) were faster than those of aromatic alkenes, producing the corresponding oxides in 96–98% after 0.5 h. This result is in accordance with the previous reports ( Citation6–8).
Table 3. Lipase-mediated epoxidation of alkenes in scCO2a.
Experimental materials
Novozym 435 was purchased from Sigma (Beijing, China). Silica gel was purchased from Qing Dao Hai Yang Chemical Industry Co. (Qingdao, China). The alkenes were of analytical reagent grade and purchased from J&K Scientific Ltd. (Beijing, China). All the other reagents were purchased from Shanghai Chemical Reagent Company (Shanghai, China). All the commercially available reagents and solvents were used without further purification. NMR spectra were taken with an Inova 500 (500 MHz) spectrometer. Carbon dioxide with a purity of 99.99% was from Changchun Juyang Gas Co., Ltd. The pressure in the system was controlled by a liquid CO2 pump (Spe-ed SFE, Applied Separations, Inc.).
General procedure for the epoxidation of alkenes
Alkene (1 mmol), H2O2 (50% aqueous solution, 1.4 mmol), n-caprylic acid (0.05 mmol) and novozym 435 (15 mg) were mixed and sealed in a batch reactor (high-pressure-resistant stainless-steel vessel, 25 mL). The reaction was conducted at 10 MPa and 40°C. CO2 gas was sent into the vessel by a CO2 pump. The vessel was stirred with a magnetic stirrer. After the reaction to the desired time, depressurization and elution by n-hexane (10 mL*3) was conducted. The combined organic phases were concentrated under vacuum, and the resulting residue was purified by flash column chromatography on silica gel with EA/hexane (1/4). The products were analyzed by 1H-NMR.
Conclusions
In summary, scCO2 was reported to be superior to other conventional organic solvents for the lipase catalyzing the epoxidation of alkenes for the first time. In comparison with other lipase-mediated oxidation systems, this new oxidation system mediated by lipase in scCO2 is greener and more efficient. Moreover, it is the first example for the application of scCO2 in enzyme catalytic promiscuity. With this interesting finding, on-going studies on scCO2 as a green medium for other enzymatic promiscuous reactions continue in our laboratory.
Supplemental_Material.docx
Download MS Word (514 KB)Acknowledgments
We gratefully acknowledge the Foundation of Changchun BC&HC Pharmaceutical Technology Co., Ltd (no. 3R117W391465).
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Jiaxin Zhang is studying as a master candidate in School of Life Sciences, Jilin University. Her research has focused on the biocatalysis in unconventional reaction media.
Wenwei Qian is studying as an undergraduate in School of Life Sciences, Jilin University. Her research has focused on the biocatalysis in unconventional reaction media.
Chunyu Wang is working at State Key Laborarory of Supramolecular Structure and Materials, Jilin University. His research has focused on the synthesis of bioactive compounds.
Ziyi Cao is studying as an undergraduate in School of Life Sciences, Jilin University. Her research has focused on the biocatalysis in unconventional reaction media.
Shanshan Chen is studying as an undergraduate in School of Life Sciences, Jilin University. Her research has focused on the synthesis and development of new drugs.
Liu Zhang is studying as a master candidate in School of Life Sciences, Jilin University. Her research has focused on the biocatalysis and biotransformation.
Yang Zhang is working at Department of Biopharmacy, School of Pharmaceutical Sciences, Jilin University. Now, she is mainly investigating the biological activity of chiral drugs.
Lei Wang is working in Key Laboratory of Molecular Enzymology and Engineering of Ministry of Education, Jilin University as a professor. Recently, his research has focused on the new synthetic methodologies to synthesize bioactive compounds.
References
- Xia, Q.H.; Ge, H.Q.; Ye, C.P.; Liu, Z.M.; Su, K.X. Chem. Rev. 2005, 105 (5), 1603–1662. doi: 10.1021/cr0406458
- Frost, C.G.; Penrose, S.D.; Gleave, R. Org. Biomol. Chem. 2008, 6 (23), 4340–4347. doi: 10.1039/b812897a
- Chandrasekhar, S.; Balaji, S.V.; Rajesh, G. Tetrahedron Lett. 2010, 51 (39), 5164–5166. doi: 10.1016/j.tetlet.2010.07.127
- Yin, G.; Buchalova, M.; Danby, A.M.; Perkins, C.M.; Kitko, D.; Carter, J.D.; Scheper, W.M.; Busch, D.H. J. Am. Chem. Soc. 2005, 127 (49), 17170–17171. doi: 10.1021/ja055413k
- Minisci, F.; Gambarotti, C.; Pierini, M.; Porta, O.; Punta, C.; Recupero, F.; Lucarini, M.; Mugnaini, V. Tetrahedron Lett. 2006, 47 (9), 1421–1424. doi: 10.1016/j.tetlet.2005.12.089
- Warwel, S. J. Mol. Catal. A-Chem. 1997, 117 (1-3), 311–319. doi: 10.1016/S1381-1169(96)00264-6
- Björkling, F.; Godtfredsen, S.E.; Kirk, O. J. Chem. Soc., Chem. Commun. 1990, 19, 1301–1303. doi: 10.1039/C39900001301
- Ankudey, E.G.; Olivo, H.F.; Peeples, T.L. Green Chem. 2006, 8 (10), 923–926. doi: 10.1039/B604984B
- Rios, M.Y.; Salazar, E.; Olivo, H.F. J Mol. Catal. B-Enzym. 2008, 54 (3-4), 61–66. doi: 10.1016/j.molcatb.2007.12.012
- Wang, Z.; Chen, X.; Wang, C.; Zhang, L.; Li, F.; Zhang, W.; Chen, P.; Wang, L. Green Chem. Lett. Rev. 2017, 10 (4), 269–273. doi: 10.1080/17518253.2017.1375159
- Yang, F.; Wang, Z.; Zhang, X.; Jiang, L.; Li, Y.; Wang, L. ChemCatChem. 2015, 7 (21), 3450–3453. doi: 10.1002/cctc.201500720
- Méndez-Sánchez, D.; Lavandera, I.; Gotor, V.; Gotor-Fernández, V. Org. Biomol. Chem. 2017, 15 (15), 3196–3201. doi: 10.1039/C7OB00374A
- Zhao, Z.; Zhang, L.; Li, F.; Tang, X.; Ma, Y.; Wang, C.; Wang, Z.; Zhao, R.; Wang, L. Catalysts 2017, 7 (12), 354. doi: 10.3390/catal7120354
- Björkling, F.; Frykman, H.; Godtfredsen, S.E.; Kirk, O. Tetrahedron 1992, 48 (22), 4587–4592. doi: 10.1016/S0040-4020(01)81232-1
- Yang, F.; Zhang, X.; Li, F.; Wang, Z.; Wang, L. Green Chem. 2016, 18 (12), 3518–3521. doi: 10.1039/C6GC00780E
- Nobeli, I.; Favia, A.D.; Thornton, J.M. Nat. Biotechnol. 2009, 27 (2), 157. doi: 10.1038/nbt1519
- Bornscheuer, U.T.; Kazlauskas, R.J. Angew. Chem. Int. Edit. 2004, 43 (45), 6032–6040. doi: 10.1002/anie.200460416
- Hult, K.; Berglund, P. Trends Biotechnol. 2007, 25 (5), 231–238. doi: 10.1016/j.tibtech.2007.03.002
- DeSimone, J.M.; Guan, Z.; Elsbernd, C.S. Science 1992, 257 (5072), 945–947. doi: 10.1126/science.257.5072.945
- Leitner, W. Accounts Chem. Res. 2002, 35 (9), 746–756. doi: 10.1021/ar010070q
- Licence, P.; Ke, J.; Sokolova, M.; Ross, S.K.; Poliakoff, M. Green Chem. 2003, 5 (2), 99–104. doi: 10.1039/b212220k
- Sheldon, R.A. Green Chem. 2005, 7 (5), 267–278. doi: 10.1039/b418069k
- Nakamura, K.; Chi, Y.M.; Yamada, Y.; Yano, T. Chem. Eng. Commun. 1986, 45 (1-6), 207–212. doi: 10.1080/00986448608911384
- Chulalaksananukul, W.; Condoret, J.S.; Combes, D. Enzyme Microb. Tech. 1993, 15 (8), 691–698. doi: 10.1016/0141-0229(93)90071-9
- Melgosa, R.; Sanz, M.T.; Solaesa, ÁG; Bucio, S.L.; Beltrán, S. J. Supercrit. Fluid. 2015, 97, 51–62. doi: 10.1016/j.supflu.2014.11.003
- Romero, M.D.; Calvo, L.; Alba, C.; Habulin, M.; Primožič, M.; Knez, Ž J. Supercrit. Fluid. 2005, 33 (1), 77–84. doi: 10.1016/S0896-8446(04)00114-7
- dos Santos, P.; Rezende, C.A.; Martínez, J. J. Supercrit. Fluid. 2016, 107, 170–178. doi: 10.1016/j.supflu.2015.08.011
- Xu, Y.; Khaw, N.R.B.J.; Li, Z. Green Chem. 2009, 11 (12), 2047–2051. doi: 10.1039/b913077b
- Törnvall, U.; Orellana-Coca, C.; Hatti-Kaul, R.; Adlercreutz, D. Enzyme Microb. Tech. 2007, 40 (3), 447–451. doi: 10.1016/j.enzmictec.2006.07.019
- Hernandez, K.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Curr. Org. Chem. 2012, 16 (22), 2652–2672. doi: 10.2174/138527212804004526
- Nolen, S.A.; Lu, J.; Brown, J.S.; Pollet, P.; Eason, B.C.; Griffith, K.N.; Gläser, R.; Bush, D.; Lamb, D.; Liotta, C.L.; Eckert, C.A. Ind. Eng. Chem. Res. 2002, 41 (3), 316–323. doi: 10.1021/ie0100378
- Rueda, N.; Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á; Fernandez-Lafuente, R. Chem. Rec. 2016, 16 (3), 1436–1455. doi: 10.1002/tcr.201600007
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Biotechnol. Adv. 2015, 33 (5), 435–456. doi: 10.1016/j.biotechadv.2015.03.006