ABSTRACT
Relatively inexpensive, stable plant extract, namely Citrus aurantium leaves, was employed as highly efficient inhibitor of carbon steel corrosion by corrosive acid. The inhibition efficiency was estimated based on the weight loss method. Inhibition impacts of researched inhibitor increase with the concentration of the plant extract increase. The inhibition efficiency depends on three factors: molecular structure, concentration, and molecular weight of the inhibitor. Inhibition efficiency of 81.2% was achieved with 20% (v/v) of the extract in 1 M hydrochloric acid during 3 h at 25°C. The effect of temperature was also investigated and activation parameters were evaluated. Inhibition adsorption characteristics were approximated by Langmuir adsorption isotherm. Chemical adsorption mechanism was proposed for the studied inhibitor from the trend of inhibition performance and temperature degree in addition to activation energy values and heat of adsorption.
GRAPHICAL ABSTRACT

1. Introduction
Corrosion is an event that was considered either chemical or physical in nature, denatures the mineral characteristic, and of alloys make them inefficient for specified function ( Citation1–5). The main reason of the corrosion of alloys is their tendency to revert to the steady state. Most of the alloys are inherently unsteady and their natural trend to seek self-demolition through interacting with the environment to reach to lower energy state via producing metal compounds. These were the states that where most of the minerals were found ( Citation6). As there are many techniques for controlling the corrosion of alloys, the employ of inhibitor is the superior way for protecting alloys from corrosive solutions ( Citation7). Inhibitors were compounds that added in little quantities to corrosive solutions in order to prevent the reaction of surface of metal with the corrosive environment ( Citation8). They were added to considerable systems, such as cooling, refinery and gas production units ( Citation9). Anticorrosion coatings are generally employed to inhibit the average of corrosion and increase the longevity of the mild steel. A broad range of organic adsorption inhibitors presently applied in the corrosion domain is expensive (Citation10–16). Inhibitor adsorption on mild steel is affected by the nature of the mild steel, type of electrolyte, and molecular structure of the inhibitor ( Citation17–21). Inhibitor molecules are adsorbed on the surface of mild steel forming a barrier and consequently preventing reactions (cathodic or anodic) from processing at the surface of mild steel. These inhibitors could react with the iron atom at the mild steel surface to form inorganic complexes, blocking the surface of mild steel ( Citation22–26). Due to the high cost, toxicity, and hazardous of synthesized corrosion inhibitor ( Citation27–29), the examination of plant extract as corrosion inhibitors is presently interesting ( Citation30–32). Herein we focus on simple, practical, and economical corrosion inhibitors, so in this study, extract of Citrus aurantium leaves was used as green corrosion inhibitor for CS “Carbon Steel” in corrosive media.
2. Experimental
2.1. Specimen preparation
CS “Carbon Steel” of thickness 3 × 10−1 cm was obtained locally and was mechanically cut into coupons with dimensions of 65 × 10−1 cm, 25 × 10−1 cm, 3 × 10−1 cm having a hole with the diameter of 3.0 mm. First, coupons were polished, washed with distilled water, acetone and dried.
2.2. Preparation of plant extract
2.2.1. Collection of plant materials
Fresh C. aurantium leaves were obtained from Citrus orchards in Iraq and identified in College of Agriculture of the University of Basra, Basra City. The leaves were air-dried, grinded, and made into a fine powder using laboratory mortar and kept in a sterile air-tight container to avoid contamination.
2.2.2. Preparation of extract
Two hundred grams of dried leaf powder was dissolved in 1000 ml of ethanol for 48 h and centrifuged at 3000 rpm to enable paper diffusion of the active ingredients into the extraction medium. Filtration was later carried out using Whatman’s filter paper and the filtrate was stored at 5oC in a refrigerator.
2.3. Chemistry of C. aurantium
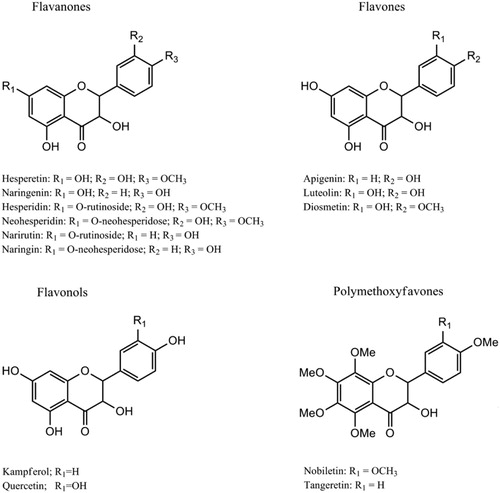
The chemical composition of C. aurantium includes vitamins, minerals, phenolic compounds, and terpenoids ( Citation33). The flavonoids contained in C. aurantium can be divided into four groups, including flavones, flavanones, flavonols, and anthocyanins (only in blood oranges) () ( Citation34, Citation35).
3. Results and discussion
3.1. Impact of inhibitor concentration
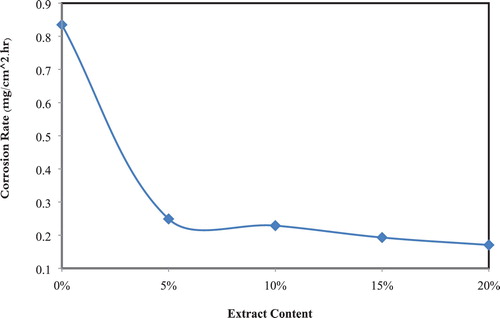
CR “Corrosion Rate” of CS in 1 M of hydrochloric acid with different contents of the extract was calculated after immersion of 3.0 h, at 25°C. CRs and IEs “Inhibition Efficiencies” were listed in . and show clearly that the CR decreases while IE increases with increasing extract content reaching the maximum value of 80.2 at 20% of the inhibitor. This behavior can be imputed to the adsorption of the extract used in this study on the surface of CS thereby inhibiting corrosion ( Citation36, Citation37).
Figure 2. Variation of percentage corrosion IE with the content of the extract in corrosive media at 25°C.
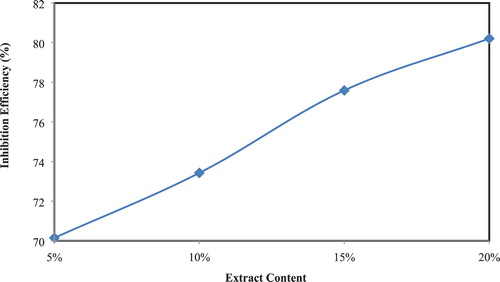
Table 1. Corrosion parameters obtained from weight loss measurements.
3.2. Impact of temperature
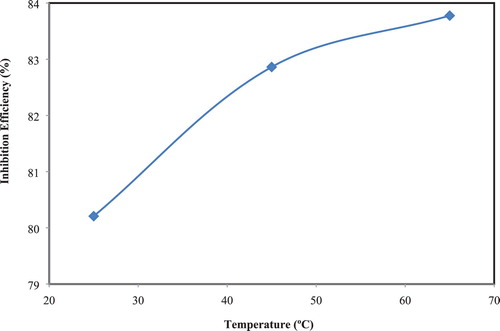
The influence of degrees of temperature on the corrosion parameters namely, WL “Weight Loss”, CR, and IE have been studied in corrosive media for 3.0 h, at temperatures 25°C, 45°C, and 65°C. The results (, and ) indicated that CR of CS without and with the extract increase with increasing in temperature, although corrosion rate is lowered in the presence of the extracts comparing with a blank solution, but the IE increases with increasing temperature. These results showed that the adsorption of the extract on the CS in corrosive solution is chemisorption ( Citation36, Citation38).
Figure 3. Plot of CR of CS in corrosive media in absence and presence of 40% of the extract versus studied temperature.
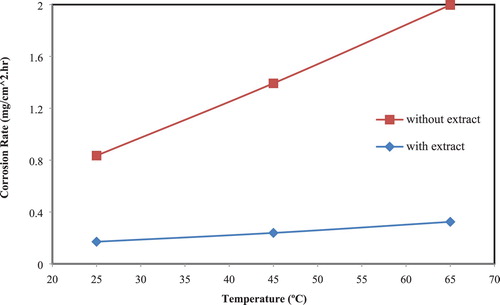
Table 2. Corrosion parameters for CS in corrosive media without and with 40% of the extract at different temperatures.
3.3. Activation parameters of the inhibition process
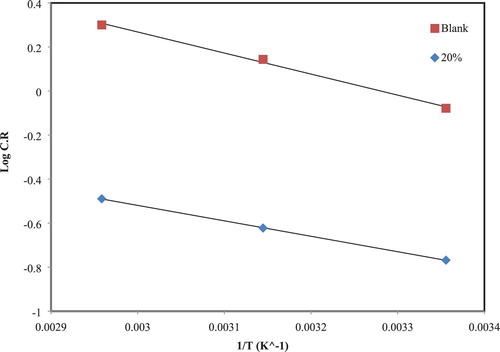
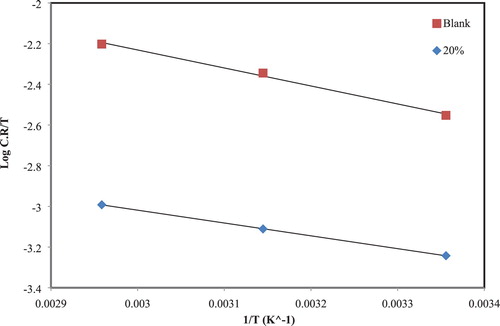
“Activation energy” and enthalpy for the corrosion of CS in absence and presence of 40% (v/v) of the extract in 1 M hydrochloric acid were estimated using Arrhenius equations (1) and (2) (
Citation39, Citation40):
(1)
(2)
As shown in , the CR of CS getting from WL measurements versus the temperatures 25°C, 45°C, and 65°C, with a slope of . In addition, has a plot of
versus
with a slope of
that the
values were calculated.
and
values are listed in .
Table 3. Activation parameters for the dissolution of CS in corrosive acid without and with 40% of the extract.
3.4. Adsorption isotherm
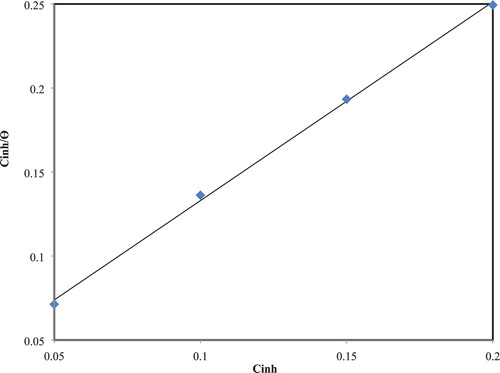
Increasing of extract molecules that adsorb on the surface of CS and the interaction could have qualified though adsorption isotherm. The data have been tested graphically though appropriate to the Langmuir model. This model is given by (
Citation41, Citation42)(3) By plotting values of
versus
(), a linear plot was obtained indicating that the adsorption of the studied plant extract as corrosion inhibitor was harmonious with the assumption of Langmuir adsorption isotherm and the slope obtained is close to unity.
Figure 7. Langmuir isotherm for the adsorption of the inhibitor on the CS surface in corrosive media at 25°C.
Scheme 1. Chemical structures of C. aurantium ( Citation35).
4. Conclusion
Citrus aurantium leaves extract acts as efficient corrosion picking inhibitor on CS in 1 M corrosive media. The extract showed maximum IE of 81.2% at the presence of 20% in v/v during 3 h at 25°C. The IE% increased with increasing temperature. Ea decreases in presence of studied plant extract which indicates the chemisorption of the inhibitor molecules on the CS surface. C. aurantium leaves extract adsorbs on CS surface approbate to the Langmuir isotherm.
Symbols
| A | = | constant |
|
| = | corrosion rate ( |
|
| = | content of the inhibitor |
|
| = | apparent activation energy (kJ mol−1
|
| H | = | Planks constant (6.626 × 10−34 Js) |
|
| = | enthalpy of activation (kJ mol−1) |
|
| = | binding constant of the adsorption reaction |
| N | = | Avogadro’s number (6.022 × 1023 mol−1) |
| R | = | gas constant (8.314 J mol−1 K−1) |
|
| = | entropy of activation (J mol−1 K−1) |
| T | = | temperature (K) |
|
| = | surface coverage |
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ahmed A. Al-Amiery http://orcid.org/http://orcid.org/0000-0003-1033-4904
Notes on contributors
Rifat Mohammed Dakhil, Assistant professor of Chemical Engineering at Technical College Basra, Southern Technical University with research interests in Mass Transfer, published 40+ peer-reviewed international papers.
Tayser Sumer Gaaz, lecturer of mechanical Engineering at Department of Machinery Equipment Engineering Techniques, Technical College Al-Musaib, Al-Furat Al Awsat Technical University, with research interests in composites, published 25+ peer-reviewed international papers.
Ahmed A. Al-Amiery, full professor of chemistry science at University of Technology, with research interests in synthesis of novel applicable molecules, published 150+ peer-reviewed international papers with 16+ patents, awarded the Medal of scientific excellence (2014), and also hold Science awards from the Ministry of Higher Education and Scientific Research for four consecutive years (2010, 2011, 2012, 2013, 2014, 2015 and 2016, selected for the Who’s Who for International Executives 2015, selected for the one of the best mentalities of the world for 2016 by the University of Cambridge and TWAS- Young Affiliates.
Abdul Amir H. Kadhum, full professor of chemistry science at Faculty of Engineering & Built Environment, Universiti Kebangsaan Malaysia with research interests in material sciences, published 250+ peer-reviewed international papers with 11+ patents.
Additional information
Funding
References
- Chauhan, R.; Garg, U.; Tak, R. Corrosion Inhibition of Aluminium in Acid Media by Citrullus colocynthis Extract. J. Chem. 2011, 8, 85–90.
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G., Virtanen, S., et al. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. doi: 10.1016/j.pmatsci.2017.04.011
- Hu, H.; Nie, X.; Ma, Y. Corrosion and Surface Treatment of Magnesium Alloys. Magnesium Alloys – Properties in Solid and Liquid States. InTech, 2014.
- Azmi, W.M.A.W. Study on Effects of Soil Properties Towards Corrosion of Carbon Steel Pipeline. UMP, 2014.
- Gusieva, K.; Davies, C.; Scully, J.; Birbilis, N. Corrosion of Magnesium Alloys: The Role of Alloying. Int. Mater. Rev. 2015, 60, 169–194. doi: 10.1179/1743280414Y.0000000046
- Rogers, T.H. Marine Corrosion, G. Newnes: London, 1968.
- Kadhim, A.; Al-Okbi, A.K.; Jamil, D.M.; Qussay, A.; Al-Amiery, A.A., Gaaz, T.S., et al. Experimental and Theoretical Studies of Benzoxazines Corrosion Inhibitors. Results Phys. 2017, 7, 4013–4019. doi: 10.1016/j.rinp.2017.10.027
- Kadhum, A.A.H.; Mohamad, A.B.; Hammed, L.A.; Al-Amiery, A.A.; San, N.H.; Musa, A.Y. Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin. Mater. (Basel) 2014, 7, 4335–4348. doi: 10.3390/ma7064335
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B.; Junaedi, S. A Novel Hydrazinecarbothioamide as a Potential Corrosion Inhibitor for Mild Steel in HCl. Mater. (Basel) 2013, 6, 1420–1431. doi: 10.3390/ma6041420
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Thermodynamic, Electrochemical and Quantum Chemical Investigation of Some Schiff Bases as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solutions. Corros. Sci. 2010, 52, 933–942. doi: 10.1016/j.corsci.2009.11.016
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A Study of the Inhibition of Iron Corrosion in HCl Solutions by Some Amino Acids. Corros. Sci. 2010, 52, 1684–1695. doi: 10.1016/j.corsci.2010.01.019
- Jamil, D.; Al Okbi, A.; Al Baghdadi, S.; Al Amiery, A.; Kadhim, A.; Gaaz, T.; Kadhum, A.; Mohamad, A,. A. Experimental and Theoretical Studies of Schiff Bases as Corrosion Inhibitors. Chem. Cent. J. 2018, 12 (7), 2–9.
- Xia, S.; Qiu, M.; Yu, L.; Liu, F.; Zhao, H. Molecular Dynamics and Density Functional Theory Study on Relationship Between Structure of Imidazoline Derivatives and Inhibition Performance. Corros. Sci. 2008, 50, 2021–2029. doi: 10.1016/j.corsci.2008.04.021
- Obayes, R.; Al-Amiery, A.; Alwan, G.; Alobaidy, A.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Quantum Chemical Assessment of Benzimidazole Derivatives as Corrosion Inhibitors. Chem. Cent. J. 2014, 8 (21), 1–8.
- Obayes, R.; Al-Amiery, A.; Alwan, G.; Abdullah, T.; Kadhum, A.; Mohamad, A. Sulphonamides as Corrosion Inhibitor: Experimental and DFT Studies. J. Mol. Struct. 2017, 1138, 27–34. doi: 10.1016/j.molstruc.2017.02.100
- Al-Azawi, K.; Al-Baghdadi, S.; Mohamed, A.; Al-Amiery, A.; Abed, T.K.; Mohammed, S.A.; Kadhum, A.A.; Mohamad, A.B. Synthesis, Inhibition Effects and Quantum Chemical Studies of a Novel Coumarin Derivative on the Corrosion of Mild Steel in a Hydrochloric Acid Solution. Chem. Cent. J. 2016, 10 (1), 23. doi: 10.1186/s13065-016-0170-3
- Junaedi, S.; Al-amiery, A.; Kadihum, A.; Mohamad, A. Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution Together with Quantum Chemical Studies. Int. J. Mol. Sci. 2013, 14, 11915–11928. doi: 10.3390/ijms140611915
- Al-Amiery, A.; Al-Majedy, Y.; Kadhum, A. Hydrogen Peroxide Scavenging Activity of Novel Coumarins Synthesized Using Different Approaches. PLoS One 2015, 10 (7), e0132175. doi: 10.1371/journal.pone.0132175
- Al-Amiery, A.A.; Al-Bayati, R.; Saour, K.; Radi, M. Cytotoxicity, Antioxidant and Antimicrobial Activities of Novel 2-Quinolone Derivatives Derived from Coumarins. Res. Chem. Intermed. 2012, 38 (2), 559–569. doi: 10.1007/s11164-011-0371-2
- Al-Amiery, A.A.; Al-MajedyKadhum, A.A.H.; Mohamad, A. Novel Macro-Molecules Derived from Coumarin: Synthesis and Antioxidant Activity. Sci. Rep. 2015, 5, 11825. doi: 10.1038/srep11825
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Al-Duhaidahawi, D.; Kadhum, A.A.H.; Mohamad, A.B. Green Antioxidants: Synthesis and Scavenging Activity of Coumarin-Thiadiazoles as Potential Antioxidants Complemented by Molecular Modeling Studies. Free Radic Antioxid 2016, 6 (2), 173–177. doi: 10.5530/fra.2016.2.7
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.; Mohamad, A.B. New Coumarin Derivative as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium. Molecules 2014, 20 (1), 366–383. doi: 10.3390/molecules20010366
- Al-Amiery AA, Al-Majedy YK, Kadhum AAH, Mohamad AB (2016) Synthesis of new Coumarins Complemented by Quantum Chemical Studies. Res. Chem. Intermed. 42(4):3905–3918 doi: 10.1007/s11164-015-2252-6
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.A. Antifungal Activities of New Coumarins. Molecules 2012, 17 (5), 5713–5723. doi: 10.3390/molecules17055713
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.; Mohamad, A. The use of Umbelliferone in the Synthesis of New Heterocyclic Compounds. Molecules 2011, 16 (8), 6833–6843. doi: 10.3390/molecules16086833
- Al-Majedy, Y.; Kadhum, K.; Al-Amiery, A.A.H. A Synthesis and Characterization of Some New 4-Hydroxy-Coumarin Derivatives. Molecules 2014, 19 (8), 11791–11799. doi: 10.3390/molecules190811791
- Jamil, D.; Al-Okbi, A.; Al-Baghdadi, S.; Al-Amiery, A.; Kadhim, A.; Gaaz, T.; Kadhum, A.; Mohamad, A. Chem. Cent. J. 2018, 12 (7), 1–9.
- Rubaye, A.; Abdulwahid, A.; Al-Baghdadi, S.B.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Cheery Sticks Plant Extract as a Green Corrosion Inhibitor Complemented with LC-EIS/MS Spectroscopy. Int. J. Electrochem. Sci. 2015, 10, 8200–8209.
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B.; Musa, A.Y.; Li, C.J. Electrochemical Study on Newly Synthesized Chlorocurcumin as an Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Mater. (Basel) 2013, 6, 5466–5477. doi: 10.3390/ma6125466
- Nwabanne, J.; Okafor, V. Inhibition of the Corrosion of Mild Steel in Acidic Medium by Vernonia amygdalina: Adsorption and Thermodynamics Study. J. Emerg. Trends Eng. Appl. Sci. 2011, 2, 619–625.
- Upadhyayula, V.K.; Meyer, D.E.; Gadhamshetty, V.; Koratkar, N. Screening-Level Life Cycle Assessment of Graphene-Poly (Ether Imide) Coatings Protecting Unalloyed Steel from Severe Atmospheric Corrosion. ACS. Sustain. Chem. Eng. 2017, 5, 2656–2667. doi: 10.1021/acssuschemeng.6b03005
- Junaedi, S.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B.; Takriff, M.S. Synthesis and Characterization of Novel Corrosion Inhibitor Derived from Oleic Acid: 2-Amino 5-Oleyl-1, 3, 4-Thiadiazol (AOT). Int. J. Electrochem. Sci 2012, 7, 3543–3554.
- Nabavi, S.F.; Khan, H., D'Onofrio, G., et al. Apigenin as Neuroprotective Agent: Of Mice and Men. Pharmacol. Res. 2018, 128, 359–365. doi: 10.1016/j.phrs.2017.10.008
- Marya, H.K.; Nabavi, S.M.; Habtemariam, S. Anti-diabetic Potential of Peptides: Future Prospects as Therapeutic Agents. Life Sci. 2018, 193, 153–158. doi: 10.1016/j.lfs.2017.10.025
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview on Citrus aurantium L. Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longevity 2018, 2018, 1–12. doi: 10.1155/2018/7864269
- Iloamaeke, I.; Onuegbu, T.; Ajiwe, V.; Umeobika, U. Corrosion Inhibition of Mild Steel by Pterocarpus soyauxi Leaves Extract in HCL Medium. Int. J. Plant, Anim. Environ. Sci. 2012, 2, 22–28.
- Oguzie, E.; Li, Y.; Wang, F. Corrosion Inhibition and Adsorption Behavior of Methionine on Mild Steel in Sulfuric Acid and Synergistic Effect of Iodide Ion. J. Colloid Interface Sci. 2007, 310, 90–98. doi: 10.1016/j.jcis.2007.01.038
- Daoud, D.; Douadi, T.; Issaadi, S.; Chafaa, S. Adsorption and Corrosion Inhibition of New Synthesized Thiophene Schiff Base on Mild Steel X52 in HCl and H2SO4 Solutions. Corros. Sci. 2014, 79, 50–58. doi: 10.1016/j.corsci.2013.10.025
- Ameh, P.O.; Magaji, L.; Salihu, T. Corrosion Inhibition and Adsorption Behaviour for Mild Steel by Ficus glumosa gum in H2SO4 Solution. Afr. J. Pure Appl. Chem. 2012, 6, 100–106.
- Dahmani, M.; Et-Touhami, A.; Al-Deyab, S.; Hammouti, B.; Bouyanzer, A. Corrosion Inhibition of C38 Steel in 1M HCl: A Comparative Study of Black Pepper Extract and its Isolated Piperine. Int. J. Electrochem. Sci 2010, 5, 1060–1069.
- Odiongenyi, A.; Odoemelam, S.; Eddy, N. Corrosion Inhibition and Adsorption Properties of Ethanol Extract of Vernonia amygdalina for the Corrosion of Mild Steel in H2SO4. Portugal Electrochim. Acta 2009, 27, 33–45. doi: 10.4152/pea.200901033
- Solmaz, R. Investigation of Adsorption and Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by 5-(4-Dimethylaminobenzylidene) Rhodanine. Corros. Sci. 2014, 79, 169–176. doi: 10.1016/j.corsci.2013.11.001