ABSTRACT
Green Chemistry principles can be used to re-cast traditional Organic chemistry experiments into more guided-inquiry based experiments. Inquiry questions related to green chemistry principles and metrics have been incorporated into our laboratory for the development of more guided-inquiry based experiments. Re-casting traditional experiments provides time for guided-inquiry by allowing students to evaluate reaction conditions and wastefulness of reactions. This includes evaluating solvent choices, heating methods, use of renewal materials, and contemplating reactants and products impacts on human health and environment. Students examine the changes as it pertains to green chemistry, the success of the reaction and the potential impacts on the mechanism. Involving students in these discoveries rooted in a guiding question made the Organic experiments guided-inquiry. Students were surveyed about their exposure to green chemistry and guided-inquiry based labs. Examples of some of the re-casted experiments, excerpts from student reports, and student impressions of the theme are presented.
GRAPHICAL ABSTRACT
Introduction
Green chemistry in organic chemistry education
The nature of Organic chemistry experiments is a natural fit for the incorporation of green chemistry principles and metrics. A survey of the chemistry education literature focused on Organic experiments shows many books ( Citation1,Citation2) and articles (some examples include 3–9) published related to green principles in recent years. Doxsee and Hutchinson have published a book on a variety of experiments which contain many revised traditional experiments ( Citation1). A collaboration of Organic chemistry educators presented a comprehensive summary of experimental and theoretical applications of green chemistry in Organic experiments in a publication edited by Dicks ( Citation2). A small subset of articles shows a variety of greener principles or methods are used in these new or revised experiments such as microwave irradiation, solvent-free conditions, and safer reagents or solvents ( Citation3–9). Montes et al. ( Citation3) have developed a greener synthesis of aspirin using microwave irradiation. Students determine the effect of catalysts on reaction time, purity, yield, and secondary products. Morsch et al. ( Citation4) implemented a greener Wittig reaction carried out in aqueous conditions at room temperature. Palleros ( Citation5) used a solvent-free aldol reaction to form a variety of chalcones. Hulce and Marks ( Citation6) implemented a solvent-free oxidation using a tungstate phase-transfer catalysts and hydrogen peroxide. Russell et al. ( Citation7) have modified their first-semester Organic curriculum to incorporate microwave assisted synthesis. The focus of their efforts was not focused specifically on greening the experiments, but rather making the lab more student centered through reduction of reaction times. Mckenzie et al. ( Citation8) showed how solvent changes (solventless to benign solvents) impacted four traditional Organic experiments. Each change exposed students to different benefits of solvent choices and impacted students from a pedagogical standpoint. Lang et al. used Oxone for a greener oxidation of borneol, in which the product would be used in a research group. The authors surveyed their students about their opinions on the experiment and found that they valued green chemistry in their curriculum ( Citation9). Other chemical disciplines are seeing greener methods, though many are published without specifically being associated with green chemistry or sustainability ( Citation10).
Inquiry in organic chemistry education
The science laboratory is used to supplement the lecture by improving conceptual understanding, developing inquiry and technical skills as well as motivating students ( Citation11). Students often leave chemistry laboratories with slightly more knowledge than they entered with ( Citation12,Citation13). One reason for this is that emphasis may be placed on the result rather than the process of achieving the result (expository instruction) ( Citation14). Inquiry experiments can provide students with the opportunity to actively engage in science through a more discovery-based learning opportunity. Providing students with authentic research type labs (open-inquiry instruction) may become frustrating for students with limited instruction and not enough time to reflect ( Citation12,Citation15). Mohrig ( Citation12) suggests that open-inquiry is not suited for introductory Organic courses, but question-first guided-inquiry is ideal to promote critical thinking and student enjoyment.
Organic inquiry-based laboratories have been published ( Citation16–20) that teach techniques and/or traditional reactions. Mistry et al. ( Citation17) had students design their own liquid-liquid extraction procedure in a guided-inquiry experiment. Students were given a mixture of three compounds to separate that contained an acid and basic compound with characteristic functional groups. Hammond et al. ( Citation18) had students conduct an aldol condensation to form chalcones and subsequent hydrogenation. Students had to determine the functional groups on the starting materials and reaction regiochemistry. The lab improved students understanding of spectroscopy. Montes et al. ( Citation19) had students evaluate the solubility of a variety of Organic compounds in various solvents and amounts of solute. Mackay et al. ( Citation20) has a guided-inquiry Wittig experiment in which students investigate how substituents impact the E/Z selectivity and then have students explain the findings.
Green chemistry and inquiry
Schoffstall and Gaddis suggest that guided-inquiry labs can be implemented by revision of expository labs ( Citation11). Several of the experiments described in the previous section took this approach in which they re-casted traditional experiments to make them guided-inquiry based ( Citation21) or greener. An approach to guided inquiry-oriented experiments would take these greener reactions and change reaction conditions or reagents to compare the metrics or effectiveness vs. safety of reagents and solvents. Providing students with the tools and principles that are used when greening reactions, guided-inquiry can benefit students understanding of course content. Using green chemistry as a theme can appeal to students providing something tangible for them to explore and develop their critical thinking skills. Cooper ( Citation22) suggests that the laboratory experience can be valuable if the instructor understand the purpose of the inquiry-laboratory and when students see their role as active. Green chemistry may provide the motivation for instructors and students to value the experiments.
Experiments can be re-cast in which the experiment is redesigned to incorporate a theme or objective. Experiments can be re-cast to allow students to explore a green inquiry question through evaluation of reagents, catalysts, solvents, separation methods, work-up conditions, and how reaction conditions impact green metrics. There are some experiments that incorporate inquiry but have some aspect of green chemistry ( Citation1,Citation23) or examine chemical hazards ( Citation23). Green principles can be incorporated into synthesis to provide compounds for bioassays. Doxsee and Hutchinson ( Citation1) use combinatorial chemistry to produce a library of compounds to test for antibiotic activity but employs green principles. Eby and Deal ( Citation23) used an electrophilic aromatic substitution experiment in which students determine the directing effects of substituent groups on salicylamide upon green iodination and rationalize the results. Students in this situation can easily use their knowledge to predict the amide and hydroxyl effects, but through the experiment must consider the size of the iodine atom.
Re-casting organic experiments
Re-casting to incorporate green chemistry inquiry: examples of experiments and qualitative analysis of reports
At Florida Southern College (FSC), green chemistry principles have been used throughout the Organic chemistry laboratories but are also heavily discussed in the lecture courses, particularly Organic II. lists examples of experiments conducted by FSC students. Incorporation of green chemistry and sustainability topics introduces our majors to these important ideas as future chemists. However, a large majority of Organic students are non-majors, primarily pre-professionals and students interested in environmental science. Green chemistry as a theme appeals to these students because of the environmental and health impacts. The green chemistry principles published by Anastas and Warner ( Citation24) are used as a guide. The mission of the experiments in our Organic chemistry course is driven by a quote by Mary Kirchhoff ( Citation25) Educating students about environmentally friendly alternatives to traditional solvents, reagents, and reaction conditions fosters critical thinking and promotes sustainability through green chemistry.
Table 1. Examples of organic experiments and related green principles used at FSC.
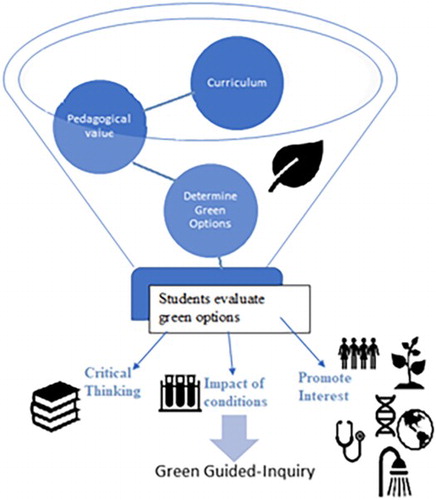
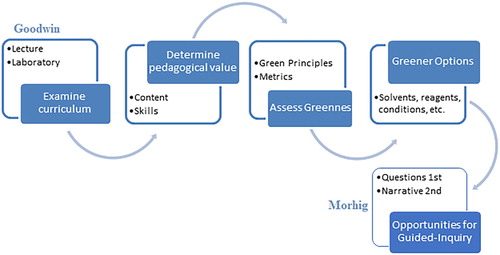
Re-casting experiments is an easy way to modify experiments currently used as green guided-inquiry experiments. Mohrig ( Citation12) suggests re-casting experiments with a question first. Goodwin ( Citation36) provides a framework for developing green Organic experiments (). I argue that students can assess alternative reagents or compare methods to green a reaction. It is important that students realize that all risks cannot be eliminated, or all 12 green principles incorporated. This process can be used for students to develop critical thinking skills, connect the results to developing greener methods, and provide students with mini-research questions. Students are asked to consider more than just the basic mechanism of a reaction. Students are asked to consider the conditions of the reaction including the environmental and human risks vs. the effect on the reaction. I argue that strictly grading students based on yield and/or purity, teaches students only the technique and/or the reaction. Though important, this does not improve their understanding of the content or how to troubleshoot a technique. Students are not only exposed to tools that can be used to green an experiment, they are also challenged to compare the reagents, solvents and conditions in the experiment. These experiments encourage students to also consider the process of achieving the results ( Citation14). Students are challenged to think more deeply about the experiments and consider the reactions holistically. The design of such an experiment lends itself easily to guided-inquiry opportunities which as Mohrig suggested promoted critical thinking and student enjoyment in the lab ( Citation12). As Goodwin ( Citation36) perfectly stated, Discussing with students the criteria used in selecting and rejecting experiments is a valuable educational experience. This was the essence of designing green guided-inquiry laboratories ().
Figure 1. Graphical representation of the flow of the development of the re-casting of traditional experiments using Goodwin’s framework (36) Mohrig’s question first approach ( Citation12).
With easy access to literature and the variety of websites to find student reports, students often Google reagents or experiments to determine what the outcome of the reactions should be. Re-casting experiments through examining changes to the green aspects of the experiments and aligning the experimental question may improve the authenticity of the guided-inquiry experiments. When these experiments were implemented, students asked more questions about other changes that could be made to the experiment. There seems to be less plagiarizing of data between academic years (harder to copy when the experimental questions differ each year). More students are asking why they get the results they do (critical thinking), and students have begun asking more about green applications to concepts discussed in lecture (applications). In the long term, it could be expected that students may use the lens of green chemistry in other courses or their own sustainability behaviors. is an overview of some experiments with green or inquiry-questions. Questions and experiments are cycled through to reduce students reviewing data or notebooks from the previous semester. A selection of laboratory reports for some experiments over the last year was reviewed ( Citation37). Quotes from student reports and the surveys are included in the discussion of the document (italics).
Table 2. Examples of guided-inquiry questions for re-casted green experiments at FSC.
Separation comparison experiment
Emphasis is rarely placed on the pros and cons of different separation methods as it applies to green chemistry. Like Revell ( Citation28), FSC students compared different separation methods of Excedrin (recrystallization, column chromatography and liquid-liquid separation) as well as different evaluation methods (melting point ranges and TLC vs spectroscopic methods) but are asked to put them in context with success and green chemistry in their reports. When we focus on greenness in the lecture, we often neglect the lack of greenness in the separation ( Citation10).
All students suggested that column chromatography or recrystallization were the better methods. They qualified these statements to include that column chromatography appeared to be more helpful when separating more than two compounds or compounds that would not be easy to separate by solubility. Some reports explicitly addressed the choice of purification relevant to green chemistry, noting that ethanol, a renewable feedstock, was used in the recrystallization and is better than ethyl acetate or silica gel in column chromatography. Others noted that recrystallization used less solvent in general, but did not give the purest product, while column chromatography allowed them to cleanly separate three components if done correctly. There may be an impact of whether column or recrystallization was better based on guidance from the instructor.
column chromatography was the best method for separation … The reasoning behind this is that it required for very small amounts to be divided into multiple test tubes and those fractions provided the most accurate data. However, it proved to be more wasteful. [than recrystallization]
CO2 extraction of limonene
Students extracted limonene from various citrus fruits and compared them using instrumentation (IR, GC-MS, refractometer, and polarimetry, but in subsequent semesters will include NMR). Carbon dioxide extraction is less time consuming than steam distillation, leaving time for discussion of the designated questions (). Students found their extracts to be impure limonene (including traces of water and other minor components). The optical rotation varied between the samples but indicated the sample primarily contained the R isomer.
The extraction of limonene, in this case, was an Organic solvent extraction and this was relatively green because carbon dioxide was used. Carbon dioxide is better than other solvents because it is safer to humans and also to the environment.
I really liked comparing the data and seeing the impurities of our limonene that we extracted from orange peel using dry ice.
This was very intriguing to actually see how pressure and temperature can be manipulated to extract limonene from lemon zest. I did learn a lot about how a polarimeter and refractometer work as well as reading the data produced from the instruments analysis.
Esterification ()
Esterification is a common reaction conducted in the Organic laboratory, especially due to the appeal of the aromas of some esters ( Citation37–40). In FSC labs, students have explored impacts of the substitution on nine benzoic acids and different reaction conditions ( Citation29). Benzoic acids were chosen because most of the products were solids and easy to separate from the alcohols. Students compared a solid-phase catalyst, sulfuric acid, use of thionyl chloride, time, conventional heating, and microwave heating (). They evaluated the collaborative data as well as the impact of the substituent (nine carboxylic acids). The reactions were evaluated by GC-MS and NMR. Students propose experiments and implement them (after consultation with the instructor).
Scheme 1. Esterification of nine benzoic acids using different heating methods, catalysts or formation of acid chloride.
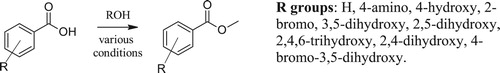
Students observed the reaction time was significantly shortened using the microwave (as they expected). Some found that the Amberlyst-15 catalyst required longer reaction times than sulfuric acid in a water bath. Though this may be due to student errors when determining if the reaction began refluxing as the Amberlyst-15 was the first catalyst they used. This was not something students speculated would occur in the microwave. The varying temperature of the water could cause some of the reaction to not reflux properly and the reaction would not have adequate conditions to fully complete. Students speculated that the substituents on the ring likely influence the electrophilicity of the carbonyl carbon atom due to inductive and resonance effects.
We try to utilize methods that are more efficient and produce less waste. An example of this was in our unit 2 lab where the first week we used a refluxing system that took a lot of energy and time and gave a larger amount of waste compared to the second week method where we used a microwave reactor that had the reaction proceed in a pressurized environment at a specific temperature. The microwave reactor took 1/4 of the time to react than the refluxing system.
A few students correctly noted that the results could be skewed as the sulfuric acid catalyst required a base work-up that may reduce some of the carboxylic acid present, hence the acid: ester ratio would be altered. A work-up added an additional step removing the beads. They found that although SOCl2 was corrosive, the reactions completed faster with higher yield ( Citation28), while solid catalysts can be re-used. This catalyst was a solid though so the ability for it to be extracted much easier than the thionyl chloride was very important. These are examples where reaction metrics could be used more in the experiment.
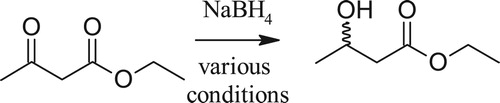
Reduction of ethyl acetoacetate ()
The reduction of ethyl acetoacetate using sodium borohydride with L-tartaric acid or use of biosynthesis has been published ( Citation34,Citation35). This experiment has been re-casted to evaluate the effectiveness of the solvents and other chiral auxiliaries. Students found the yeast reduction method (using sucrose) to be difficult to work with, wasteful and did not seem as effective in terms of yield ( Citation35). Students varied the solvent of the NaBH4-tartaric acid reduction as well as replacing tartaric acid with a variety of amino acids. The amino acids were less effective ( Citation41), but students did not find a clear consensus with the solvents. This may be due to the scale of the reaction and their inexperience with microextractions. However, this resulted in students thinking about the role of the solvent in the reaction. Upon looking at the GSK Solvent guide, I learned that there are several factors that are taken into account [considered] when evaluating how green a solvent is. Some of these factors include life cycle score, waste, acute/chronic health impact, and environmental impact.
Scheme 2. Chiral reduction of ethyl acetoacetate using a variety of chiral auxiliaries and solvent conditions.
Though students predicted that the D-tartaric acid would give the opposite orientation in excess than the L-tartaric acid (result of L-tartaric acid is heavily published), some students obtained the opposite rotations. One issue a few students encountered was not washing their product well enough and leaving tartaric acid. It was important that they analyzed their NMR before making judgements about the polarimetry. With relatively pure products, students found D-tartaric acid gave a lower %ee, but opposite rotation. The yeast method produced a more enantiomerically pure product, but they could appreciate that they have less waste in the NaBH4-tartatic acid reduction from the reaction (other isomer). One specific enantiomer of tartaric acid is used to produce an enantiomeric excess of a single enantiomer to reduce waste.
Solid-support synthesis and Boc deprotection
Peptide synthesis was conducted as a multi-week experiment in which the material of one week is used for the next. … we often tried to make use of what we made in the last lab for the next one instead of making material and then using other sources of the material. Students could conclude the solid-support method had less waste as it did not require chromatography at each step. Various Boc deprotections were used. Benign methods published were compared to the hydrolysis in HCl. Students found that aqueous and MeOH-potassium phosphate methods conducted in the microwave worked well ( Citation32,Citation33).
Within these methods, green chemistry techniques can be observed through the use of the microwave reactor and methanol. The microwave reactor is energy efficient while methanol is a safer solvent [than ethyl acetate/HCl]. … The microwave reactor reduces the time of a reaction, produces little to no side products and increases the product yield.
This unit provided me with the opportunity to learn more about solid-state synthesis, … The principles behind solid-state chemistry are simple: rather than performing the reaction in solution, perform the reaction on a solid. … I am beginning to take interest in solid-state synthesis from a materials perspective. … This may be something I look into as a senior research project.
We also take extra effort to recycle things that are recyclable, such as the beads in the peptide synthesis lab. … that saves a lot considering how many students use the beads every year, not just in our class, but classes worldwide.
A result not captured in the reports are the questions students ask in lab or lecture. FSC students are asking questions about green chemistry and environmental issues, including recycling of polymers, materials in electronics, reactions in the laboratory they see in lecture, and things they can do on campus to promote green chemistry. Students asked general questions such as what the differences in solvent choices are, what other substances can be used in a reaction, what can they do to improve reaction efficiency, and why did some groups produce different results. Other than learning the content, all instructors want students to think critically about the content and consider applications of it.
Student thoughts on green chemistry and guided inquiry experiments
Organic students were asked their opinion about green chemistry (n = 44 respondents, 76% response rate) and the guided-inquiry experiments through surveys and assignments (2017–2018). The inquiry survey was given for four experiments in Organic II (n = 116 total responses, 61.3% response rate average). Three experiments are reported here. Organic II students in Fall 2017 and Spring 2018 were asked about inquiry-based and green chemistry labs in Spring 2018. Former Organic chemistry students (n = 13 respondents, 72% response rate) who were enrolled in medicinal chemistry were also asked about green chemistry after learning about large-scale production for the pharmaceutical industry. Students were asked their thoughts on green chemistry (). These responses used a Likert scale: 1 – strongly disagree; 5 – strongly agree. No student absolutely disagreed with any criteria.
Table 3. Student perceptions on green chemistry using a Likert scale: 1 – strongly disagree; 5 – strongly agree.
Most positive responses were absolutely agree in each criteria, where 100% of students agreed that the Organic course taught them a lot about green chemistry. Two students who did not find green chemistry important to their lives or courses stated in their free response that it was important, but only at a large industrial scale. When asked if they would like to see applications of green chemistry in other courses, 91.2% said “yes” or “some.” summarizes the concepts students mentioned in their discussion on the relevance of green chemistry.
Table 4. Students descriptions on the relevance of green chemistry – “In your own words, what is the relevance of learning about green chemistry (to you personally, in general and/or as a scientist)?”.
When Organic students were asked to describe applications of green chemistry, all comments discussed laboratory or theoretical (general) applications and two (4.5%) students mentioned the discussions from lecture. Most students’ descriptions were general (not specific to an experiment), with lab specific comments on the esterification experiment with mention to the comparison of reagents, catalysts and heating methods (). Most of the comments were related to hazard reduction. 21 students had some mention of “use of microscale” for hazard reduction and safety. Microscale chemistry can complement green chemistry pedagogy as a pollution prevention method ( Citation42). It is important to note that microscale reactions are not hazard-free, as small scale for students may cause them to relax their safety concerns or reduce the proper waste disposal practices. This is often seen in the hazard section of their notebook, as the severity of the hazards relative to the quantity is not clear to the students. Some safety concerns may be exaggerated or underrated. Duarte et al. proposed a safety assessment as green metrics (ecological paradigm) do not completely capture the risk paradigm of microscale chemistry ( Citation43) and Beyond Benign has a Chemical Hazard Awareness module ( Citation44).
Table 5. Student descriptions of some applications of green chemistry – “Describe some applications of green chemistry in your labs and lectures that you learned. Add examples you may have to explain”.
Below are some examples of student comments on the relevance of green chemistry as well as applications to lab and lecture.
Being thoughtful and responsible in scientific endeavors in an attempt to reduce waste and make reactions more efficient, using ‘greener’ reagents and solvents. (social/ethical, waste, safety)
… I believe that it is our responsibility to work towards becoming a greener society and it starts in our education. Green chemistry is more of mind set that can flow into other aspects of your life in ‘green’ ways. (social/ethical)
… we have learned about drugs which act differently depending on the enantiomer. Using green chemistry to find ways to insure [ensure] the right regiochemistry and stereochemistry is very important. (social/ethical, waste, safety)
… The creation of usable materials for everyday life requires an immense amount of energy as well as the output of toxic materials … Finding new ways to synthesize products and reducing the possibility of harm toward industry workers is essential for our future as mankind. Living on this earth is a privilege, not a right, and the sooner we figure that out the better. (energy, safety, social/ethical)
Green chemistry is necessary to optimize our use of resources and ultimately save money and the environment. (environment, cost, waste)
… using Ethanol as a solvent. It is much greener than other options, it is also less harmful … This works particularly well for educational purposes because we are not researching, we just need to understand the concepts. (reduce by-products)
The survey regarding guided-inquiry experiments did not have any questions regarding green chemistry, however ten of the 116 responses explicitly discussed applications of green chemistry as it related to the guided-inquiry labs and some others seem to allude to it. Sixteen percent of student comments explicitly stated that the guided-inquiry labs were either research or relevant to research. 24% of comments discussed how making changes to the reaction and the impact on mechanism, product formation (including yield), or usefulness of reagents or solvents. Since the experiments required making observations on how reagent, solvent or condition changes impact the reaction or greenness, it is not surprising twelve percent of comments had to do with behaviors in the lab such as careful observations, cleanliness, preparation, teamwork, and collaborative data collections as key to determine impacts on reactions. The comments indicate the need for clearer statements or explanations in the experiments that the reaction may not give the desired product, as students often thought they did something wrong (though on occasion they did). As stated by Andraos and Dicks ( Citation10), texts often place emphasis on yield, rather than quality of the reactions. Below are some examples of responses related to green chemistry and research.
I learned that as a chemist looking at substituents is very important so that you are able to create the greenest synthesis possible as it affects products:
Being a scientists (sic) comes with alot (sic) of failure, and trial and error. I believe (sic) it is a fun experience (sic) and intriguing concept.
I finally experienced the actual feeling of doing research and how most of the time it doesn’t actually work or you have to keep trying new things until it does.
This lab addressed green chemistry from a perspective I do not recall learning much about so I enjoyed the opportunity understand the use of specific catalysts and the impacts they have on reaction yields.
That most reactions do not work and that a lot of the labs that er (sic) are given in classes have been performed many times before us and are proven to work or not to work. So we can not expect to see promising results all the time.
Students stated they would be interested in more discovery style experiments (). It may be implied that they had enough variables to draw reasonable conclusions or any additional parameter would be burdensome of the study. For example, students collaboratively examined nine carboxylic acids, two heating methods, and two catalysts vs. acid chloride for the esterification of benzoic acids, in addition to proposing experiments.
Table 6. Students interest in discovery style experiments and examining additional data
Conclusion
Green chemistry was used as a theme in developing guided-inquiry-based experiments. The information presented here provides some examples of experiments that were adapted or developed in which students can answer some key questions related to green principles. Student reports showed that students are thinking about the green principles even in experiments when they are not explicitly asked to discuss them. Students successfully completed the experiments, wrote reports and completed quizzes like traditional experiments. FSC students asked general questions about solvent choices, changes in reagents, why a chemist might conduct a certain reaction with harmful reagents, and what can they do to make the reaction more effective. Further studies on the effectiveness of green chemistry inquiry-based labs need to be examined.
Throughout the years, it is assumed students enjoyed the implementation of greener methods in their experiments. Student perceptions about the green chemistry theme could influence the investment students have in the guided-inquiry experiences. Students have a favorable opinion of green chemistry and guided-inquiry from the two assessments. Though the inquiry or green chemistry assessments were not connected, some discussions between the assessments imply students can see the connections between them. Some students compared the changing of variables to research and it is important for students to make the connections between these skills and the applications. This lab involved conducting the same experiment … each time changing different variables. … this experiment offers students a glimpse into actual research.
Acknowledgements
I would like to thank the editors for the opportunity to share my experience, the reviewers for their feedback, my colleagues who have been patient with the curriculum changes I made often to FSC labs, and students who provided feedback and comments. The support for how to successfully re-cast experiments was though the expertise of the cCWCS workshop on Teaching Guided-Inquiry Organic Labs (2016).
Disclosure statement
No potential conflict of interest was reported by the author.
Notes on contributor
Deborah Bromfield Lee is an Associate Professor of Chemistry at Florida Southern College. Deborah received her doctorate at North Carolina State University under the supervision of Dr. Maria Oliver-Hoyo. Her teaching responsibilities include the Organic chemistry sequence (lecture and laboratories), research courses, and a Food Chemistry course. Her research interest includes incorporating greener methods in the lab, as well as observing student perceptions and learning gains. She is an active member in the ACS Chemical Education Division (CHED). She has co-chaired the CHED programs for the ACS meetings in Boston (2018) and Orlando (2019). She has presented at ACS regarding Organic laboratories focusing on green chemistry, electronic notebooks, and inquiry.
ORCID
Deborah Bromfield Lee http://orcid.org/0000-0002-5270-7206
References
- Doxsee, K.M.; Hutchinson, J.E. Green Organic Chemistry: Strategies, Tools, and Laboratory Experiments, 1st ed.; Brooks/Cole Cengage Learning: University of Oregon, 2004.
- Dicks, A.P., Ed. Green Organic Chemistry in Lecture and Laboratory, 1st ed.; CRC Press: Boca Raton, FL, 2012.
- Montes, I.; Sanabria, D.; García, M.; Castro, J.; Fajardo, J. J. Chem. Educ. 2006, 83 (4), 628. doi: 10.1021/ed083p628
- Morsch, L.A.; Deak, L.; Tiburzi, D.; Schuster, H.; Meyer, B. J. Chem. Educ. 2014, 91 (4), 611–614. doi: 10.1021/ed400408k
- Palleros, D.R. J. Chem. Educ. 2004, 81 (9), 1345. doi: 10.1021/ed081p1345
- Hulce, M.; Marks, D.W. J. Chem. Educ. 2001, 78 (1), 66. doi: 10.1021/ed078p66
- Russell, C.B.; Mason, J.D.; Bean, T.G.; Murphree, S.S. J. Chem. Educ. 2014, 91 (4), 511–517. doi: 10.1021/ed400064f
- McKenzie, L.C.; Huffman, L.M.; Hutchison, J.E.; Rogers, C.E.; Goodwin, T.E.; Spessard, G.O. J. Chem. Educ. 2009, 86 (4), 488. doi: 10.1021/ed086p488
- Lang, P.T.; Harned, A.M.; Wissinger, J.E. J. Chem. Educ. 2011, 88 (5), 652. doi: 10.1021/ed100853f
- Andraos, J.; Dicks, A.P. Chem. Educ. Res. Pract. 2012, 13 (2), 69–79. doi: 10.1039/C1RP90065J
- Schoffstall, A.M.; Gaddis, B.A. J. Chem. Educ. 2007, 84 (5), 848. doi: 10.1021/ed084p848
- Mohrig, J.R. J. Chem. Educ. 2004, 81 (8), 1083. doi: 10.1021/ed081p1083
- Gallet, C. J. Chem. Educ. 1998, 75 (1), 72. doi: 10.1021/ed075p72
- Domin, D.S. J. Chem. Edu. 1999, 76 (4), 543–547. doi: 10.1021/ed076p543
- Hofstein, A.; Lunetta, V.N. Sci. Educ. 2004, 88 (1), 28–54. doi: 10.1002/sce.10106
- Mohrig, J.R.; Alberg, D.; Hofmeister, G.; Schatz, P.F.; Hammond, C.N., Laboratory Techniques in Organic Chemistry: Supporting Inquiry-Driven Experiments, 4th ed.; W. H. Freeman and Company: New York, 2014.
- Mistry, N.; Fitzpatrick, C.; Gorman, S. J. Chem. Educ. 2016, 93 (6), 1091–1095. doi: 10.1021/acs.jchemed.5b00691
- Hammond, C.N.; Schatz, P.F.; Mohrig, J.R.; Davidson, T.A. J. Chem. Educ. 2009, 86 (2), 234. doi: 10.1021/ed086p234
- Montes, I.; Lai, C.; Sanabria, D. J. Chem. Educ. 2003, 80 (4), 447. doi: 10.1021/ed080p447
- MacKay, J.A.; Wetzel, N.R. J. Chem. Educ. 2014, 91 (5), 722–725. doi: 10.1021/ed3003836
- Davidson, T.A.; Wissinger, J. cCWCS Workshop - Teaching Guided-Inquiry Organic Labs - Recasting Experiments, June 2016.
- Cooper, M.M.; Kerns, T.S. J. Chem. Educ. 2006, 83 (9), 1356. doi: 10.1021/ed083p1356
- Eby, E.; Deal, S.T. J. Chem. Educ. 2008, 85 (10), 1426. doi: 10.1021/ed085p1426
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998.
- Kirchhoff, M.M. J. Chem. Educ. 2013, 90 (6), 683–684. doi: 10.1021/ed400295g
- McKenzie, L.C.; Thompson, J.E.; Sullivan, R.; Hutchison, J.E. Green Chem. 2004, 6 (8), 355–358. doi: 10.1039/b405810k
- Kradtap Hartwell, S. Chem. Educ. Res. Pract. 2012, 13 (2), 135–146. doi: 10.1039/C1RP90070F
- Revell, K.D. J. Chem. Educ. 2011, 88 (10), 1413–1415. doi: 10.1021/ed101195v
- Bromfield Lee, D. C. A. K. To be submitted for publication 2019.
- Leung, S.H.; Angel, S.A. J. Chem. Educ. 2004, 81 (10), 1492. doi: 10.1021/ed081p1492
- Bockman, M.R.; Miedema, C.J.; Brennan, B.B. J. Chem. Educ. 2012, 89 (11), 1470–1473. doi: 10.1021/ed2008813
- Thaqi, A.; McCluskey, A.; Scott, J.L. Tetrahedron Lett. 2008, 49 (49), 6962–6964. doi: 10.1016/j.tetlet.2008.09.027
- Wang, G.; Li, C.; Li, J.; Jia, X. Tetrahedron Lett. 2009, 50 (13), 1438–1440. doi: 10.1016/j.tetlet.2009.01.056
- Yotagai, M.; Ohnuki, T. J. Chem. Soc., Perkin Trans. 1990, 1 (6), 1826–1828. doi: 10.1039/P19900001826
- Pohl, N.; Clague, A.; Schwarz, K. J. Chem. Educ. 2002, 79 (6), 727. doi: 10.1021/ed079p727
- Goodwin, T.E. J. Chem. Educ. 2004, 81 (8), 1187–1190. doi: 10.1021/ed081p1187
- Bromfield Lee, D.C. Presented at 255th National Meeting of the American Chemical Society, New Orleans, LA, March 18–22nd 2018; Paper CHED 2003.
- Minter, D.E.; Villarreal, M.C. J. Chem. Educ. 1985, 62 (10), 911. doi: 10.1021/ed062p911
- Bromfield-Lee, D.C.; Oliver-Hoyo, M.T. J. Chem. Educ. 2009, 86 (1), 82. doi: 10.1021/ed086p82
- Janssens, N.; Wee, L.; Martens, J. A. J. Chem. Educ. 2014, 91 (6), 876–879. doi: 10.1021/ed400356j
- Kim, J.; Suri, J. T.; Cordes, D. B.; Singaram, B. Org. Process Res. Dev. 2006, 10 (5), 949–958. doi: 10.1021/op060079d
- Singh, M. M.; Szafran, Z.; Pike, R. M. J. Chem. Educ. 1999, 76 (12), 1684. doi: 10.1021/ed076p1684
- Duarte, R. C. C.; Ribeiro, M. G. T. C.; Machado, A. A. S. C. J. Chem. Educ. 2017, 94 (9), 1255–1264. doi: 10.1021/acs.jchemed.7b00056
- Benign by Design. – Module: Chemical Hazard Awareness. https://www.beyondbenign.org/lessons/modulechemicalhazard-awareness/ (accessed July 2018) 2018.
